Abstract
To date, only adults of Zercon forsslundi have been known. According to the description of this species, it has been the first report of Zercon with clear differences in opisthonotal chaetotaxy between females and males, a unique character in the genus. At the same time, Z. forsslundi belongs to a unique group of Zercon species with dorsal setae J5 in females clearly longer than setae J1–J4, the latter ones being short and of similar length. Our description is the first-ever report on the full morphological ontogeny of Z. forsslundi, the mite species, the adult which is the most similar to the recently described Zercon hamaricus and to a lesser extent to Zercon polonicus. The morphological closeness of the above-mentioned species has been confirmed in immature stages. We also studied the geographic distribution of Z. forsslundi and its above-mentioned congeners with remarks on their evolutionary affinity on the background of dispersal abilities and faunal dispersal after the Last Glacial Maximum. We also report the occurrence of Z. forsslundi and Z. hamaricus in the Northern Norway for the first time. Ranges of Z. forsslundi and Z. hamaricus overlap in the area of N Norway. Also, the microenvironmental sympatry was confirmed for these taxa. We also proved the niche overlap of these two species, which is probably limited to the northern verge of Eurasia. Zercon polonicus, not present in the current Arctic fauna, probably is a post-glacial relict, inhabiting more southern mountainous areas of Europe (Carpathians and Alps).
Similar content being viewed by others
Introduction
Until now, nearly 500 species of Zercon have been described, mostly inhabiting the Northern Hemisphere and the Holarctic Realm, however, some species have also been found on the Central Mexican Plateau (Neotropical realm) representing the southernmost occurrence of the family1,2,3. So far, only the adults of Zercon forsslundi Sellnick, 19584 have been described. The separation of males and females of Z. forsslundi in the Sellnick’s4 key resulted from the peculiar sexual dimorphism of this species. Males and females of this species differ in opisthonotal chaetotaxy, which is unusual in the genus5. This peculiar character is partly confirmed in the group of ten Zercon species with females possessing dorsal setae J5 clearly longer when compared with short and similar in length setae J1–J4. Kaczmarek et al.5 distinguished two subgroups of these species, based on morphology of setae S1–S3, r4–r5–s6 and R1–R6. In the first subgroup, there are the Palearctic Z. forsslundi, Z. hamaricus Kaczmarek et al., 20215 and Z. polonicus Błaszak, 19706. The second subgroup is comprised of Nearctic Z. canadensis Halašková, 19777, Z. carolinensis Halašková, 19698, Z. columbianus Berlese, 19109, Z. fenestralis Evans, 195510, Z. lindquisti Halašková, 19777, Z. lucidus Sikora, 201411, and Neotropical Z. mexicanus Ujvári, 20111; the latter one representing the southernmost occurrence of the family and the only known occurrence of Zerconidae outside the Holarctic.
The similarity between Z. forsslundi, Z. polonicus and Z. hamaricus was proved by Kaczmarek et al.5. Here, we provide a description of all the ontogenetic stages of Z. forsslundi expanding the morphological comparison with its most similar congeners. We also widen zoogeographical data and discuss the distribution of Z. forsslundi and closely related taxa, hypothesizing their evolutionary connections.
Material and methods
Individuals of Zercon forsslundi (det. S. Kaczmarek) used in present description were collected on June 5, 2018, by Steffen Roth in Spitsbergen, Svalbard, Norway from two sites: Longyearbyen, Bjørndalen (78.20371 N, 15.31903 E); Kapp Linne, near Isfjord Radio Station (78.03989 N, 13.6457 E). New localities of occurrence of Z. forsslundi and Z. hamaricus in the Northern Norway are listed in Table A1 (see Additional material).
Materials are deposited in collections of the University Museum Bergen and Department of Evolutionary Biology, Faculty of Biological Sciences, Kazimierz Wielki University.
A total of 21 females, 10 males, 12 deutonymphs, 8 protonymphs and 10 larvae of Z. forsslundi were studied. Mites were extracted using Tullgren funnels for 7 days and mounted in PVA mounting medium (Lactic Acid, Poly Vinyl Acetate, and Phenol Solution, BioQuip Products, Inc., CA, USA). The drawings were made using a Nikon Eclipse E200 microscope equipped with a Nikon Y-IDT drawing tube, and then edited with Corel Draw 2017. Measurements and transmitted-light photomicrographs were made using a Leica DM3000 equipped with a Leica DFC420 camera and Leica Application Suite 3.8. For scanning electron microscopy (SEM), the mites were air-dried, sputter coated with Au in an Agar Scientific AGB7340, and placed on Al-stubs with double-sided adhesive tape. Observations and SEM micrographs were made with a Thermo Fisher Scientific Phenom Pure microscope. All measurements are given in micrometres (μm). The terminology follows Lindquist and Evans12 and Lindquist and Moraza13 for idiosomal setation and Johnston and Moraza14 for the notation of dermal glands and lyrifissures. The setal terminology and notation of dermal glands and lyrifissures used in the descriptions of other Zerconidae species discussed in this paper have been converted to the above-mentioned systems if necessary.
Results
Morphological ontogeny of Zercon forsslundi (Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16)
Zercon forsslundi, unusual opisthonotal chaetotaxy in male. (A, B) both J5 longer than usual (intermediate length); (C) left J5 of typical length, right seta longer (intermediate length); (D) left J5 very long, right seta longer than usual (intermediate length) and displaced posteriorly. Scale bars: 20 µm.
-
Superorder: Parasitiformes Reuter, 1909
-
Order: Mesostigmata G. Canestrini, 1891
-
Family: Zerconidae G. Canestrini, 1891
-
Zercon forsslundi Sellnick, 1958
Diagnosis (adult female)
Anterior margin of ventrianal shield with two pairs of setae (Zv1 present). Pores gv3 located posteriorly to line connecting para-anal setae close to line connecting setae Jv4. Podonotal shield with 21 pairs of setae, covered entirely with irregular tile-like sculpture. Setae j1, s3 and r4–r5, and s6 barbed. Seta r3 barbed, located on margin of idiosoma. Opisthonotal shield with 21 pairs of setae (Z2 present). All opisthonotal setae without apical hyaline endings. Setae J1–J4, Z1–Z3, and S2–S3 short, smooth and pointed, never reach the insertions of next setae in series and never extend beyond the edge of opisthonotum. Seta S3 similar in length to S2, smooth and pointed. Other opisthonotal setae long and barbed. Seta J5 never reach beyond posterior edge of opisthonotum, but reach posterodorsal cavities. Pores gdZ1 (Po2) close and posterior to line connecting insertions of setae Z2 and S3, closer to Z2. Pores gdZ3 (Po3) close to the line connecting the insertions of setae J5 and Z4, closer to Z4. Pores gdZ3 and insertions of setae J5 and Z4 located distinctly anterior to posterodorsal cavities.
Description
Female (n = 13)
Idiosoma length 524–572, width (at level of anterior edge of ventrianal shield) 410–445.
