Abstract
Brain metastasis occurs in approximately 50% of patients with advanced HER2-positive breast cancer. Despite improved prognosis, survival remains limited. This study aims to investigate the clinicopathological characteristics of HER2-positive breast cancer brain metastasis (BCBM) patients and their prognostic associations, to identify personalized treatment strategies to enhance survival. This retrospective study included HER2-positive BCBM patients treated at three institutions: the Fifth Medical Center of the Chinese PLA General Hospital, Peking University First Hospital, and the First Affiliated Hospital of Xi’an Jiaotong University. Clinical, pathological, and treatment data were collected. A prognostic model was developed using recursive variable selection, starting with significant variables from univariate analysis and refining them through a recursive loop in multivariate Cox regression. The model stratified patients into three risk groups: low-risk (score 0–1), intermediate-risk (score 2–3), and high-risk (score 4–6), based on various independent prognostic factors. The median survival times for the low-, intermediate-, and high-risk groups were 30, 20, and 10 months, respectively (P < 0.0001, HR1 = 1.35, 95% CI: 0.94–1.94; HR2 = 4.02, 95% CI: 2.4–6.73). The mean ROC curve AUC values for the 1-year, 2-year, and 3-year prognostic predictions were 0.69, 0.70, and 0.61, respectively. In the independent validation cohort of 75 patients, prognostic stratification into low-, intermediate-, and high-risk groups revealed significant differences in outcomes (73 months vs. 35 months vs. 9 months, P < 0.001, HR1 = 2.68, 95% CI: 1.30–5.52; HR2 = 8.82, 95% CI: 3.89–19.97). The mean AUC value for the 1-year and 2-year prognostic predictions in the validation cohort was 0.94, whereas the mean AUC value for the 3-year prognostic prediction was 0.81. This study developed a prognostic stratification model for HER2-positive BCBM patients based on clinicopathological characteristics.
Similar content being viewed by others
Introduction
Breast cancer is the most prevalent malignant tumor among women worldwide, and with its incidence rising annually in China, where it ranks as the leading cause of cancer in females1. Approximately 10–30% of breast cancer patients ultimately develop brain metastasis, with a median survival time of less than 6 months, a 1-year survival rate of approximately 20%, and a 2-year survival rate of only 2%. The treatment of breast cancer has now advanced significantly with the advent of molecular subtyping. Breast cancer is classified into luminal, HER2-positive, and triple-negative breast cancer subtypes on the basis of hormone receptor (HR) status, human epidermal growth factor receptor 2 (HER2) status, and Ki-67 expression2,3. On the basis of hormone receptor (HR) status, HER2 status, and Ki-67 expression, breast cancer is classified into luminal, HER2-positive, and triple-negative subtypes. Among these patients, approximately 50% with advanced HER2-positive breast cancer will develop brain metastasis, with an even higher incidence in those who also present with multiple extracranial metastases, such as in the bones, liver, and lungs4.
The primary treatment options for brain metastasis continue to be local therapies, including surgery, whole-brain radiotherapy (WBRT), and stereotactic radiosurgery (SRT). While radiotherapy continues to play a pivotal role in the management of brain metastasis, its dosage and frequency are limited1. Consequently, drug treatment for brain metastasis, especially for HER2-positive breast cancer patients, has become a focal point of research in recent years5,6. Small-molecule tyrosine kinase inhibitors (TKIs), such as lapatinib, neratinib, pyrotinib, and tucatinib, have demonstrated significant intracranial efficacy in treating HER2-positive brain metastasis. Additionally, novel antibody-drug conjugates (ADCs), such as T-Dxd, have shown promising results. Despite these advances and improvements in the prognosis of patients with HER2-positive brain metastasis, the survival time remains limited7. Accurately predicting patient prognosis and effectively integrating treatments, including drug therapy, radiotherapy, and surgery, continue to be major challenges8,9.
