Abstract
This study aimed to construct a nomogram for predicting overall survival in patients with bone metastases (BM) from hepatocellular carcinoma (HCC), leveraging data from the Surveillance, Epidemiology, and End Results (SEER) database spanning the period from 2010 to 2015. The patient cohort was randomly partitioned into a training set (n = 338) and a validation set (n = 145). Kaplan–Meier analysis and multivariate Cox proportional hazards regression identified marital status, liver surgery, and radiotherapy/chemotherapy as significant prognostic factors (P ≤ 0.05), which were subsequently integrated into the nomogram. The nomogram exhibited a concordance index of 0.72 (95% confidence interval: 0.70–0.75) and demonstrated robust calibration between predicted and observed survival rates. This nomogram has the potential to accurately forecast prognosis and assist clinicians in formulating appropriate treatment strategies for patients with HCC bone metastasis.
Similar content being viewed by others
Introduction
Liver cancer represents a substantial global health challenge, ranking as the sixth most prevalent malignant tumor worldwide and the third leading cause of cancer-related mortality. Hepatocellular carcinoma (HCC) is the predominant form of liver cancer, accounting for approximately 80% of all liver cancer cases1,2. The etiology of HCC is primarily attributed to infections with Hepatitis B virus (HBV) and Hepatitis C virus (HCV), excessive alcohol consumption, dietary exposure to aflatoxins, smoking, and obesity3,4,5. Although the United States is categorized as a low-risk area for HCC, the incidence of HCC in the U.S. has more than tripled since the 1980s, with projections indicating a continued upward trend through 20306. Despite recent advancements in diagnostic and therapeutic technologies, which have improved early detection and treatment of HCC and consequently enhanced patient survival rates, the long-term prognosis remains poor due to the high risk of intrahepatic recurrence and extrahepatic metastasis7,8,9,10,11,12.
Bone metastasis (BM) is the second most frequent site of metastasis in HCC and is associated with a poor prognosis13,14. Historically, the incidence of BM from HCC has been lower compared to other malignant tumors with bone metastasis15,16,17,and research in this area has been limited due to the poor prognosis and short survival of patients with advanced HCC. However, with advancements in diagnostic technology and the extension of survival periods for patients with HCC, the diagnosis rate of BM from HCC has increased in recent years17,18. Bone has become the second most common metastatic site for HCC, with BM accounting for approximately 30% of extrahepatic metastases, second only to lung metastases19. In addition to the primary tumor burden, patients with BM suffer from skeleton-related events (SREs), loss of mobility, reduced quality of life, increased medical costs, and shortened overall survival (OS)20,21,22,23. Consequently, early diagnosis and the development of effective treatment strategies remain global challenges, and the clinical management of BM from HCC is garnering increasing attention.
Therapeutic options for HCC with BM are mainly confined to palliative treatments, such as external beam radiotherapy (EBRT), bone-targeting agents (BTAs), biologically targeted therapy, and surgery. Given that these strategies are rarely curative for HCC with BM, disease progression prevention and palliative symptom reduction are the main goals. Previous research has indicated that the independent risk factors for BM in newly diagnosed HCC patients are sex, grade, T stage, and N stage. The independent prognostic factors for HCC patients with BM are radiotherapy, chemotherapy, and lung metastasis24. However, this study has focused exclusively on cancer-specific survival rates, neglecting to examine overall survival rates. Moreover, the study was limited to a cohort of only 190 individuals, which is insufficient for a robust case study analysis. Most importantly, critical variables such as tumor size and alpha-fetoprotein (AFP) levels were not considered and modeled, casting significant doubt on the conclusions drawn.
Nomograms have been utilized for survival prognosis in various types of cancer and are recognized as reliable tools capable of providing personalized survival predictions25,26. Nomograms offer significant assistance in various clinical aspects, including personalized treatment decision-making, risk stratification, prognostic assessment, and early intervention. For instance, nomograms can predict overall survival rates based on specific patient characteristics, such as age, tumor grade, T-stage, N-stage, M-stage, surgical status, and alpha-fetoprotein levels, thereby enabling physicians to devise more personalized treatment plans for each patient. Furthermore, physicians can utilize nomograms for risk stratification to identify patients who require closer monitoring or more aggressive treatment. These applications can substantially enhance the efficiency of clinical therapy.
