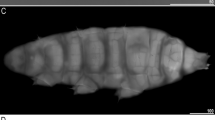
Abstract
Tardigrades are microscopic panarthropods renowned for their ability to undergo cryptobiosis. While integrative taxonomy of tardigrades has been intensively applied in the description of tardigrade species over the past two decades, many details of their external morphology remain poorly recognized and under-described. This limitation is largely due to their small size and the limited morphological features useful for classical taxonomy. In particular, compared to heterotardigrades, the external sensory structures of eutardigrades are less studied. In this study, we present a detailed morphological analysis using FE-SEM of two Greenlandic apochelan species, Milnesium grandicupula sp. nov. and M. cf. longiungue. We identified several previously unobserved anatomical structures in milnesiids, including minute sensory structures in the cephalic region. Detailed microphotographs revealed that apochelan tardigrades also possess a set of external sensory structures on the head sensory fields, similar to those observed in other major evolutionary lineages within tardigrades. This finding corroborates the hypothesis of the homology of head sensory organs between eutardigrades and heterotardigrades. Notably, these small sensory structures exhibit a distinctive pattern: a relatively large central organ surrounded by smaller pores, which may be comparable to the sensory dorsal organ (SDO) of crustaceans and the cephalic median organ (CMO) of trilobites.
Similar content being viewed by others
Introduction
Tardigrades, commonly known as water bears, are minute metazoans (50–1,200 μm), typically characterized by an elongated or cylindrical body with four pairs of legs, usually ending with claws and/or digits, with few exceptions. They are found in diverse terrestrial and aquatic habitats, including lowlands, high mountains, lacustrine, and marine environments across various latitudes and altitudes1. Tardigrades play a multitrophic role in ecosystems, often reaching high densities and, in some cases, dominating specific habitats2,3,4. Due to their ability to enter cryptobiosis (an ametabolic state typical for tardigrades under unfavourable conditions), tardigrades are renowned for their remarkable survival in hostile environments5. In this state, they can withstand extreme conditions and re-enter an active state when conditions become favorable again6. As a result, tardigrades have attracted attention from various scientific fields, including astrobiology, toxicology, and medical sciences7,8,9,10,11,12. Although interest in tardigrade physiology and their potential applications in biotechnology or medical science is increasing, fundamental knowledge regarding their origin, evolution, systematics, and morphology still requires further detailed investigation.
Along with Onychophora and Arthropoda, Tardigrada constitutes Panarthropoda, the most species-rich animal group, which is thought to have originated from the Cambrian lobopodians13. Morphological comparisons between tardigrades and Cambrian lobopodians suggest potential homology of several characters, including the features of head sensory organs, the mouth, and claws14. However, due to the small size and soft bodies of tardigrades, combined with the scarcity of tardigrade fossil records15 many detailed morphological traits remain underexplored, and knowledge regarding the homology of these characters is still largely incomplete. Comparisons with other panarthropod groups can aid in tracing the homology of morphological characters within panarthropods14,16.
The Phylum Tardigrada is divided into two classes, Heterotardigrada and Eutardigrada, and currently includes over 1,500 described species, with this number continuing to grow17,18,19. The cuticle of many heterotardigrades is covered by plates equipped with cirri and clavae, whereas eutardigrades lack both armour and cirri/clavae. Notably, heterotardigrades exhibit several external sensory organs on the head, whereas these organs are typically reduced or absent in eutardigrades20. Instead, eutardigrades possess sensory fields on the head, which are innervated by nerves rooted in the brain21. However, several homologous external head sensory organs have been recently described22. The Class Eutardigrada is divided into two orders: Parachela, which includes aquatic and limno-terrestrial species, and Apochela, which comprises exclusively limno-terrestrial species.
Apochela comprises a single family, Milnesiidae, which includes four genera and 54 species17. Except for Milnesium, the remaining three genera are monotypic taxa. Milnesium is a cosmopolitan and exclusively carnivorous genus, feeding on rotifers, nematodes, protozoans, or other tardigrades23,24,25. This group includes the largest tardigrades, with body lengths often exceeding 1,000 μm, and is characterized by a unique set of morphological traits: six peribuccal lamellae, six peribuccal papillae, a pair of lateral papillae, a relatively simple buccal-pharyngeal apparatus without teeth or sclerotized elements, such as placoids, and, more importantly, primary and secondary branches of apochelan claws are separated, unlike in parachelan tardigrades26,27,28. Due to the limited number of useful morphological traits, Milnesium has been regarded as a challenging group for taxonomy23. Traditionally, two primary characters have been used to define morphogroups in Milnesium: claw configuration (CC)29 and dorsal cuticular sculpturing27. A recent study on Milnesium made a significant advancement by distinguishing pseudopores from other cuticular structures on the dorsal surface, suggesting that the details of pseudopores could serve as a valuable taxonomic key character23 as well as pseudoplates on the dorsal cuticular surface. Despite this progress, the weak correlation between taxonomically important morphological traits and the internal phylogeny of the genus continues to obscure our understanding the evolution of Milnesium, highlighting the need for future researches and additional data.