In clinical practice, treatment strategies are often established on the basis of the patient’s expected survival time10. A survey on the selection of local treatments for multiple brain metastases revealed that one-third of clinicians use recursive partitioning analysis (RPA) and graded prognostic assessment (GPA) scores to choose SRT11,12. However, these models do not incorporate breast cancer-specific prognostic or treatment factors. The Breast GPA, an optimized version of the original GPA score proposed by Sperduto et al., is more widely recognized11,13. The initial Breast GPA categorized breast cancer brain metastasis into three prognostic grades on the basis of the Karnofsky Performance Scale (KPS) score, breast cancer molecular subtype, and age (applicable only to patients with KPS scores between 60 and 80)14. In 2015, the MD Anderson Cancer Center revised the Breast GPA to create the MDACC-GPA, refining the prognostic grades into four categories and incorporating the number of brain metastases as a prognostic factor, increasing the concordance index from 0.78 (95% CI, 0.77–0.80) to 0.84 (95% CI, 0.83–0.85)15,16. In 2020, Sperduto et al. updated the Breast GPA17including extracranial metastasis and the time interval from primary cancer diagnosis to metastatic cancer diagnosis, as prognostic factors, maintaining the four-grade prognostic system18. However, with advancements in comprehensive treatment approaches for brain metastasis, particularly progress in drug treatments for HER2-positive brain metastasis patients, the existing scoring systems cannot accurately predict patient prognosis19,20. It is necessary to establish a more refined scoring system tailored to breast cancer brain metastasis, enabling clinicians to set treatment goals and guide treatment strategies in a stratified, rational, and orderly manner.
Therefore, our center conducted a real-world study focused on patients with HER2-positive breast cancer brain metastasis (BCBM). The objective of this study is to investigate the clinicopathological characteristics of HER2-positive BCBM patients and their association with prognosis. Additionally, this study aims to identify personalized treatment strategies for different risk groups in order to improve the survival outcomes of HER2-positive BCBM patients.
Patients and methods
Study design
This retrospective study included patients diagnosed with breast cancer brain metastasis who received treatment at three institutions: the Fifth Medical Center of the Chinese PLA General Hospital, Peking University First Hospital, and the First Affiliated Hospital of Xi’an Jiaotong University. The training cohort comprised HER2-positive breast cancer brain metastasis patients who met the predefined inclusion criteria. These patients were treated at the Fifth Medical Center of the Chinese PLA General Hospital from June 2003 to June 2022. The validation cohort was drawn from two hospitals: Peking University First Hospital, with patients treated between 2008 and 2022, and the First Affiliated Hospital of Xi’an Jiaotong University, with patients treated from 2012 to 2022.
Given the retrospective nature of the study, informed consent was waived by the Ethics Committee of the Fifth Medical Center of Chinese PLA General Hospital. The study was conducted in accordance with local ethical guidelines and was approved by the Ethics Committee of the Fifth Medical Center of Chinese PLA General Hospital (No. 2012L01067).
Patient selection
Patients included in this study met the following criteria: (1) histopathologically confirmed HER2-positive breast cancer of the primary lesion; (2) brain metastasis confirmed by CT or MRI; (3) at least one measurable intracranial lesion according to the RECIST 1.1 criteria; (4) age ≥ 18 years; and (5) female breast cancer patients.
HER2-positive status was defined as an immunohistochemistry (IHC) membrane staining score of 3+, HER2-negative status was defined as 0, and low HER2 expression was defined as a membrane staining score of 1+. For ambiguous HER2 IHC scores (2+), fluorescence in situ hybridization (FISH) was performed. According to the ASCO/CAP standards, patients with a HER2/CEP17 ratio ≥ 2.0 and a HER2 gene copy number ≥ 4 or a HER2/CEP17 ratio < 2 but a HER2 gene copy number ≥ 6 were considered HER2 positive. A HER2/CEP17 ratio < 2 and HER2 gene copy number < 4 or a HER2/CEP17 ratio ≥ 2 but HER2 gene copy number < 4 were considered low HER2 expression. The estrogen receptor (ER) and progesterone receptor (PR) statuses were detected via immunohistochemistry (IHC), with a positivity threshold of ≥ 1%. Hormone receptor (HR) positivity was defined as being ER/PR positive, and HR negativity was defined as being ER and PR negative. Brain metastasis overall survival (BMOS) was defined as the time from the diagnosis of brain metastasis to death or the end of observation.
Data collection
The data collection period began at the time of breast cancer diagnosis, and the subsequent observation period was conducted through telephone interviews or outpatient visits. Clinical and pathological data were systematically collected, including general patient demographics, the date of breast cancer diagnosis, histological type and grade at initial diagnosis, tumor stage, molecular subtype, immunohistochemical markers, the date of BCBM diagnosis, the number and location of extracranial metastasis at diagnosis, the number of BCBM lesions, and post-diagnosis treatment and efficacy indicators, including progression-free survival (PFS) and overall survival (OS).