Given the importance of cancer survival estimations for assessing prognosis and improving cancer treatment, in this study, we employed the Surveillance, Epidemiology, and End Results (SEER) database from the National Cancer Institute to examine 483 patients with hepatocellular carcinoma and bone metastasis, to develop and validate a nomogram for predicting the overall survival of this patient cohort.
Materials and methods
Patient selection and data processing
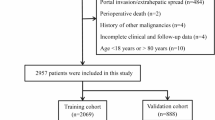
We identified eligible patients from the SEER database (Version: 8.4.3, released on 1/18/2024). The primary site of the hepatocellular carcinoma was labeled C2227. From 2010 to 2015, a total of 1079 samples were included in the study according to the International Classification of Diseases in Oncology (3rd edition) with the histological codes hepatocellular carcinoma (8170/3, 8171/3, 8172/3,8173/3,8174/3 and 8175/3). The following variables were evaluated: age at diagnosis, sex, race, T stage, radiation, chemotherapy, marital status, surgery, tumor size, Alpha-fetoprotein status, survival time, and status. The exclusion criteria in this study were: (1) race recode is unknown (n = 4); (2) marital status at diagnosis is unknown (n = 55); (3) AFP pretreatment interpretation Recode is unknown (n = 293); (4) T stage is T0 or TX (n = 44); (5) tumor size is inexact (n = 200). Ultimately, we identified 483 eligible patients for our study, the research flowchart is shown in Fig. 1. Overall survival (OS) was the major outcome of this study. All data from the SEER database are freely accessible for research purposes.
Nomogram development and statistical analyses
To construct and validate the nomogram, we randomly divided the subjects into training and validation groups in a ratio of 7:3. Chi-square test for variance analysis of count data percentages. Cumulative survival time was calculated using the Kaplan–Meier method, and the differences in survival curves were analyzed using the log-rank test. The cutoff values for age and tumor size were calculated using the X-tile software, the results are shown in Fig. 2. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify variables that significantly affected BM from HCC overall survival. The stepwise regression based on the Akaike information criterion (AIC) minimum was used to select variables for inclusion in the nomogram. Using these identified prognostic factors, we constructed a nomogram for predicting the one-year survival rates of patients with BM from HCC.
We used the concordance index (C-index) and the receiver operating characteristic curve (ROC) and determined the area under the curve (AUC) to evaluate the discriminative ability of the nomogram. The forest plot illustrates the visual outcomes of univariate and multivariate regression analyses. The calibration curves were used to compare the association between the actual outcomes and predicted probabilities. The clinical usefulness and benefits of the predictive model were estimated through decision curve analysis (DCA)28,and use Delong test to compare different ROC or Nomogram based on the same dataset. All statistical analyses were performed using R language (Version 4.4.1, https://www.r-project.org/) in the RStudio environment. P-values < 0.05 denoted statistically significant differences.
Results
Patient characteristics
We included 483 HCC with BM patients from 2010 to 2015. Among all 483 patients, males constituted 414 individuals (85.71%), and 392 patients (81.16%) tested positive for alpha-fetoprotein (AFP). Approximately half of the patients underwent radiotherapy, either alone or in combination with chemotherapy. The overall one-year survival rate for the patients was 15.8% (95% confidence interval: 12.8–19.4%). The results of the univariate regression analysis in the overall population indicate that factors associated with overall cancer survival include marital status at diagnosis, radiotherapy status, chemotherapy status, tumor size, and surgical intervention, among others (Table 1). Subsequently, the dataset was randomly divided into a training group (n = 338) and a validation group (n = 144) in a ratio of 7:3. There is no difference in the percentage of each indicator between the training group and the validation group, and the two groups are comparable (Table 2).
Associations between variables and HCC with BM overall survival
The log-rank test demonstrated that marital status at diagnosis, liver surgery, and the application of radiotherapy and chemotherapy significantly were linked to cumulative survival than other parameters (Fig. 3).