Dorsal idiosoma (Figs. 1, 3A,B, 4). Podonotal shield with 21 pairs of setae; all without hyaline endings. Lengths of podonotal setae of females summarized in Table 1. Seta j1 longest in j-series, other j-series setae of comparable length. Setae j1 with subapical barbs. Setae j2–j6 smooth and pointed. All z-series setae of comparable length, smooth and pointed. Seta s1 shortest of the s-series. Setae s3 and s6 of comparable length and longest of s-series. Seta s3 with subapical barbs, seta s6 with one subapical barb, other s-series setae smooth and pointed. Setae r1 and r2 smooth and pointed, setae r4–r5 with one subapical barb. Seta r1 shortest and r5 longest of r-series on podonotal shield. Podonotal shield covered entirely with irregular tile-like sculpture. Location of podonotal glands: gdj2 (po1) posterior to insertion of seta s1, adaxial and usually clearly closer to line connecting setae s1 and z2; gdj4 (po2) always close and posterior to the line connecting insertions of setae j4 and z4, usually equally distant from insertions of j4 and z4 (if not then slightly moved towards j4); gdz5 (po3) always posterior to line connecting insertions of setae z5 and s4, always closer to s4. Location of podonotal poroids: idj1 always abaxial to j2 and anterior to s1; ids4 close to lateral margin of podonotal shield, close to the level of insertion of seta r5; idj3, idj6 and idz4 not visible. Opisthonotal shield with 21 pairs of setae; all without hyaline endings. Lengths of opisthonotal setae and longitudinal distances between insertions of setae in specific series of females summarized in Table 2. Setae J1–J4 short, smooth and pointed. Seta J5 clearly longer, with subapical barbs. Seta J5 never reach beyond posterior edge of opisthonotum and clearly reach posterodorsal cavities. Setae Z1–Z3 of comparable length, short, smooth and pointed. Setae Z4 and Z5 clearly longer, with subapical barbs. Seta Z4 clearly reach beyond lateral edge of opisthonotum, and almost reach the insertion of Z5. Seta Z5 slightly longer (1.1 times) than Z4. Distance between pair of setae Z5 184–202. Setae S2 and S3 of comparable length and shortest of S-series, smooth and pointed. Setae S1 with one subapical barb, S4 with subapical barbs. Setae S2 and S3 never reach the insertion of next seta in series. Seta S4 clearly longer (2.5–3.2 times) than S2 and S3. Seta S5 with subapical barbs, longer (1.1–1.3 times) than S4. Setae R1–R6 of similar length (17–30), pointed, R1 and R2 with subapical barb. Opisthonotal shield covered with tile-like structure from the anterior edge to J4–S4 line. The area between lines J4–J4 and J5–J5 with irregular lines and some bright spots in the axial part. Remaining posterior surface of opisthonotum without sculpture, finely dotted. Posterodorsal cavities crescent-like with undulating anterior margins. Location of opisthonotal glands: gdz6 (Po1) anterior to Z1 (abaxial or adaxial to Z1); gdZ1 (Po2) always close and posterior to line connecting insertions of setae Z2 and S3, always closer to insertion of Z2; gdZ3 (Po3) close to the line connecting insertions of setae J5 and Z4 (usually on the line but sometimes also slightly above or below), always closer to insertion of Z4; gdZ4 (Po4) always between the insertions of setae S5 and Z5; gdJ3 not visible. Location of opisthonotal poroids: idz6 close to insertion of Z1 and gdz6 (usually adaxial, rarely on the line Z1–gdz6); idJ1 close to the line connecting insertions of setae J1 and J2 (usually abaxial, sometimes adaxial or on the line); idJ2 always abaxial to line connecting insertions of setae J2 and J4, on the level of insertion of J3; idJ3 always posterior to line connecting insertions of setae J4 and Z3, always closer to Z3; idJ4 always posterior to line connecting insertions of setae J5 and Z4, always adaxial to gdZ3; idJ5 always close and abaxial to inner posterodorsal cavities; idZ4 always between gdZ4 and outer dorsal cavity; ids6 always close to antero-abaxial corner of opisthonotal shield, close to anterior edge of shield; idS1 postero-abaxial to ids6, close to lateral edge of opisthonotal shield; idS3 single, abaxial to line connecting setae S3 and S4, close to level of insertion of J3; idS4 postero-adaxial to seta S4, at the level of setae Z4; idR3 rarely visible, postero-adaxial to idS3, at the level of insertion of R3.
Gnathosoma. Epistome (Fig. 2A–D) typically shaped for genus, with medial process bifurcated. One female with medial process tripartite.
Ventral idiosoma (Fig. 5). Epigynal and peritrematal shields shaped typically for genus. The peritrematal groove is directed laterally, with fine and dense pilae inside (Fig. 3C). Seta r3 with subapical barbs. Sternal shield fully covered with reticulate ornamentation, setae st1–st5 similar in length, 15–27, smooth and pointed. Ventrianal shield length 204–225, width (at level of Jv2 setae) 315–333, with 21 setae; Jv4, Jv5, para-anal and postanal setae with single subapical barb. Ventrianal shield covered with barely reticulate ornamentation in the anterior part (to level of Jv3 setae), remaining posterior surface of ventrianal shield without reticulate ornamentation, finely dotted. Setae Jv1, Jv2, Zv1–Zv4 15–23, Jv3 20–30, Jv4 25–30, para-anal setae 31–38, Jv5 and postanal setae 31–40. Three parallel lines of denser dots visible posterior to postanal seta (length of each line does not exceed distance between para-anal setae). Anus length 21–25, width 17–21. Location of ventral glands: gv1 close to insertion of st3, gv2 multiple and typically located; gvi in the inguinal region; gv3 (posterior para-anal glands) posterior to line connecting para-anal setae, close to line connecting setae Jv4; gp anterior to peritreme. Location of ventral poroids: iv1 abaxial to insertion of st1; iv2 postero-abaxial to insertion of st2; iv3 close to the posterior edge of sternal shield, adaxial to insertion of st3; iv5 on the genital shield, posterior to insertion of seta st5; ip1 anterior to gp; ip2 postero-adaxial to stigma; ivo1 and ivo2 located at level of Jv2–Zv2 setae (usually ivo1 anterior to ivo2, rarely on the one side of the body ivo1 abaxial to ivo2); ivo3 close to lateral edge of ventrianal shield, close and posterior to line connecting insertions of setae Zv4; ivo4 not visible; ivi in the inguinal region; ivp single, anterior to line connecting setae Jv5, close to insertion of Jv5. The inguinal region has clearly visible, dense cuticular spines directed ventro-anterally (towards the coxae) (Fig. 6).
Male (n = 6)
Idiosoma length 398–445, width (at level of anterior edge of ventrianal shield) 303–330.