Model construction process
A recursive method was employed for variable selection. Step 1: Variables that showed statistical significance in univariate regression analysis were included in the candidate queue. Step 2: A recursive loop function was constructed, and variables were sequentially included in the multivariate Cox regression model. Each variable was assessed for its statistical significance in terms of both its contribution and hazard ratio. If a variable demonstrated statistical significance, it was retained in the model; otherwise, it was moved to the candidate eliminated queue, and the next iteration commenced. Step 3: When the candidate queue was exhausted, variables from the eliminated queue were re-assessed. This process continued until no further variables could be incorporated into the multivariate Cox regression model. At this point, the model training process was conducted, yielding the optimal model.
Statistical analysis
All the statistical analyses and survival analyses were performed via SPSS 22.0 software and R version 4.2.1. A P value < 0.05 was considered to indicate statistical significance. Clinical baseline data included quantitative data (median and range) and categorical data (total and frequency of subcategories). Univariate and multivariate Cox regression models were used to determine the relationships between prognostic factors and endpoint events in patients with BCBM. To address multicollinearity in recursive feature selection, we first assessed multicollinearity among all predictor variables by calculating variance inflation factors (VIF) and Pearson correlation coefficients. Variables with VIF > 5 or pairwise correlations > 0.8 were iteratively removed, starting with the variable showing the highest VIF value, until all remaining variables met the multicollinearity thresholds. Only after this pre-screening step was recursive feature elimination applied to the filtered dataset, ensuring that the final selected features were not only predictively important but also statistically independent, thereby avoiding model instability and interpretation issues associated with multicollinear predictors. Each characteristic was analyzed univariately, and those with statistical significance were included in the multivariate regression analysis. Survival curves were plotted via the log-rank test.
Results
Patient characteristics
Between June 2003 and June 2022, a total of 700 patients with BCBM were treated at the Fifth Medical Center of the Chinese PLA General Hospital. Of these, 226 patients with HER2-positive BCBM met the inclusion criteria for the training cohort (Supplementary Figure S1). The validation cohort included 32 patients treated at Peking University First Hospital from 2008 to 2022, and 43 patients treated at the First Affiliated Hospital of Xi’an Jiaotong University from 2012 to 2022. Data collection was completed in June 2022, with a median follow-up period of 57 months.
The median age of patients in the test cohort was 44.4 years (range: 25.0–75.3 years), while the median age in the validation cohort was 47.8 years (range: 24.0–70.0 years). The TNM stages for the test cohort were as follows: stage I, 25 patients (11%); stage II, 93 patients (43%); stage III, 74 patients (33%); and stage IV, 34 patients (15%). In the validation cohort, 9 patients (12%) were in stage I, 29 patients (39%) in stage II, 25 patients (33%) in stage III, and 12 patients (16%) in stage IV. HR positivity was observed in 105 patients (46%) in the test cohort and 34 patients (45%) in the validation cohort. Extracranial metastasis was present in 124 patients (55%) in the test cohort and in 47 patients (63%) in the validation cohort. Multiple brain metastases were identified in 69 patients (31%) in the test cohort and 27 patients (36%) in the validation cohort. In the test cohort, 20 patients (9.0%) underwent surgery following the diagnosis of brain metastasis, 181 patients (80%) received radiotherapy for brain lesions, 151 patients (67%) were treated with trastuzumab, 53 patients (23%) received trastuzumab combined with pertuzumab, 92 patients (41%) were treated with pyrotinib, and 26 patients (12%) received T-DM1. In the validation cohort, 11 patients (15%) underwent surgery following the diagnosis of brain metastasis, 56 patients (75%) received radiotherapy for brain lesions, 49 patients (65%) were treated with trastuzumab, 12 patients (16%) received trastuzumab combined with pertuzumab, 49 patients (65%) were treated with pyrotinib, and 10 patients (13%) received T-DM1. The detailed baseline characteristics of the patients are presented in Table 1.