Univariate and multivariate Cox regression analyses of HCC with BM overall survival
The log-rank test and univariate Cox regression analysis gave the same conclusion. We included all variables in the multivariate Cox model, and the stepwise regression method model with the smallest AIC was the optimal model. Univariate Cox regression analysis demonstrated that marital status at diagnosis, radiotherapy status, chemotherapy status, tumor size, and surgical intervention affected OS in patients with BM from HCC. However, the optimal model and multivariate Cox regression analyses demonstrated that marital status at diagnosis, radiotherapy status, chemotherapy status, and surgical intervention affected OS in patients with BM from HCC (Table 3). Subsequently, a total of 4 predictors: marital status at diagnosis, radiotherapy status, chemotherapy status, and surgical intervention were utilized to construct the nomogram (Fig. 4a). Furthermore, considering the clinical impact of tumor size on patient prognosis, we also constructed a nomogram incorporating the tumor size variable based on the results of univariate regression analysis for reference (Fig. 4b). We have conducted a Delong test to compare the ROC curves of Nomogram a (constructed based on multivariate regression analysis) and Nomogram b (which includes the tumor size variable for clinical considerations). The results indicate that there is no significant statistical difference between the two nomograms (P = 0.7444), the results can be seen in the supplementary materials. However, all subsequent research results are based on the analysis of Nomogram a. The forest plot results of Univariate and multivariate Cox regression analyses are shown in Fig. 5.
Nomogram predicting the one-year overall survial (OS) of patients with HCC BM. (a) is a nomogram constructed based on the results of multivariable regression analysis. (b) is a nomogram that incorporates the variable of primary tumor size, which was considered after Univariate regression analysis and clinical considerations, and is provided for reference only).
Nomogram construction
A nomogram based on the selected prognostic factors was developed to predict the OS of patients with HCC BM at one year. The nomogram demonstrated that surgical intervention was the strongest prognostic factor, followed by chemotherapy status, radiotherapy status, and marital status at diagnosis. By adding the scores of all indicator levels, a total score was obtained. The prediction corresponding to this total score assisted in estimating the one-year OS for each patient. The nomogram results for HCC with BM OS are shown in Fig. 4.
Validation and calibration of the nomogram
The C-index of nomogram (training group: 0.72, 95%CI: 0.69–0.74, validation group: 0.71, 95%CI: 0.66–0.76). The calibration plots indicate the level of agreement between the predicted value of the nomogram and the actual value for the OS of patients with BM from HCC. In this study, the calibration plots of the nomogram showed good agreement between the actual observations and the predicted outcomes both in the training (Fig. 6) and validation cohorts (Fig. 6) for one-year HCC with BM OS. The receiver operating characteristic (ROC) curves for both the training group (one-year AUC = 0.76) and validation group (one-year AUC = 0.73) demonstrate that our model possesses good discriminative capacity (Fig. 7).
Calibration curves and DCA curves for training and validation sets columns. (a,c) represent the calibration plots for the training set, while (e) depicts the decision curve analysis (DCA) plot for the training set. (b,d) are the calibration plots for the validation set, and (f) illustrates the decision curve analysis (DCA) plot for the validation set.)
Discussion
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer (PLC), with an incidence rate that has been steadily increasing29,30,31. In recent years, the rise in non-alcoholic fatty liver disease (NAFLD), in conjunction with metabolic syndrome and obesity, has further heightened the risk of developing HCC32,33. Although bone metastasis in HCC patients is not uncommon in clinical practice, research in this area remains limited. Therefore, the development of an accurate and practical prognostic model is crucial for guiding clinical practice and informing therapeutic decisions. Leveraging the extensive clinical data available in the SEER database, this study analyzed 483 cases of bone metastasis (BM) in HCC patients, revealing a one-year overall survival rate of approximately 15.8% (95% CI: 12.8–19.4%), which aligns with previous reports. We constructed a nomogram to predict overall survival in these patients, achieving an adjusted concordance index of 0.72 (0.69–0.74).