Dorsal idiosoma (Figs. 7, 9, 10). Podonotal shield with 21 pairs of setae; all without hyaline endings. Lengths of podonotal setae of males summarized in Table 1. Seta j1 longest of j-series, other j-series setae of comparable length. Seta j1 with subapical barbs. Setae j2–j6 smooth and pointed. All z-series setae smooth and pointed, z2 and z6 of comparable length and slightly shorter than z3–z5. Seta s1 shortest of the s-series. Setae s3 and s6 of comparable length and longest of s-series. Seta s3 with subapical barbs, seta s6 with one subapical barb, other s-series setae smooth and pointed. Setae r1 and r2 smooth and pointed, setae r4 and r5 with one subapical barb. Seta r1 shortest and r5 longest of r-series on podonotal shield. Podonotal shield covered entirely with irregular tile-like sculpture. Location of podonotal glands: same as in female. Location of podonotal poroids: same as in female. Opisthonotal shield with 21 pairs of setae; all without hyaline endings. Lengths of opisthonotal setae and longitudinal distances between insertions of setae in specific series of males summarized in Table 2. Setae J1–J5 short, smooth and pointed. Seta J5 clearly thickened when compared to J1–J4. In addition, we have observed four males with J5 setae longer than usual: two males with both J5 setae longer and thicker than in other males, but still considerably shorter than those in females (16–19, intermediate length, barbed); one male with one of the J5 setae short (10, typical length, smooth) and the other longer (21, intermediate length, barbed); one male with one of the J5 considerably longer than usual in males (58, resembling the female’s J5, barbed), and the other of intermediate length (18, barbed) (Fig. 9A–D). Setae of Z-series similar to those in female. Seta Z4 clearly extends beyond lateral edge of opisthonotum, and insertion of Z5. Seta Z5 slightly longer (1.01–1.14 times) than Z4. In studied males, the length of Z3 seta ranged from 9 to 16, with both setae of comparable length in specific individuals. In one male we found unusual length of Z3 setae; one Z3 seta was short (12) while the other one was clearly longer (20) (Fig. 10). Distance between pair of setae Z5 144–156. Setae of S-series similar to those in female. Seta S4 clearly longer (2.5–3.2 times) than S3. Seta S5 with subapical barbs, longer (1.1–1.2 times) than S4. Setae R1–R6 13–23, R1 and R2 with one subapical barb. Opisthonotal shield covered with tile-like structure from the anterior edge to J4–S4 line. The area between lines J4–J4 and J5–J5 with irregular lines and some bright spots in the axial part. Remaining posterior surface of opisthonotum without sculpture, finely dotted. Posterodorsal cavities crescent-like with undulating anterior margins. Location of opisthonotal glands: gdZ1 (Po2) usually close and posterior to the line connecting insertions of setae Z2 and S3 (sometimes on the line or slightly above), always closer to insertion of Z2; gdZ3 (Po3) usually anterior to the line connecting insertions of setae J5 and Z4 (sometimes on the line, never posterior), always closer to insertion of Z4; other glands as in female. Location of opisthonotal poroids: idz6 close to insertion of Z1 and gdz6, always adaxial to the line Z1–gdz6; idJ1 close to the line connecting insertions of setae J1 and J2, usually abaxial (rarely on the line); idJ3 always posterior to line J4–Z3, at the level of insertions of J4 and S4, often closer to Z3, rarely equally distant to J4 and Z3; other poroids similar as in female.
Gnathosoma. Epistome (Fig. 2E–I) typically shaped for genus with medial process bifurcated. One male with medial process tripartite, the other one male with undivided medial process.
Ventral idiosoma (Fig. 8). Genital and peritrematal shields shaped typically for genus. Seta r3 with subapical barbs. Sternal shield covered with irregular ornamentation. Sternal setae smooth and pointed, st1–st5 13–21 long. Ventrianal shield length 164–177, width (at level of Jv2 setae) 250–268, with 21 setae. Para-anal setae with one subapical barb, other setae smooth. Ventrianal shield covered with barely reticulate ornamentation in the anterior part (to level of Jv3 setae), remaining posterior surface of ventrianal shield without reticulate ornamentation, finely dotted. Setae Jv1, Jv2, Zv1–Zv4 10–19, Jv3 18–23, Jv4 21–26, para-anal setae 23–30, Jv5 and postanal setae 28–35. Three parallel lines of denser dots visible posterior to postanal seta (length of each line does not exceed distance between para-anal setae). Anus length 18–20, width 15–16. Location of ventral glands: gv1 adaxial to line connecting insertions of setae st3 and st4; location of other glands same as in female. Location of ventral poroids: iv1 postero-abaxial to insertion of st1; iv3 postero-adaxial to insertion of st3; iv5 on the sternal shield, posterior to insertion of seta st5; location of other poroids same as in female.
Deutonymph (n = 6)
Idiosoma length 358–414, width (at level of anterior edge of ventrianal shield) 264–314.
Dorsal idiosoma (Fig. 11). Podonotal shield with 21 pairs of setae; all without hyaline endings. Lengths of podonotal setae of deutonymphs summarized in Table 1. Seta j1 longest of j-series, other j-series setae of comparable length. Seta j1 with subapical barbs, other j-series setae smooth and pointed. All z-series setae smooth and pointed, z6 shortest and z4 longest. Seta s1 shortest of s-series. Setae s2 slightly longer than s1, s4 and s5 clearly longer and of comparable length. Setae s3 and s6 of comparable length and longest of s-series. Seta s3 with subapical barbs, seta s6 with one subapical barb, other s-series setae smooth and pointed. Setae r1 and r2 smooth and pointed, setae r4–r5 with one subapical barb. Seta r1 shortest and r5 longest of r-series on podonotal shield. Podonotal shield covered with irregular net-like sculpture. Location of podonotal glands and poroids: same as in male and female. Opisthonotal shield with 21 pairs of setae; all without hyaline endings. Lengths of opisthonotal setae and longitudinal distances between insertions of setae in specific series of deutonymphs summarized in Table 2. Setae J1–J5 as in male: short, smooth and pointed; seta J5 slightly thickened when compared to J1–J4. Setae of Z-series similar to those in female. Seta Z4 clearly extends beyond lateral edge of opisthonotum, and insertion of Z5. Setae Z4 and Z5 of comparable length. Distance between pair of setae Z5 114–124. Seta S1 with one subapical barb, S4 with subapical barbs. Setae S2 and S3 of comparable length and shortest of S-series, smooth and pointed, never reach the insertion of next seta in series. Seta S4 clearly longer (3.9–5.8 times) than S2 and S3. Seta S5 with one subapical barb, slightly longer (1.02–1.16 times) than S4. Setae R1–R6 of similar length (7–15), pointed, R1 with one subapical barb. Opisthonotal shield covered with irregular net-like sculpture from the anterior edge to J4–S4 line. The area between lines J4–J4 and J5–J5 with irregular lines and some bright spots in the axial part, remaining posterior surface of opisthonotum without sculpture, finely dotted. Posterodorsal cavities crescent-like with undulating anterior margins. Location of opisthonotal glands: gdZ1 (Po2) often close and posterior to line Z2–S3 (sometimes on the line; rarely slightly anterior), often equally distanced to Z2 and S3, sometimes clearly closer to Z2; gdZ3 (Po3) close and always anterior to the line connecting insertions of setae J5 and Z4, always closer to insertion of Z4; other opisthonotal glands same as in male and female. Location of opisthonotal poroids: idJ4 close to line J5–Z4 (usually on the line, rarely posterior), always adaxial to gdZ3; idz6 and idJ3 same as in male; idJ1 same as in female; idR3 not visible.
Gnathosoma. Epistome (Fig. 2J–L) typically shaped for genus. One deutonymph with medial process tripartite.