Prognostic model for patients with BCBM indicator selection
Given that the age distribution of the patient cohort approximated a normal distribution (Fig. 1A), with a mean age of 48.5 years, a first quartile of 37 years, and a third quartile of 53 years, age cutoff thresholds were established at 40, 45, 50, 55, and 60 years. Univariate analysis was performed to evaluate the prognostic impact of different age thresholds on patient outcomes. The results indicated that an age cutoff of 60 years provided the best prognostic performance (Fig. 1B). Following the determination of the optimal age threshold, a Cox proportional hazards regression analysis was conducted to identify significant prognostic factors. Univariate analysis showed that the following factors were associated with survival outcomes in patients with brain metastasis: age ≥ 60 years at the time of brain metastasis diagnosis (HR = 1.97, 95% CI: 0.99–3.90, P = 0.05), KPS ≥ 60 (HR = 0.18, 95% CI: 0.08–0.45, P < 0.001), multiple brain metastases (HR = 1.71, 95% CI: 1.14–2.59, P = 0.01), more than three extracranial metastases at the time of brain metastasis diagnosis (multi extracranial metastasis) (HR = 1.55, 95% CI: 1.11–2.07, P = 0.01), and receipt of radiotherapy for brain metastasis (HR = 0.40, 95% CI: 0.26–0.62, P < 0.001) (Fig. 1C). Multivariate analysis further revealed the following independent prognostic factors for survival after brain metastasis diagnosis: age ≥ 60 years at diagnosis (HR = 2.36, 95% CI: 1.09–5.12, P = 0.03), KPS ≥ 60 (HR = 0.19, 95% CI: 0.08–0.44), multiple brain metastases (HR = 1.65, 95% CI: 1.12–2.44, P = 0.01), multiple extracranial metastases (HR = 1.40, 95% CI: 1.0–1.95, P = 0.05), and receipt of radiotherapy for brain metastasis (HR = 0.45, 95% CI: 0.30–0.69, P < 0.001) (Fig. 1D).
Selection of Prognostic Factors for HER2-Positive BCBM Patients. (A) Age distribution curve and histogram for 226 HER2-positive BCBM patients. (B) Univariate Cox regression analysis of different age thresholds and prognosis in BCBM patients. (C) Univariate Cox regression of various clinical and pathological characteristics related to prognosis. (D) Multivariate Cox regression identifying the optimal prognostic factors and their associations with prognosis.
Development of a prognostic model for patients with BCBM
Based on the identified independent prognostic risk factors, we developed a stratified assessment system (Table 2). For patients with HER2-positive BCBM, we assigned 1 point for age > 60 years at diagnosis, 1 point for a KPS score < 60, 1 point for multiple brain metastases, 1 point for not receiving radiotherapy for brain metastasis after diagnosis, and 2 points for multiple extracranial metastases (Table 2). The total risk score ranged from 0 to 6, with higher scores indicating a worse prognosis (Supplementary Figure S2). Patients were categorized into three groups based on the risk score: low-risk (0 ≤ score ≤ 1), intermediate-risk (2 ≤ score ≤ 3), and high-risk (4 ≤ score ≤ 6) ) (Fig. 2A). The median overall BMOS values for the low-, intermediate-, and high-risk groups in the test set were 30, 20, and 10 months, respectively (P < 0.0001, HR1 = 1.35 [95% CI: 0.94–1.94], HR2 = 4.02 [95% CI: 2.4–6.73]). The average area under the curve (AUC) values for the 1-year, 2-year, and 3-year prognostic predictions were 0.69, 0.70, and 0.61, respectively (Fig. 2B).
Evaluation of Prognostic Model Performance. (A) Prognostic differences among patients stratified by risk groups: high-risk (red), intermediate-risk (yellow), low-risk (green). (B) ROC curves evaluating the model’s predictive performance at different survival time points. (C) Prognostic Model Performance in 75 Independent Test Sets of HER2-Positive BCBM Patients. (D) ROC curve analysis of the model’s prediction performance at various survival time thresholds. E-F) Comparison of the Chinese Population Data Model with the GPA Model Comprehensive Assessment Index.
To further evaluate the generalizability of our model, we collected clinical data from 75 patients from Peking University First Hospital and the First Affiliated Hospital of Xi’an Jiaotong University. These patients were stratified into low-, intermediate-, and high-risk groups on the basis of the risk score. The median OS times for these groups were 73 months, 35 months, and 9 months, respectively (P < 0.001, HR1 = 2.68 [95% CI: 1.30–5.52], HR2 = 8.82 [95% CI: 3.89–19.97]). In the independent validation cohort, the average AUC values for the 1-year and 2-year prognostic predictions were both 0.94, and the 3-year AUC was 0.81 (Fig. 2C-D).