The median overall survival time for patients with HCC bone metastasis was four months, comparable to a previously published retrospective study34but lower than that reported in a 2015 Chinese retrospective study with a sample size of 103 cases (14.5 months). This discrepancy may be partly due to the fact that the smaller study included only patients who received intrahepatic treatment, whereas our study encompassed patients with initial bone metastasis of liver cancer, regardless of treatment status. Moreover, the previous study focused on primary liver cancer bone metastasis (PLC BM), while our study specifically targeted HCC BM. Importantly, the lack of clear diagnostic guidelines for HCC BM may introduce diagnostic biases, and racial differences could also contribute to these variations.
In our study, multivariate survival analysis revealed that marital status, chemotherapy use, radiotherapy use, and surgical intervention are independent predictive factors for improved overall survival in patients with HCC BM. Notably, in the univariate and multivariate regression analyses of the overall study population, the size of the primary tumor significantly impacted overall survival (P < 0.05). Consequently, we incorporated the tumor size variable into our model construction and developed a nomogram (Fig. 4B). Interestingly, patients with larger tumors exhibited a higher one-year overall survival rate compared to those with smaller tumors, which may be attributed to the more aggressive nature of smaller tumors35.
The relationship between tumor size and cancer prognosis is complex and can vary depending on the type of cancer and individual differences, involving intricate interactions between the tumor microenvironment and the bone milieu36,37. Angiogenesis and epithelial-mesenchymal transition (EMT) play pivotal roles in the metastatic cascade, with the destruction of the bone microenvironment further exacerbating disease progression38. While it is traditionally believed that larger tumors are associated with a poorer prognosis, this is not a universal rule. Some small tumors exhibit high invasiveness and metastatic potential, leading to adverse outcomes, particularly in high-risk malignancies. Conversely, some larger tumors grow slowly and are less likely to metastasize, resulting in a relatively better prognosis for the patient.
Our study reveals that married patients exhibit a significantly higher overall cancer survival rate compared to their single or divorced counterparts, a finding that aligns with recent publications in the field39,40,41,42. This association may stem from the fact that married patients typically have a more robust social support system, superior economic conditions, and healthier dietary and lifestyle practices. They are also more inclined to adhere to medical advice, take medication on schedule, and undergo regular check-ups. Importantly, married patients receive greater emotional and psychological support from their partners, which aids in their coping with the stress and anxiety associated with illness, a phenomenon observed in other diseases as well43,44,45,46.
The study also found that women had a better overall cancer survival rate than men. Compared with patients with HCC stages T1 to T2, patients with stages T3 to T4 had a poorer overall survival. Patients with positive AFP had a higher overall mortality rate than those who tested negative. For patients with HCC bone metastasis, systemic radiotherapy or chemotherapy is more common than surgery; approximately half of the cohort received chemotherapy or radiotherapy, and patients who underwent chemoradiotherapy had a better prognosis than those who did not receive chemotherapy. However, the number of patients who underwent surgical treatment was limited, which may be due to the consideration of prognosis, overall condition, and other factors by the patients’ families, to improve the patients’ ultimate quality of life. Although for patients with HCC who have progressed to BM, radical surgical interventions such as hepatectomy and liver transplantation are generally no longer viable treatment options, our research nonetheless indicates that surgical procedures and chemoradiotherapy still offer some benefit in improving the overall survival rate of these patients.
Compared to the published study47this study offers several innovative aspects and advantages. Firstly, we focused on predicting overall survival rather than early mortality, providing a more comprehensive prognostic tool for long-term clinical decision-making. Secondly, we employed a nomogram, which is more accessible and user-friendly for clinicians compared to the complex ensemble machine-learning model used in the published study. Thirdly, we highlighted the impact of marital status on survival outcomes and incorporated tumor size into the analysis, offering additional clinically relevant insights. Lastly, we included decision curve analysis (DCA) to evaluate the clinical utility of the nomogram, demonstrating its practical value in real-world scenarios.
This study also has several limitations. Firstly, this was a retrospective study without dynamic control. Secondly, in the sample selection process, we excluded patients with missing data on the collected variables, which may have led to selection bias. Thirdly, the samples were obtained from the SEER database and external validation was not performed, despite the absence of external validation, my study leverages the robust SEER database and provides detailed methodological transparency, setting a strong foundation for future validation efforts. Given the deficiencies of retrospective research, it is necessary to carry out large-scale prospective randomized controlled trials.