Ventral idiosoma (Fig. 12). Seta r3 with subapical barbs. Sternal shield without visible ornamentation, finely dotted. Setae st1–st5 8–17, smooth and pointed (st5 off the shield). Ventrianal shield length 130–148, width (at level of Jv3 setae) 195–213, with 17 pointed setae (Jv1 and Zv1 off the shield). Postanal seta with subapical barb, other setae smooth. Ventrianal shield covered with barely visible ornamentation and finely dotted. Setae Jv1–Jv4 and Zv3 11–19, Zv1, Zv2 and Zv4 7–10, para-anal setae 21–23, and postanal seta 33–44 long. Three parallel lines of denser dots visible posterior to postanal seta (length of each line does not exceed distance between para-anal setae). Anus length 16–17, width 13–15. Location of ventral glands: gv1 between insertions of setae st3 and st4; gv2 multiple and typically located; gv3 (posterior para-anal glands) posterior to line connecting para-anal setae, posterior to line connecting setae Jv4; gp close to peritreme, at the level of coxa III; gvi close to posterior edge of coxa IV. Location of ventral poroids: iv1–iv2 posterior to insertions of st1 and st2 respectively; iv3 not visible; iv5 posterior to insertion of seta st5, at the level of gv2; ip1 at the level of anterior edge of coxa II; ip2 similar to those of adults; ivo1 always outside the ventrianal shield, at the level of Zv1; ivo2 always on the shield, posterior to level of Zv2 and antero-abaxial to Zv4; ivo3 close to lateral edge of ventrianal shield, close to line adjacent to anterior edge of anus; ivo4 not visible; ivp single, anterior to line connecting setae Jv5, close to insertion of Jv5; ivi close to posterior edge of coxa IV. Soft cuticular area between ventral shields with visible parallel corrugations.
Protonymph (n = 5)
Idiosoma length 360–390, width (at level of anterior edge of opisthonotal shield) 273–310.
Dorsal idiosoma (Fig. 13). Podonotal shield with 12 pairs of setae; all without hyaline endings. Lengths of podonotal setae of protonymphs summarized in Table 1. Seta j1 longest of j-series, j2 shortest, other j-series setae of comparable length. Seta j1 with subapical barbs, other j-series setae smooth and pointed. Seta z4 longest of z-series, other z-series setae of comparable length, all z-setae smooth and pointed. Setae of s-series of comparable length. Seta s6 with one subapical barb and off the podonotal shield, other s-setae smooth. Seta r2 on the podonotal shield, longest on podonotal shield, with subapical barbs. Seta r5 off the podonotal shield, short, smooth and pointed. Podonotal shield covered with irregular lines (delicately corrugated). Location of podonotal glands: gdj2 (po1) postero-abaxial to insertion of seta j2, adaxial and close to line connecting insertions of setae j2 and z2; other podonotal gland same as in female and male. Location of podonotal poroids: idj1 antero-abaxial to insertion of j2; ids4 antero-abaxial to insertion of seta s4, posterior to insertion of seta r2. Soft cuticular area between dorsal shields with visible parallel corrugations. Opisthonotal shield with 14 pairs of setae; all without hyaline endings. Lengths of opisthonotal setae and longitudinal distances between insertions of setae in specific series of protonymphs summarized in Table 2. Setae J1–J5 short, smooth and pointed. Seta J5 visibly thicker when compared to J1–J4. Setae Z1–Z3 short, smooth and pointed. Setae Z4 and Z5 clearly longer, with barbs located at about one third or one fourth from seta termination. Seta Z4 clearly reach beyond lateral edge of opisthonotum, and reach beyond insertion of Z5. Setae Z4 and Z5 of similar length. Distance between pair of Z5 setae 93–101. Seta S2 shortest of S-series, smooth and pointed. Setae S3–S5 longer with subapical barbs. Seta S3 clearly longer (2.9–4.4 times) than S2. Seta S2 never reach insertion of S3. Setae S3–S4 clearly reach the insertions of next setae in series. Setae S4 longer (1.2–1.5 times) than S3. Setae S4 and S5 more similar in length (S5 1.02–1.2 times longer than S4). Seta R1 off the opisthonotal shield, short (8–10), smooth and pointed. Opisthonotal shield covered with barely visible corrugations between J- and Z-series. Remaining posterior surface of opisthonotum finely dotted. Posterodorsal cavities with undulated anterior margin; outer cavities crescent-like, inner ones more rounded. Location of opisthonotal glands: gdZ1 (Po2) usually anterior to insertions of setae Z2 and S3, usually slightly closer to insertion of S3; gdZ3 (Po3) always anterior to line J5–Z4, always closer to insertion of seta Z4; other glands same as in adults and deutonymph. Location of opisthonotal poroids: idJ1 close to line connecting insertions of setae J1 and J2, usually on the line (rarely adaxial to the line); idJ3 close to line (usually anterior) connecting insertions of setae J4 and Z3; idJ4 always anterior to line connecting insertions of setae J5 and Z4, always adaxial to gdZ3; idS3 single, usually slightly adaxial to line connecting insertions of setae S3 and S4, at the level of insertions of J3; idS4 close and postero-adaxial to insertion of S4; idz6 same as in males and deutonymphs; other poroids as in adults and deutonymphs; idR3 not visible.
Gnathosoma. Epistome (Fig. 2M–O) typically shaped for genus.
Ventral idiosoma (Fig. 14). Setae r3 with subapical barbs. Sternal shield without ornamentation. Setae st1–st3 11–15, st5 off the sternal shield and shorter 4–6. Ventrianal shield with 9 smooth and pointed setae (Jv1 off the shield), with weak ornamentation (corrugations). Setae Jv1, Jv2 and Zv2 of comparable length (11–16), para-anal setae longer 19–21, postanal seta 34–36. Seta Jv5 26–33 long with subapical barb. Three parallel lines of denser dots visible posterior to postanal seta (length of each line does not exceed distance between para-anal setae). Anus length 15–20, width 12–13. Location of ventral glands: gv2 single, abaxial to Jv1 setae, close to the line connecting insertions of these setae; gv3 (posterior para-anal glands) posterior to line connecting para-anal setae; gp anterior to short peritreme, at the level of posterior edge of coxa III. Location of ventral poroids: ip1 at the level of anterior edge of coxa II; ip2 postero-abaxial to stigma; iv1 posterior to insertion of st1; iv2 posterior to insertion of st2; ivo1 always outside the ventrianal shield, postero-abaxial to gv2; ivo2 usually on the anterior edge of the shield at the level of Jv2, sometimes outside the shield; ivo3 close to lateral edge of ventrianal shield, at the level of para-anal setae; ivo4 not visible; ivp single, antero-abaxial to insertion of seta Jv5. Soft cuticular area between ventral shields with visible parallel corrugations.
Larva (n = 7)
Idiosoma length 220–290, width (at level of setae S3) 186–226.