We compared our model with the previously established GPA model via the integrated discrimination improvement (IDI) index. The results demonstrated that our brain metastasis risk assessment model, specifically tailored to the Chinese population, improved the average prognostic prediction accuracy by 0.063 for one year and by 0.084 for two years compared with the GPA model (Fig. 2E-F).
Evaluation of treatment strategies in different patient groups
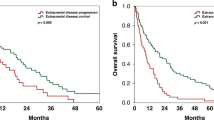
After the diagnosis of brain metastasis, patients receiving trastuzumab combined with pertuzumab achieved a median BMOS of 49 months, whereas those who did not receive this treatment had a median BMOS of 18 months. These findings indicate that trastuzumab combined with pertuzumab improves the prognosis of patients with HER2-positive BCBM (P < 0.0001, HR = 0.28, 95% CI: 0.15–0.5) (Fig. 3A). However, treatment with pyrotinib, T-DM1, or trastuzumab alone did not significantly improve the prognosis of patients with HER2-positive breast cancer brain metastasis (P > 0.05) (Supplementary Figure S3, A-C).
Impact of Individualized Drug Treatments on Survival Prognosis. A-B) Effect of trastuzumab + pertuzumab and trastuzumab on prognosis in different risk groups. C-E) Effect of trastuzumab + pertuzumab, pyrotinib, and T-DM1 on prognosis in low-risk patients. F) Impact of trastuzumab + pertuzumab on intermediate-risk patients.
We further assessed the efficacy of drug treatments across different risk groups, as stratified by our prognostic model. In the low-risk group, any anti-HER2 therapy resulted in a significant improvement in prognosis (Fig. 3, C-E). Although the improvement with trastuzumab alone did not reach statistical significance, a trend toward overall survival benefit was observed (Fig. 3B). In the intermediate-risk group, trastuzumab combined with pertuzumab improved the prognosis (P = 0.01, hazard ratio (HR) = 0.43, 95% CI: 0.2–0.89) (Fig. 3F, Supplementary Figure S3, D-F). For the high-risk group, there was no statistically significant difference in survival prognosis between patients who received drug treatment and those who did not (P > 0.05) (Supplementary Figure S4, A-D).
Additionally, we observed that combining drug therapy with radiotherapy extended BMOS in breast cancer patients. The median BMOS in the drug plus radiotherapy group was 26 months, whereas it was 10 months in the drug-only group (P < 0.01, HR = 0.45, 95% CI: 0.30–0.69) (Supplementary Figure S5). Radiotherapy alone significantly improved survival following the diagnosis of brain metastasis. Further analysis of the impact of WBRT, SRT, and combined WBRT + SRT on survival across the low-, intermediate-, and high-risk groups showed no statistically significant differences in prognosis among these radiotherapy modalities (all P > 0.05) (Supplementary Figure S6).
Discussion
The diagnosis and treatment of patients with brain metastasis from breast cancer remains a critical and unresolved challenge in clinical practice8. However, the exclusion of these patients from most clinical trials and drug development studies has significantly hindered the determination of optimal treatment strategies and the advancement of individualized prognostic management for BCBM21,22. In this study, we developed a survival prognostic stratification model by collecting and analyzing the clinicopathological characteristics of patients with HER2-positive BCBM. We also investigated the efficacy of different therapeutic regimens in various patient subgroups, providing individualized treatment recommendations to guide clinical prognostic management and drug development for BCBM, with the ultimate goal of improving overall survival in this population.