Conclusion
To enhance the accuracy of predicting overall survival (OS) in patients with bone metastasis (BM) from hepatocellular carcinoma (HCC), we have developed and validated a nomogram for predicting one-year OS using a large cohort of real-world samples. The nomogram incorporates four independent risk factors: marital status at diagnosis, radiotherapy status, chemotherapy status, and surgical intervention. This nomogram demonstrates excellent discriminative ability and holds potential for clinical application. Future work should include multicenter prospective studies and validation with a larger number of cases to confirm our findings.
Data availability
The data supporting the findings of this study are publicly available through the Surveillance Epidemiology, and End Results (SEER) Program, managed by the National Cancer Institute(NCl). The SEER database can be accessed via the following URL: https://seer.cancer.gov/. The main data generated or analyzed during this study are included in this article and its supplementary information files.
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48. https://doi.org/10.3322/caac.21763 (2023).
Siegel, R. L., Giaquinto, A. N., Jemal, A., Cancer statistics & Cancer CA Cancer J. Clin. 74 (2), 203. https://doi.org/10.3322/caac.21830 (2024).
Li, X. et al. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer. 21 (9), 541–557. https://doi.org/10.1038/s41568-021-00383-9 (2021).
McGlynn, K. A., Petrick, J. L. & El-Serag, H. B. Epidemiology of hepatocellular carcinoma. Hepatology 73 (1), 4–13. https://doi.org/10.1002/hep.31288 (2021).
Mejia, J. C. & Pasko, J. Primary liver cancers: intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Surg. Clin. N. Am. 100 (3), 535–549. https://doi.org/10.1016/j.suc.2020.02.013 (2020).
Petrick, J. L., Kelly, S. P., Altekruse, S. F., McGlynn, K. A. & Rosenberg, P. S. Future of hepatocellular carcinoma incidence in the united States forecast through 2030. J. Clin. Oncol. 34 (15), 1787–1794. https://doi.org/10.1200/JCO.2015.64.7412 (2016).
Imamura, H. et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 38 (2), 200–207. https://doi.org/10.1016/s0168-8278(02)00360-4 (2003).
Nevola, R. et al. Predictors of early and late hepatocellular carcinoma recurrence. World J. Gastroenterol. 29 (8), 1243–1260. https://doi.org/10.3748/wjg.v29.i8.1243 (2023).
Ishizawa, T. et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 134 (7), 1908–1916. https://doi.org/10.1053/j.gastro.2008.02.091 (2008).
Portolani, N. et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann. Surg. 243 (2), 229–235. https://doi.org/10.1097/01.sla.0000197706.21803.a1 (2006).
Roayaie, S. et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 137 (3), 850–855. https://doi.org/10.1053/j.gastro.2009.06.003 (2009).
Zhang, X., Li, J., Shen, F. & Lau, W. Y. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 33 (2), 347–354. https://doi.org/10.1111/jgh.13843 (2018).
Feng, J. et al. Identification of topoisomerase 2A as a novel bone metastasis-related gene in liver hepatocellular carcinoma. Aging (Albany NY). 15 (22), 13010–13040. https://doi.org/10.18632/aging.205216 (2023).
Lu, Y., Hu, J. G., Lin, X. J. & Li, X. G. Bone metastases from hepatocellular carcinoma: clinical features and prognostic factors. Hepatobil. Pancreat. Dis. Int. 16 (5), 499–505. https://doi.org/10.1016/S1499-3872(16)60173-X (2017).
Fukutomi, M. et al. Increased incidence of bone metastases in hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 13 (9), 1083–1088. https://doi.org/10.1097/00042737-200109000-00015 (2001).
Kim, S. U. et al. Hepatocellular carcinoma presenting with bone metastasis: clinical characteristics and prognostic factors. J. Cancer Res. Clin. Oncol. 134 (12), 1377–1384. https://doi.org/10.1007/s00432-008-0410-6 (2008).