Dorsal idiosoma (Fig. 15). Podonotal shield with nine pairs of setae; all without hyaline endings. Lengths of podonotal setae of larvae summarized in Table 1. All j-series setae of comparable length, j1 with subapical barbs, j2–j6 with one subapical barb. Setae z2 and z5 short, smooth and of comparable length, seta z4 clearly longer, with subapical barbs. Seta s4 longest of podonotal shield, with subapical barbs. Seta s6 off the shield, diminutive. Location of podonotal glands: gdj4 and gdz5 not visible. Location of podonotal poroids: idj3, idj6, idz4 and ids4 not visible. Soft cuticular area between dorsal shields with visible parallel corrugations. Opisthonotal shield with 6 pairs of setae; all without hyaline endings. Lengths of opisthonotal setae in larvae summarized in Table 2. Setae J2 and S3 off shield, diminutive. Setae J3, J4 and S4 similar in length, diminutive, smooth and pointed. Setae Z3, Z4 and J5 with subapical barbs and usually curved terminations. Seta Z4 longer (1.1–1.3 times) than Z3. Seta J5 longest of opisthonotal shield, longer (1.04–1.31 times) than Z4. Location of opisthonotal glands: gdJ3 not visible; gdZ3 (Po3) always close and posterior to line connecting insertions of setae J4 and Z3, usually equally distant from their insertions; sometimes slightly closer to insertion of Z3, rarely closer to insertion of J4. Location of opisthonotal poroids: idz6 close to line connecting insertions of setae S3, abaxial to insertion of J2; idJ3, idJ5 and idR3 not visible. Podonotal and opisthonotal shields with irregular pattern, delicately corrugated. Soft cuticular area between dorsal shields with visible parallel corrugations. Posterodorsal cavities circular with smooth edges, arranged typically for the genus.
Gnathosoma. Epistome (Fig. 2P) typically shaped for genus.
Ventral idiosoma (Fig. 16). Sternal shield without ornamentation. Setae st1–st3 10–16. Ventrianal shield subtriangular, bell-shaped (not measured), with three setae (Jv1, Jv2, Jv5 and Zv2 off the shield). Setae Jv5 4–6, Jv1 and Zv2 7–12, Jv2 19–24, para-anal setae 19–26 and postanal seta 42–51 long. Anal opening poorly visible (not measured). Setae Z5 and S5 diminutive, abaxial to ventrianal shield, at the level of para-anal setae. Location of ventral glands: gdZ4 (Po4) anterior to line connecting insertions of para-anal setae, abaxial to line connecting insertions of setae Jv5 and S5; gp close to lateral edge of the idiosoma, at the level of setae Jv1 and Zv2; gv3 not visible. Location of ventral poroids: ivo4 and ivp not visible. Soft cuticular area between ventral shields with visible parallel corrugations.
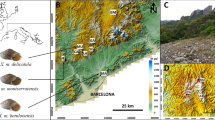
Distribution of Zercon forsslundi, Z. polonicus and Z. hamaricus (Fig. 17)
Distribution map of Zercon forsslundi, Z. hamaricus and Z. polonicus. Empty markers (earlier records): circles—Z. forsslundi; diamonds—Z. polonicus; squares—Z. hamaricus. Filled markers (new records): black circles—Z. forsslundi; black squares—Z. hamaricus; gray stars – sympatric populations of Z. forsslundi and Z. hamaricus, dotted line—Arctic Circle.
Altogether, 50 presence points of Z. forsslundi were used for analysis, including six new localities4,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 (for new localities see Additional material, Table A1). The majority presence points (42%) were located in Svalbard (21, including two localities used for the current description). One third of localities (15 presence points) were located in Russia, with most of them (14) in the northern part of the country (the Murmansk and Arkhangelsk Oblasts, the Nenets and Yamalo-Nenets Autonomous Okrugs), with the exception of the southernmost finding in Russia (the Pechora-Ilych Nature Reserve in Komi Republic). The other 14 presence points are located in Finland (7, throughout the country), Sweden (2, northern and central parts), Norway (4, northern part, new data) and Latvia (1, the southernmost presence point of Z. forsslundi). Except for the localities of Zercon polonicus used by Błaszak6,36 for the species description (S Poland, Tatra and Pieniny Mountains) it was also found in NE Austria (Saubrunn, Lower Austria)37. Zercon hamaricus was described by Kaczmarek et al.5 based on material from S Norway, here we proved its presence in five new localities in N Norway.
Discussion
The newly collected adults of Z. forsslundi agree very well with the description of Sellnick4; however, we found some minor differences (Table 3) that widens our knowledge of the intraspecific variability of the studied species. The most pronounced are: (1) the placement of dorsal cavities that in our individuals are relatively close to the posterior edge of opisthonotum when compared with drawings of Sellnick4; (2) insertions of J5 and Z4 according to Sellnick’s description are on the same transverse line, while in most of our individuals the line connecting insertions of these setae is arcuate. Sellnick described setae Z4, Z5, S4 and S5 as distally flattened; terminations of these setae are just like that; however, the SEM micrographs show, that each of these setae possesses twisted sutures running from the insertion and almost reach the distal tip of seta, which is also true for other long opisthonotal setae (Fig. 4).
According to Sellnick’s4 drawing, r4–r5–s6 and S1 are barbed in Z. forsslundi. Our present study revealed that r4–r5–s6 and S1–R1–R2 are barbed; therefore, the characteristics of marginal setae shows more close relation between Z. hamaricus and Z. forsslundi. It is worthy to emphasize that in Z. hamaricus we found that setae S1–R6 possess one subapical barb, whereas in Z. forsslundi we found single barb only on S1–R2 setae. These barbs are, however, not always visible (in both light and scanning microscopy), apparently because of the various barbs locations (dorsally, ventrally, laterally). Nevertheless, we have not observed barbs on R3–R6 setae in Z. forsslundi. Therefore, the smoothness of r4–r5–s6 and S1–R1–R6 in Z. hamaricus, Z. polonicus and Z. forsslundi is as follows: all barbed in Z. hamaricus, all smooth in Z. polonicus and some setae (r4–r5–s6–S1–R1–R2) barbed in Z. forsslundi.
Kaczmarek et al.5 pointed out close relationship of Z. hamaricus to Z. forsslundi as well as Z. polonicus (the similarity between the latter species has also been emphasized in the key of Karg38). The morphological closeness of Z. forsslundi, Z. hamaricus and Z. polonicus has been confirmed in immature stages (Table 4); however, to fully analyze the similarities between these species, the supplemental description of the larvae of the latter species is needed.