We collected and analyzed clinical data and treatment-related information from 226 HER2-positive BCBM patients treated at the Chinese PLA General Hospital. Cox multivariate regression analysis identified five prognostic indicators strongly associated with the management of brain metastasis: age, KPS score, multiple brain metastases, receipt of radiotherapy for brain metastasis after diagnosis, and multiple extracranial metastases. The most recent GPA score has shown that age, KPS, the number of extracranial metastases, and breast cancer subtype are independently associated with patient prognosis23. However, these models were not specifically designed for HER2-positive subtypes and do not incorporate the therapeutic effects of HER2-targeted agents. Additionally, their development was primarily based on data from Western populations, which may limit their generalisability to Chinese patients. In our study, we focused specifically on HER2-positive BCBM and developed a prognostic model based on real-world data from Chinese institutions. By integrating both clinicopathological characteristics and HER2-targeted treatment variables, our model offers improved prognostic discrimination and greater clinical relevance for stratifying patients and guiding treatment decisions. Based on the model’s evaluation, patients were stratified into low-, intermediate-, and high-risk groups, which effectively predicted the 1-year, 2-year, and 3-year survival rates for the brain metastasis population. Compared with the GPA model, our model demonstrated a modest improvement in accuracy based on data from our center. Moreover, the results from the independent validation cohort indicated that our model exhibits strong generalizability and effective classification performance across different centers. These findings underscore the reliability of our HER2-positive BCBM prognostic model, which is based on clinical indicators. Notably, the independent test set presented a greater AUC value than the larger cohort, primarily due to the smaller sample size in the independent test cohort and the limited number of low-risk patients, many of whom had incomplete data. Despite these limitations, our comprehensive evaluation demonstrates that our model effectively differentiates between low-, intermediate-, and high-risk groups, achieving high accuracy and generalizability.
Our study revealed that anti-HER2 therapy significantly prolongs BMOS in HER2-positive breast cancer patients diagnosed with brain metastasis. Due to their relatively low molecular weight and ability to cross the blood‒brain barrier, small-molecule TKIs represent promising treatments for brain metastasis24,25. Pyrotinib, a small-molecule TKI developed in China, has demonstrated central nervous system (CNS) efficacy rates ranging from 42.1 to 74.6%, with a median PFS of 5.6–11.3 months in the latest PERMEATE study involving BCBM patients26. Similarly, the latest ADC drug, T-Dxd, has shown outstanding efficacy in stabilizing brain metastasis. In the DESTINY-Breast01/03 studies,67 patients with stable, asymptomatic brain metastasis following local therapy achieved a PFS of 15 to 18.1 months, with a CNS objective response rate (CNS-ORR) ranging from 46.7 to 67.4% in patients with measurable intracranial lesions27,28. The PHEREXA study indicated a trend toward a PFS benefit in the brain metastasis subgroup treated with trastuzumab and pertuzumab combined with capecitabine. In the PERMEATE study, pyrotinib combined with capecitabine achieved a CNS-ORR of up to 74.6% and a PFS of up to 11.3 months in patients with brain metastasis progression after radiotherapy. Additionally, two phase III studies involving 443 asymptomatic brain metastasis patients treated with T-DM1 reported a median PFS of 5.5 to 5.9 months. These findings highlight the importance of anti-HER2 therapy for BCBM patients, although the optimal individualized treatment strategy remains to be determined29. On the basis of our model, any treatment with trastuzumab, trastuzumab combined with pertuzumab, pyrotinib, or T-DM1 significantly prolonged survival in low-risk patients. For intermediate-risk patients, the combination of trastuzumab and pertuzumab provides the most substantial survival benefit. However, our prognostic model, which is based on clinicopathological features at the initial diagnosis of brain metastasis, suggests that this benefit may largely result from effective control of extracranial lesions. For high-risk patients or those with recurrent brain metastasis after initial diagnosis, no definitive treatment guidelines exist, although small-molecule TKIs may be preferred due to their ability to cross the blood-brain barrier. Further prospective studies with larger sample sizes are needed to confirm these findings.
A previous retrospective study demonstrated that BCBM patients treated with pyrotinib in combination with radiotherapy had an intracranial PFS of 15 months, whereas those treated with pyrotinib alone had a survival of 9 months30. This highlights the substantial survival benefit of combining drug therapy with radiotherapy in BCBM patients. However, there is currently no clear guidance on identifying the patient subgroups most likely to benefit from this combination. Our study revealed that combining drug therapy with radiotherapy significantly improved the survival following a brain metastasis diagnosis across the entire patient population. For low-risk patients, overtreatment should be avoided, emphasizing the value of our model in guiding treatment strategies for HER2-positive BCBM patients and advancing the precision treatment approach.
In summary, our study systematically analyzed the HER2-positive BCBM population and integrated clinicopathological characteristics and anti-HER2 treatment data to develop a survival prognostic scoring model. This model offers optimized treatment recommendations for various patient subgroups, promoting individualized care and improving survival outcomes for HER2-positive BCBM patients. However, as a retrospective study, our findings may be influenced by confounding factors and bias. Ongoing data collection, along with the expansion of the sample size and inclusion of more multicenter datasets, is essential for further refinement of the prognostic model. These efforts will be crucial in ensuring the most effective individualized treatment options for BCBM patients and enhancing the generalizability and robustness of the model.