Natsuizaka, M. et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J. Gastroenterol. Hepatol. 20 (11), 1781–1787. https://doi.org/10.1111/j.1440-1746.2005.03919.x (2005).
Uchino, K. et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 117 (19), 4475–4483. https://doi.org/10.1002/cncr.25960 (2011).
Schlageter, M. et al. Clinicopathological features and metastatic pattern of hepatocellular carcinoma: an autopsy study of 398 patients. Pathobiology 83 (6), 301–307. https://doi.org/10.1159/000446245 (2016).
Coleman, R. E. et al. Bone metastases. Nat. Rev. Dis. Primers. 6 (1), 83. https://doi.org/10.1038/s41572-020-00216-3 (2020).
Huang, Z. et al. Bone metastasis of hepatocellular carcinoma: facts and hopes from clinical and translational perspectives. Front. Med. 16 (4), 551–573. https://doi.org/10.1007/s11684-022-0928-z (2022).
Lin, S., Hoffmann, K. & Schemmer, P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 1 (3–4), 144–158. https://doi.org/10.1159/000343828 (2012).
Attili, V. S. et al. Bone metastasis in hepatocellular carcinoma: need for reappraisal of treatment. J. Cancer Res. Ther. 4 (2), 93–94. https://doi.org/10.4103/0973-1482.42257 (2008 Apr-Jun).
Hu, C. et al. Diagnostic and prognostic nomograms for bone metastasis in hepatocellular carcinoma. BMC Cancer. 20 (1), 494. https://doi.org/10.1186/s12885-020-06995-y (2020).
Shen, J., Zhou, Y., Yu, B., Zhao, K. & Ding, Y. Construction and validation of a nomogram for patients with multiple hepatocellular carcinoma: A SEER-based study. Eur. J. Surg. Oncol. 49 (10), 106966. https://doi.org/10.1016/j.ejso.2023.06.018 (2023).
Su, K. et al. Evaluation of lactate dehydrogenase and alkaline phosphatase as predictive biomarkers in the prognosis of hepatocellular carcinoma and development of a new nomogram. J. Hepatocell Carcinoma. 10, 69–79. https://doi.org/10.2147/JHC.S398632 (2023).
Steliarova-Foucher, E., Stiller, C., Lacour, B. & Kaatsch, P. International classification of childhood cancer, third edition. Cancer 103 (7), 1457–1467. https://doi.org/10.1002/cncr.20910 (2005).
Vickers, A. J. & Elkin, E. B. Decision curve analysis: a novel method for evaluating prediction models. Med. Decis. Mak. 26 (6), 565–574. https://doi.org/10.1177/0272989X06295361 (2006 Nov-Dec).
Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet 391 (10127), 1301–1314. https://doi.org/10.1016/S0140-6736(18)30010-2 (2018).
Ganesan, P. & Kulik, L. M. Hepatocellular carcinoma: new developments. Clin. Liver Dis. 27 (1), 85–102. https://doi.org/10.1016/j.cld.2022.08.004 (2023).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 7, 6. https://doi.org/10.1038/s41572-020-00240-3 (2021).
ByrneCD & TargherG NAFLD: a multisystem disease. J. Hepatol. 62 (1 Suppl), S47–64. https://doi.org/10.1016/j.jhep.2014.12.012 (2015).
Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15 (1), 11–20. https://doi.org/10.1038/nrgastro.2017.109 (2018).
Seong, J., Koom, W. S. & Park, H. C. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 25 (2), 261–265. https://doi.org/10.1111/j.1478-3231.2005.01094.x (2005).
Li, X. et al. Prognostic value of tumor size in 3 114 patients with stage II colorectal cancer. Chin. J. Colorectal Dis. 10 (02), 149–157. https://doi.org/10.3877/cma.j.issn.2095-3224.2021.02.007 (2021).
Reichl, P., Haider, C., Grubinger, M. & Mikulits, W. TGF-β in epithelial to mesenchymal transition and metastasis of liver carcinoma. Curr. Pharm. Des. 18 (27), 4135–4147. https://doi.org/10.2174/138161212802430477 (2012).