The glacial fluctuations throughout the Quaternary undoubtedly influenced the fauna and flora of high and even mid-latitudes in the Holarctic39. The harsh climatic conditions have been dramatic not only for the species not adapted to low temperatures as e.g. the mite Labidostomma luteum Kramer, 189740 that has originated from France, and the following glaciations influenced its dispersion and range of both the parthenogenetic and bisexual populations41,42,43. In parallel, changes in glacier range also influenced the Arctic that were pushed more to the south and then dispersed to the north according to ice sheets advance and retreat, respectively. Most of the presence points of Z. forsslundi used in this work are located within the Barents Sea region, which is an area of biotic convergence between the Palearctic and Nearctic44. Dispersal throughout the continental part is relatively easy to explain in mites when compared with inhabiting the Arctic islands of the Barents Sea. Phoresy is not present in all Mesostigmata. Three distinctive types of phoretic relationships were defined in this order at the superfamily level, with only four regarded as exclusively free-living (i.e. non-phoretic) taxa, including Veigaioidea, Arctacaroidea, Epicrioidea and Zerconoidea45. The lack of phoresy, decreasing dispersal ability, undoubtedly influenced the immigration of these invertebrates to the Arctic islands since the last glaciation44. In general, the invertebrates inhabiting the Barents Sea islands are regarded as relatively young fauna (acc. to the tabula rasa hypothesis) that dispersed to Svalbard, Novaya Zemlya and Franz Josef Land after the Last Glacial Maximum (LGM), however, in situ survival hypotheses (i.e. nunatak and cryptic refugia) are also under debate44,46 (for a discussion of tabula rasa vs nunatak hypothesis see Brochmann et al.47). As zoochory is not present in Zerconidae, the other possible dispersal vectors are anemochory, hydrochory and anthropochory (i.e. wind-, water- and human-induced dispersion). Anemochory has not been previously reported in Zerconidae. The survival of some Collembola and Oribatida in seawater was defined as sufficient to allow successful dispersal from N Norway to Svalbard48, however, no zerconid mite species have been studied in this context. The negative effect of seawater can be decreased if driftwood is considered a dispersal agent. The rafting of organic matter from Siberian rivers, supported by ocean currents, influenced the distribution of plants and, very likely, invertebrates within the Arctic since the last glaciation29,44,48,49. With respect to human-induced dispersal, this is not excluded, but the former studies on soils introduced to Pyramiden (Svalbard) focused on chernozem-type soils imported from southern European Russia and Ukraine, regions that cannot be considered possible Z. forsslundi sources. Moreover, the Z. forsslundi inhabits only undisturbed natural soils at Pyramiden, which can prove the vulnerability of this species to anthropogenic disturbances30.
In northwestern Eurasia, the LGM ice-sheet covered Fennoscandia, Svalbard, Franz Josef Land and Novaya Zemlya up to the northwestern part of Taymyr Peninsula, with the southern ice extending much to the south in the western (i.e. European) part compared with the eastern region; however, the eastern ice extent is debatable50,51. The LGM ice sheet extent reconstructions suggest, that North Eurasia was not glaciated from the eastern part of the Kanin Peninsula and further east to Taymyr52. In parallel, most of the area of N Eurasia was covered with two main vegetation types i.e. steppe-tundra and polar-alpine deserts53. These areas were the continental refugia for the Arctic species that subsequently spread north after ice sheet retreat.
The more southern findings of the Arctic species of Zercon are undoubtedly populations of glacial relicts. For these cold-adapted taxa the present climatic conditions in southern mountainous areas are suitable for survival as the equivalent of the Arctic, which was also confirmed in e.g. Ceratozetes spitsbergensis (Oribatida)54. During the periods of south-Polish glaciation (Sanian 1 period, the equivalent of the Glacial C period during the first half of middle Pleistocene: 780–420 ka BP) the ice sheet occupied most of the Polish territory, reached the Carpathians and the Sudetes and entered mountain river valleys55. Zercon polonicus, closely related to Z. forsslundi and Z. hamaricus, so far has been recorded in Tatra Mountains and Pieniny Mountains, S Poland as well as in Saubrunn (540 m a.s.l.), NE Austria6,36,37. Therefore, we hypothesize that Z. polonicus can be considered a post-glacial relict among other species with J5 setae considerably longer than short and similar J1–J4 in females. For Z. polonicus, which is not present in the current Arctic fauna, it could be hypothesized that it evolved e.g. from Z. forsslundi or other related Arctic species (e.g. Z. hamaricus?) that inhabited southern Europe during glaciation, and its descendant, Z. polonicus, formerly considered the Carpathian endemic36,56, migrated between the Carpathians and Alps.
Conclusions
Here we reported that the ranges of Z. forsslundi and Z. hamaricus overlap. Moreover, we confirmed the sympatry of these two species in the area of N Norway (Additional material, Table A1). According to current data, NW Europe could be regarded as the area of interaction at the southern edge of the range of Z. forsslundi and the northern edge of the range of Z. hamaricus. We also proved that microhabitats of these species overlap in N Norway (both species were recorded in the same samples at some locations). The interactions of taxa at the edges of biogeographical ranges are extremely interesting as they influence the local/regional biodiversity. North Eurasia is the region of interaction of the Arctic fauna and more-southern non-Arctic Palearctic fauna at the northern verge of the latter, which was also previously observed in mites20. This region is, therefore, a large-scale transitional zone between the Arctic and non-Arctic faunas. The influence of edge effects on the biodiversity of mites was previously observed also at the local-scale57,58,59. At the southeastern verge of the Palearctic (i.e. the Korean Peninsula), the diversity of Zerconidae is also shaped by extremely diverse environmental conditions, moreover, strengthened by isolation at the southern edge of the family range60.
Furthermore, genetic studies should be performed on Z. forsslundi, Z. hamaricus and Z. polonicus to determine the phylogenetic relationships among these Palearctic species. It could then be used to study Nearctic members of Zercon from the same, unique morphological group. Both would be useful for studying the speciation center(s) and dispersal processes of the whole unique group in the Holarctic.
Data availability
The datasets supporting the conclusions of this article are included within the article and its additional file.
References
Ujvári, Z. First records of Zerconidae (Acari: Mesostigmata) south of the Tropic of Cancer, Mexico, with description of five new species. Int. J. Acarol. 37, 201–215. https://doi.org/10.1080/01647954.2010.502907 (2011).
Urhan, R., Duran, E. H. & Karaca, M. Three new species of Zercon C. L. Koch (Acari: Zerconidae) from the Coastal Aegean section of Turkey. J. Nat. Hist. 54, 2323–2341. https://doi.org/10.1080/00222933.2020.1844328 (2020).
Mohammad-Doustaresharaf, M., Karaca, M., Bagheri, M. & Urhan, R. A taxonomic study on the zerconid mites (Acari: Zerconidae) in northwestern Iran: Descriptions of three new species with three new records. Syst. Appl. Acarol. 28, 429–430. https://doi.org/10.11158/saa.28.3.3 (2023).
Sellnick, M. Die Familie Zerconidae Berlese. Acta Zool. Acad. Sci. Hung. 3, 313–368 (1958).
Kaczmarek, S., Marquardt, T. & Seniczak, A. A new species of Zercon (Parasitiformes: Mesostigmata) from Norway, with notes on sexual dimorphism in Zerconidae. Syst. Appl. Acarol. 26, 1676–1702. https://doi.org/10.11158/saa.26.9.5 (2021).
Błaszak, C. Zercon polonicus sp. N. (Acari, Zerconidae) a new species of mite from Poland. Bull. Int. Acad. Pol. Sci. 28, 265–268 (1970).
Halašková, V. A revision of the genera of the family Zerconidae (Acari: Gamasides) and descriptions of new taxa from several areas of Nearctic Region (Academia, 1977).
Halašková, V. Some new species of the family Zerconidae from North America (Acari: Mesostigmata). Acta Soc. Zool. Bohemoslov. 33, 115–127 (1969).
Berlese, A. Lista di nuove specie e nuovi generi di Acari. Redia 6, 242–271 (1910).
Evans, G. O. A collection of mesostigmatid mites from Alaska. Bull Br Mus Nat Hist Zool. 2, 285–307 (1955).