Conclusions
This study developed a survival prognostic stratification model based on the clinicopathological characteristics of patients with HER2-positive breast cancer brain metastasis.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ADCs:
-
Antibody Drug Conjugates
- AUC:
-
Area Under Curve
- BCBM:
-
Breast Cancer Brain Metastasis
- BMOS:
-
Brain Metastases Overall Survival
- CI:
-
Confidence Interval
- CNS:
-
Central Nervous System
- CT:
-
Computed Tomography
- ER:
-
Estrogen Receptor
- FISH:
-
Fluorescence In Situ Hybridization
- GPA:
-
Graded Prognostic Assessment
- HER2:
-
Human Epidermal Growth Factor Receptor 2
- HR:
-
Hazard Ratio
- HR:
-
Hormone Receptor
- IHC:
-
Immunohistochemistry
- IDI:
-
Integrated Discrimination Improvement
- KPS:
-
Karnofsky Score
- MRI:
-
Magnetic Resonance Imaging
- ORR:
-
Objective Response Rate
- OS:
-
Overall Survival
- PFS:
-
Progress Free Survival
- PR:
-
Progesterone Receptor
- ROC:
-
Receiver Operating Characteristic Curve
- RPA:
-
Recursive Partitioning Analysis
- SRT:
-
Stereotactic Radiotherapy
- T-Dxd:
-
Trastuzumab Deruxtecan
- TKIs:
-
Tyrosine Kinase Inhibitors
- WBRT:
-
Whole Brain Radiotherapy
References
Xia, C. et al. Cancer statistics in China and united states, 2022: profiles, trends, and determinants. Chin. Med. J. (Engl). 135 (5), 584–590 (2022).
Cacho-Diaz, B. et al. Clinical manifestations and location of brain metastases as prognostic markers. Curr. Probl. Cancer. 43 (4), 312–323 (2019).
Lin, N. U., Amiri-Kordestani, L., Palmieri, D., Liewehr, D. J. & Steeg, P. S. CNS metastases in breast cancer: old challenge, new frontiers. Clin. Cancer Res. 19 (23), 6404–6418 (2013).
Ahn, H. K. et al. Clinical implication of time to brain metastasis (TTBM) according to breast cancer subtypes. SpringerPlus 2 (1), 136 (2013).
Liang, Y., Zhang, H., Song, X. & Yang, Q. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Sem. Cancer Biol. 60, 14–27 (2020).
Laakmann, E. et al. Characteristics of patients with brain metastases from human epidermal growth factor receptor 2-positive breast cancer: subanalysis of brain metastases in breast Cancer registry. ESMO Open. 7 (3), 100495 (2022).
Lin, H., Wu, Y., Liang, G. & Chen, L. Establishing a predicted model to evaluate prognosis for initially diagnosed metastatic Her2-positive breast cancer patients and exploring the benefit from local surgery. PloS One. 15 (11), e0242155 (2020).
Choong, G. M., Cullen, G. D. & O’Sullivan, C. C. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA Cancer J. Clin. 70 (5), 355–374 (2020).
Kim, Y. J., Kim, J. S. & Kim, I. A. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J. Cancer Res. Clin. Oncol. 144 (9), 1803–1816 (2018).
Ramakrishna, N. et al. Management of advanced human epidermal growth factor receptor 2-Positive breast Cancer and brain metastases: ASCO guideline update. J. Clin. Oncology: Official J. Am. Soc. Clin. Oncol. 40 (23), 2636–2655 (2022).
Kann, B. H., Park, H. S., Johnson, S. B., Chiang, V. L. & Yu, J. B. Radiosurgery for brain metastases: changing practice patterns and disparities in the united States. J. Natl. Compr. Cancer Network: JNCCN. 15 (12), 1494–1502 (2017).
Corti, C. et al. Targeting brain metastases in breast cancer. Cancer Treat. Rev. 103, 102324 (2022).
Wang, T. et al. CSCO expert consensus on the diagnosis and treatment of breast cancer brain metastasis. Transl. Breast Cancer Res. 3, 156 (2022).