Longo, V. et al. Bone metastases in hepatocellular carcinoma: an emerging issue. Cancer Metastasis Rev. 33 (1), 333–342. https://doi.org/10.1007/s10555-013-9454-4 (2014).
Zhang, J. & Zhang, C. Bone metastases from hepatocellular carcinoma: clinical features and prognostic factors. Chin. J. Clin. Oncol. 46 (22), 1189–1192. https://doi.org/10.3969/j.issn.1000-8179.2019.22.114 (2019).
Chen, F., Wu, Y., Xu, H., Song, T. & Yan, S. Impact of marital status on overall survival in patients with early-stage hepatocellular carcinoma. Sci. Rep. 12 (1), 19923. https://doi.org/10.1038/s41598-022-14120-1 (2022).
Yan, B. et al. Does marital status impact postoperative survival in patients with less differentiated hepatocellular carcinoma? A population-based study. Cancer Med. 8 (14), 6272–6279. https://doi.org/10.1002/cam4.2536 (2019).
Zhang, W. et al. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci. Rep. 7, 41695. https://doi.org/10.1038/srep41695 (2017).
Zheng, Y., Zhang, X., Lu, J., Liu, S. & Qian, Y. Association between socioeconomic status and survival in patients with hepatocellular carcinoma. Cancer Med. 10 (20), 7347–7359. https://doi.org/10.1002/cam4.4223 (2021).
Lee, C. N., Merrill, A. L. & Peters, E. The role of emotion in Cancer surgery decisions: applying concepts from decision psychology. Ann. Surg. 273 (6), e265–e267. https://doi.org/10.1097/SLA.0000000000004574 (2021).
Yi, J. C. & Syrjala, K. L. Anxiety and depression in Cancer survivors. Med. Clin. N. Am. 101 (6), 1099–1113. https://doi.org/10.1016/j.mcna.2017.06.005 (2017).
Kruse, J. L. & Strouse, T. B. Sick and tired: mood, fatigue, and inflammation in cancer. Curr. Psychiatry Rep. 17 (3), 555. https://doi.org/10.1007/s11920-015-0555-3 (2015).
Wu, C. et al. Effect of marital status on the survival of patients with hepatocellular carcinoma treated with surgical resection: an analysis of 13,408 patients in the surveillance, epidemiology, and end results (SEER) database. Oncotarget 7 (48), 79442–79452. https://doi.org/10.18632/oncotarget.12722 (2016).
Long, Z. et al. Development and validation of an ensemble machine-learning model for predicting early mortality among patients with bone metastases of hepatocellular carcinoma. Front. Oncol. 13, 1144039. https://doi.org/10.3389/fonc.2023.1144039 (2023).
Funding
This research was funded by the National Nature Science Foundation of China (No. 82274393, 82374230, 82405209), the Natural Science Foundation of Guangdong Province China (No.2022A1515011577,2021A1515012173), the China Postdoctoral Science Foundation (No. 2024M751135) Fundamental Research Funds for the Central Universities, China (No. 21624359), the Traditional Chinese Medicine Bureau of Guangdong Province, China (No. 20231085), the Construction Project of National Famous and Old Chinese Medicine Expert Inheritance Studio (No. (2022)75), the Guangdong provincial Key Laboratory of Traditional Chinese Medicine Informatization (No. 2021B1212040007)and the Guangzhou Key Laboratory of Formula-Pattern of Traditional Chinese Medicine (No. 202102010014), Guangzhou Region Traditional Chinese Medicine Major Science and Technology Project (NO. 2025CX006, 2025CX018).
Author information
Authors and Affiliations
Contributions
QS, YK, and CZ conceived the study design. WF, DP, and JZ performed the statistical analysis. QS wrote the manuscript and performed the data visualization. JP, LL, SH, FZ, YZ, QY, and MP supervised the study. All authors provided critical revisions of the draft and approved the submitted draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, Q., Kong, Y., Zheng, C. et al. Nomogram prediction of overall survival in patients with bone metastases from hepatocellular carcinoma: a SEER-based retrospective cohort study. Sci Rep 15, 21767 (2025). https://doi.org/10.1038/s41598-025-06665-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06665-8