Sikora, B. Mites of the family Zerconidae (Acari: Mesostigmata) of the Nearctic Region. Ann. Zool. 64, 131–250. https://doi.org/10.3161/000345414x682463 (2014).
Lindquist, E. E. & Evans, G. O. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the gamasina (Acarina: Mesostigmata). Mem Entomol Soc Can. 97, 5–66. https://doi.org/10.4039/entm9747fv (1965).
Lindquist, E. E. & Moraza, M. L. Observations on homologies of idiosomal setae in Zerconidae (Acari: Mesostigmata), with modified notation for some posterior body setae. Acarologia 39, 203–226 (1998).
Johnston, D. E. & Moraza, M. L. The idiosomal adenotaxy and poroidotaxy of Zerconidae (Mesostigmata: Zerconina). In Modern Acarology (eds Dusbábek, F. & Bukva, V.) 349–356 (Academia, 1991).
Byzova, J. B., Uvarov, A. V. & Petrova, A. D. Seasonal changes in communities of soil invertebrates in tundra ecosystems of Hornsund, Spitsbergen. Pol. Polar Res. 16, 245–266 (1995).
Salmane, I. Investigation of the seasonal dynamics of soil gamasina mites (Acari: Mesostigmata) in Pinaceum myrtilosum, Latvia. Ekol. Bratisl. 19, 245–252 (2000).
Makarova, O. L. Gamasid mites (Parasitiformes, Mesostigmata), dwellers of bracket fungi, from the Pechora-Ilychskii reserve (Republic of Komi). Entomol. Rev. 84, 1335–1340 (2004).
Makarova, O. L. The fauna of free-living gamasid mites (Parasitiformes, Mesostigmata) in the northern Taiga: An analysis of the zonal specificity. Entomol. Rev. 89, 1177–1193. https://doi.org/10.1134/s0013873809090176 (2009).
Makarova, O. L. A review of gamasid mites (Parasitiformes, Mesostigmata) dwelling in the taiga of the Pechoro-Ilychskii nature reserve (northern Cis-Ural Region) with analysis of their assemblages in spruce forests. Entomol. Rev. 91, 915–931. https://doi.org/10.1134/s0013873811070128 (2011).
Makarova, O. L. Gamasid mites (Parasitiformes, Mesostigmata) of the European Arctic and their distribution patterns. Entomol. Rev. 93, 113–133. https://doi.org/10.1134/s0013873813010156 (2013).
Makarova, O. L. Free-living mites (Acari) of the Franz Josef Land Archipelago, the coldest Old World territory: Diversity, geographic distributions, assemblages. Acarologia 63, 1163–1186. https://doi.org/10.24349/p6wb-pcni (2023).
Huhta, V., Siira-Pietikäinen, A., Penttinen, R. & Räty, M. Soil fauna of Finland: Acarina, Collembola and Enchytraeidae. Memo Soc. Fauna Flora Fenn. 86, 59–82 (2010).
Makarova, O. L., Osadtchy, A. V. & Melnikov, M. V. Gamasid mites (Parasitiformes, Mesostigmata) in nests of passerine birds on the Arctic Seven Islands Archipelago, the Barents Sea. Entomol. Rev. 90, 643–649. https://doi.org/10.1134/s0013873810050118 (2010).
Makarova, O. L., Ermilov, S. G., Yurtaev, A. A. & Mansurov, R. I. The first data on the soil mites (Acari) of the Arctic Belyi Island (Northern Yamal, the Kara Sea). Entomol. Rev. 95, 805–810. https://doi.org/10.1134/s0013873815060147 (2015).
Salmane, I. & Brumelis, G. Species list and habitat preference of Mesostigmata mites (Acari, Parasitiformes) in Latvia. Acarologia 50, 373–394. https://doi.org/10.1051/acarologia/20101978 (2010).
Ávila-Jiménez, M. L., Gwiazdowicz, D. J. & Coulson, S. J. The mesostigmatid mite (Acari: Parasitiformes) fauna of Svalbard: a revised inventory of a high Arctic archipelago. J Zootaxa. 3091, 33–41. https://doi.org/10.11646/zootaxa.3091.1.2 (2011).
Ávila-Jiménez, M. L. et al. The terrestrial invertebrate fauna of Edgeøya, Svalbard: Arctic landscape community composition reflects biogeography patterns. Polar Biol. 42, 837–850. https://doi.org/10.1007/s00300-019-02471-x (2019).
Gwiazdowicz, D. J. & Coulson, S. J. High-arctic gamasid mites (Acari, Mesostigmata): community composition on Spitsbergen, Svalbard. Polar Res. 30, 8311. https://doi.org/10.3402/polar.v30i0.8311 (2011).
Coulson, S. J., Schatz, H., Gwiazdowicz, D. J. & Solhøy, T. On the oribatid and mesostigmatid mites (Acari) of the High Arctic island of Hopen. Pol. Polar Res. 35, 133–139. https://doi.org/10.2478/popore-2014-0002 (2014).
Coulson, S. J. et al. Microarthropod communities of industrially disturbed or imported soils in the High Arctic; the abandoned coal mining town of Pyramiden, Svalbard. Biodivers. Conserv. 24, 1671–1690. https://doi.org/10.1007/s10531-015-0885-9 (2015).
Pilskog, H. E., Solhøy, T., Gwiazdowicz, D. J., Grytnes, J.-A. & Coulson, S. J. Invertebrate communities inhabiting nests of migrating passerine, wild fowl and sea birds breeding in the High Arctic, Svalbard. Polar Biol. 37, 981–998. https://doi.org/10.1007/s00300-014-1495-9 (2014).
Huhta, V. Catalogue of the Mesostigmata mites in Finland. Memo Soc. Fauna Flora Fenn. 92, 129–148 (2016).
Gwiazdowicz, D. J. et al. Changing microarthropod communities in front of a receding glacier in the high arctic. Insects 11, 226. https://doi.org/10.3390/insects11040226 (2020).
Makarova, O. L. & Bizin, M. S. Littoral mesostigmatic mites (Acari, Parasitiformes) from the Kola Peninsula. Polar Biol. 43, 1503–1518. https://doi.org/10.1007/s00300-020-02724-0 (2020).
Bizin, M. & Makarova, O. L. Free-living mites (Acari) of the Shokalsky Island, off the northern Gyda Peninsula, Kara Sea, High Arctic. Acarologia 64, 172–191. https://doi.org/10.24349/634f-jb4i (2024).
Błaszak, C. Zerconidae (Acari, Mesostigmata) Polski. Monogr Faun Pol. Warszawa, Kraków: PWN (1974).
Čoja, T. & Bruckner, A. The maturity index applied to soil gamasine mites from five natural forests in Austria. Appl. Soil Ecol. 34, 1–9. https://doi.org/10.1016/j.apsoil.2006.01.003 (2006).
Karg, W. Acari (Acarina), Milben. Parasitiformes (Anactinochaeta) Cohors Gamasina Leach. Raubmilben. 2nd ed. Gustav Fischer Verlag (1993).
Batchelor, C. L. et al. The configuration of Northern Hemisphere ice sheets through the Quaternary. Nat. Commun. 10, 3713. https://doi.org/10.1038/s41467-019-11601-2 (2019).