Venur, V. A. & Leone, J. P. Targeted therapies for brain metastases from breast Cancer. Int J. Mol. Sci ;17, 9 (2016).
Kann, B. H., Park, H. S., Johnson, S. B., Chiang, V. L. & Yu, J. B. Radiosurgery for brain metastases: changing practice patterns and disparities in the united States. J. Natl. Compr. Canc Netw. 15 (12), 1494–1502 (2017).
Subbiah, I. M. et al. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast Cancer and brain metastases. J. Clin. Oncol. 33 (20), 2239–2245 (2015).
Sperduto, P. W. et al. Beyond an updated graded prognostic assessment (Breast GPA): A prognostic index and trends in treatment and survival in breast Cancer brain metastases from 1985 to today. Int. J. Radiat. Oncol. Biol. Phys. 107 (2), 334–343 (2020).
Kim, H., Rajagopalan, M. S., Beriwal, S. & Smith, K. J. Cost-effectiveness analysis of stereotactic radiosurgery alone versus stereotactic radiosurgery with upfront whole brain radiation therapy for brain metastases. Clin. Oncol. (R Coll. Radiol). 29 (10), e157–e164 (2017).
Costa, R. et al. Developmental therapeutics for patients with breast cancer and central nervous system metastasis: current landscape and future perspectives. Ann. Oncol. 28 (1), 44–56 (2017).
Ou, D., Cao, L., Xu, C., Kirova, Y. & Chen, J-Y. Upfront brain radiotherapy May improve survival for unfavorable prognostic breast cancer brain metastasis patients with Breast-GPA 0–2.0. Breast J. 25 (6), 1134–1142 (2019).
Chiec, L. & Kumthekar, P. Targeting HER2 + Breast Cancer brain metastases: a review of brain-Directed HER2-Directed therapies. CNS Drugs. 36 (2), 167–179 (2022).
Escudero, L., Martínez-Ricarte, F. & Seoane, J. ctDNA-Based liquid biopsy of cerebrospinal fluid in brain Cancer. Cancers (Basel) ;13, 9 (2021).
Sperduto, P. W. et al. Survival in patients with brain metastases: summary report on the updated Diagnosis-Specific graded prognostic assessment and definition of the eligibility quotient. J. Clin. Oncol. 38 (32), 3773–3784 (2020).
Lin, N. U. et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-Positive metastatic breast Cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. 9 (2), 197–205 (2023).
Duchnowska, R., Loibl, S. & Jassem, J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat. Rev. 67, 71–77 (2018).
Yan, M. et al. Pyrotinib plus capecitabine for human epidermal factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl. Breast Cancer Res. 1, 145 (2020).
Jerusalem, G. et al. Trastuzumab Deruxtecan in HER2-Positive metastatic breast Cancer patients with brain metastases: A DESTINY-Breast01 subgroup analysis. Cancer Discov. 12 (12), 2754–2762 (2022).
Hurvitz, S. A. et al. Trastuzumab Deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401 (10371), 105–117 (2023).
Montemurro, F. et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann. Oncol. 31 (10), 1350–1358 (2020).
Chen, J. et al. Efficacy and safety of Pyrotinib and radiotherapy vs. Pyrotinib-based therapy in patients with HER2 + breast cancer with brain metastasis: a retrospective cohort study. Ann. Transl Med. 10 (22), 1228 (2022).
Author information
Authors and Affiliations
Contributions
Shanhu Li, Jin Yang, LingXu, and Tao Wang are joint corresponding authors and contributed equally to this work. Jiaxin Chen conceived and designed the project, wrote the manuscript, and collected and analyzed the data. Yuan Sh conceived and designed the project and analyzed the data. Danfeng Dong and Huicui Yan collected and analyzed the data. Huiqiang Zhang, Zisheng Wu, Jinmei Zhou, Xuexue Wu and Fei Chu collected the data. Zefei Jiang revised the manuscript. All listed authors meet authorship criteria, and no others meeting the criteria have been omitted. Shanhu Li, Jin Yang, LingXu, and Tao Wang are the guarantors of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, J., Sh, Y., Dong, D. et al. Development of a prognostic risk stratification model for HER2-positive breast cancer brain metastasis and its implications in guiding treatment decisions. Sci Rep 15, 22623 (2025). https://doi.org/10.1038/s41598-025-06645-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06645-y