Kramer, P. Neue Acariden. Arch. Für Naturgeschichte. 45, 13–16. https://doi.org/10.11158/saa.22.6.9 (1897).
Błoszyk, J., Książkiewicz-Parulska, Z., Adamski, Z. & Napierała, A. Influence of Pleistocene glaciation on the distribution of three species of Labidostomma in Europe (Acari: Labidostommatidae). Syst. Appl. Acarol. 22, 841–857 (2017).
Błoszyk J, Adamski ZA, Napierała A. 2018 Notes on the biology and ecology of Labidostomma (Acari Prostigmata Labidostommidae) in Poland. J Redia. 3:2
Błoszyk, J., Napierała, A., Adamski, Z. & Zacharyasiewicz, M. Range of occurrence of bisexual and parthenogenetic populations of Labidostomma luteum (Acari: Prostigmata) in Europe. Diversity 14, 504. https://doi.org/10.3390/d14070504 (2022).
Coulson, S. J. et al. The terrestrial and freshwater invertebrate biodiversity of the archipelagoes of the Barents Sea; Svalbard, Franz Josef Land and Novaya Zemlya. Soil Biol. Biochem. 68, 440–470. https://doi.org/10.1016/j.soilbio.2013.10.006 (2014).
Seeman, O. D. & Walter, D. E. Phoresy and mites: More than just a free ride. Annu. Rev. Entomol. 68, 69–88. https://doi.org/10.1146/annurev-ento-120220-013329 (2023).
Ávila-Jiménez, M. L. & Coulson, S. J. A holarctic biogeographical analysis of the Collembola (Arthropoda, Hexapoda) unravels recent post-glacial colonization patterns. Insects 2, 273–296. https://doi.org/10.3390/insects2030273 (2011).
Brochmann, C., Gabrielsen, T. M., Nordal, I., Landvik, J. Y. & Elven, R. Glacial survival or tabula rasa? The history of North Atlantic biota revisited. Taxon 52, 417–450. https://doi.org/10.2307/3647444 (2003).
Coulson, S. J., Hodkinson, I. D., Webb, N. R. & Harrison, J. A. Survival of terrestrial soil-dwelling arthropods on and in seawater: implications for trans-oceanic dispersal. Funct. Ecol. 16, 353–356. https://doi.org/10.1046/j.1365-2435.2002.00636.x (2002).
Johansen, S. & Hytteborn, H. A contribution to the discussion of biota dispersal with drift ice and driftwood in the North Atlantic. J. Biogeogr. 28, 105–115. https://doi.org/10.1046/j.1365-2699.2001.00532.x (2001).
Alexanderson, H., Hjort, C., Möller, P., Antonov, O. & Pavlov, M. The North Taymyr ice-marginal zone, Arctic Siberia—A preliminary overview and dating. Glob. Planet Change 31, 427–445. https://doi.org/10.1016/s0921-8181(01)00133-3 (2001).
Polyak, L., Niessen, F., Gataullin, V. & Gainanov, V. The eastern extent of the Barents-Kara ice sheet during the Last Glacial Maximum based on seismic-reflection data from the eastern Kara Sea. Polar Res. 27, 162–172. https://doi.org/10.3402/polar.v27i2.6174 (2008).
Svendsen, J. I. et al. Late quaternary ice sheet history of northern Eurasia. Quat. Sci. Rev. 23, 1229–1271. https://doi.org/10.1016/s0277-3791(03)00342-1 (2004).
Ray, N. & Adams, J. A GIS-based vegetation map of the world at the last glacial maximum (25,000–15,000 BP). Internet Archaeol. https://doi.org/10.11141/ia.11.2 (2001).
Seniczak, A., Seniczak, S., Schwarzfeld, M. D., Coulson, S. J. & Gwiazdowicz, D. J. Diversity and distribution of mites (Acari: Ixodida, Mesostigmata, Trombidiformes, Sarcoptiformes) in the Svalbard Archipelago. Diversity 12, 323. https://doi.org/10.3390/d12090323 (2020).
Marks, L. et al. Quaternary stratigraphy and palaeogeography of Poland. Acta Geol. Pol. 66, 410–434. https://doi.org/10.1515/agp-2016-0018 (2016).
Pawłowski, J. Endemic Invertebrates of the Carpathians. Rocz Bieszczadzkie. 17, 89–128 (2009).
Seniczak, S., Kaczmarek, S. & Seniczak, A. Soil mites (Acari) of ecotones between a shelterbelt and cultivated fields in the agricultural landscape near Turew, Poland. Bull. Pol. Acad. Sci. 46, 7–12 (1998).
Kaczmarek, S. The role of ecotones in biological exchange with the surroundings, with particular reference to soil mites. In Ecology of Forest Islands (ed. Banaszak, J.) 253–258 (Wydawnictwo Akademii Bydgoskiej im. Kazimierza Wielkiego, 2000).
Kaczmarek S, Marquardt T, Marcysiak K. Mesostigmata (Acari) of ecotone zones within Bagno Stawek Reserve (Tuchola Forest, Poland). In: Bertrand M, Kreiter S, McCoy KD, Migeon A, Navajas M, Tixier MS, Vial L, editors. Integrative Acarology. In Proceedings of 6th Congress of the European Association of Acarologists. Montpellier: EURAAC, 188–196 (2008). https://hdl.handle.net/1813/22603
Kaczmarek, S. & Marquardt, T. Speciation processes within the Korean Peninsula based on the mites from the family Zerconidae (Acari: Mesostigmata). J. Asia-Pac. Entomol. 9, 223–226. https://doi.org/10.1016/s1226-8615(08)60294-0 (2006).
Acknowledgements
This article is dedicated to Czesław Błaszak (1942-2021), former full professor of the Faculty of Biology of Adam Mickiewicz University in Poznań (Poland) and Universität Heidelberg (Germany), doctor honoris causa of the Universität Vechta (Germany), honorary member of the Polish Acarological Society, zoologist, ecologist and acarologist. Author of nearly 100 descriptions of new mite species, including Zerconidae, and monographic studies of this family in the Holarctic. The Authors would like to thank the all collaborators included in the Table A1 for supporting the research material.
Funding
This study was funded by the Kazimierz Wielki University, Department of Evolutionary Biology, project from Norwegian Taxonomy Initiative (Grant No. 35–16, 811030) and Soil Organisms in the Subarctic Project of Norwegian Institute of Bioeconomy Research (Grant No. 9–21, 70184244).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, supervision and writing – original draft, S.K. and T.M; visualization and formal analysis, T.M.; investigation, S.K., T.M., C.F.C.K., S.R. and A.S.; resources, S.K., T.M., S.B.H., C.F.C.K., S.R. and A.S.; funding acquisition, S.K., S.B.H. and A.S.; writing – review and editing, all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Mites (invertebrates) were used for this study. No vertebrate or human individuals or tissues were used.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaczmarek, S., Marquardt, T., Hagen, S.B. et al. Complete instars’ morphology of Zercon forsslundi Sellnick, 1958 (Parasitiformes: Mesostigmata) with notes on distribution and evolution. Sci Rep 15, 23425 (2025). https://doi.org/10.1038/s41598-025-06732-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06732-0