In this study, we present an integrative description of two Milnesium species discovered on Ella Island, East Greenland, utilizing morphology and partial molecular sequences of four genes (the small ribosome subunit (18S rRNA), the large ribosome subunit (28S rRNA), the internal transcribed spacer 2 (ITS2), and the cytochrome oxidase c subunit I (COI)). Remarkably, detailed SEM observations of these Arctic species provide comprehensive insights into cuticular structures and external cephalic sensory structures of Milnesium, with implication for the evolution of morphological features within the phylum Tardigrada.
Results
-Taxonomic account.
Phylum Tardigrada Doyère, 184030.
Class Eutardigrada Richters, 192631.
Order Apochela Schuster et al., 198032.
Family Milnesiidae Ramazzotti, 196233.
Genus Milnesium Doyère, 184030.
Milnesium cf. longiungue Tumanov, 200634.
-Examined material.
Nine animals on slide in Hoyer’s medium, seven animals mounted on stubs for SEM observations, and two animals for DNA extraction.
-Repositories.
Seven specimens in Hoyer’s medium (slide codes: KOPRIF 2019-Ella-Milnesium cf. longiungue 01–07) along with seven specimens on three SEM stubs (stub codes: Milnesium Ella Ø Lake Sep 230121-1, Milnesium Ella Ø Lake Sep 230121-2 and Milnesium Ella Ø Lake Sep 230214), are deposited in the KOPRI Paleontology collection (Division of Glacier & Earth Sciences, KOPRI, Korea). Additionally, two specimens (slide codes: KOPRIF 2019-Ella-Milnesium cf. longiungue 08–09), are archived at the Department of Taxonomy and Ecology at Adam Mickiewicz University, Poznań, Poland.
-Locality.
72°50’51.23"N, 25°5’8.71"W, 471 m a.s.l.: the limestone outcropping at the margin of the Lake September, Ella Island, Greenland.
-General description of mature females (morphometrics in Supplementary Table S1 online and Supplementary Data S1 online).
The body slender white to slightly yellow in life, turning brownish in Hoyer’s medium (Fig. 1). Eyes present in both live and fixed specimens in Hoyer’s medium (Fig. 1a, b). Cuticle smooth, lacking granulation or pores; weakly outlined pseudoplates visible on the caudo-dorsal cuticle surface in some specimens, observed only under SEM (Fig. 1c). Six peribuccal papillae present (Fig. 2a–c), all of identical size except for a smaller ventral one (Fig. 2b). Six triangular peribuccal lamellae with longitudinal stripes (Fig. 2a, b), of unequal size (Fig. 2b), arranged in a 4 + 2 configuration; two lateral lamellae slightly smaller than the others. Two lateral papillae present (Fig. 2b, c). The buccal-pharyngeal apparatus with a straight-shaped buccal tube (Fig. 2a); valvular system of type 1 (tardigradum type28): characterized by three relatively short caudal expansions with the thin flaps. Anterolateral sensory field exhibits multiple pores above the lateral papilla (Fig. 2c, d). Under FE-SEM observation, a distinctive cephalic sensory organ complex (CSC) present in the upper part of the anterolateral sensory field (Fig. 2d), composed of a relatively large central organ (approx. 250 nm in diameter) surrounded by several small pores (diameter < 50 nm) (Fig. 2e); total CSC diameter ~ 1 μm. Due to its minute size, the CSC not clearly visible under DIC microscopy. Posterolateral sensory field with scattered pores; CSC not observed (Fig. 2f, g). Median sensory field marked by a mound structure; CSC present slightly anterior part of this field (Fig. 2f, h). Claws slender, with primary branches of all legs lacking accessory points (Supplementary Fig. S1 online). Each secondary branch possesses three points, resulting in CC of [3-3]-[3-3]. A cuticular thickening present near the base of the primary branch of all claws, and cuticular bars occur only beneath claws I–III (Supplementary Fig. S1a online).
Buccal-pharyngeal apparatus and head part of Milnesium cf. longiungue. DIC image (a) and SEM images (b–h). (a) Buccal-pharyngeal apparatus. (b) Mouth part. (c) Head part in dorsal view. (d) Anterolateral sensory field. (e) Cephalic sensory organ complex on anterolateral sensory field. (f) Head part in anterior view. (g) Posterolateral sensory field. (h) Cephalic sensory organ complex on median sensory field. ASF: Anterolateral sensory field; CMAS: Cribriform muscle attachment site; MED: Median sensory field; PSF: Posterolateral sensory field.
-Morphological comparison.
Milnesium cf. longiungue do not differ by morphology and morphometry from the original description of Milnesium longiungue originally described from Lahaul, Himalaya, India34.
-Genetic comparison.
All sequences obtained for M. cf. longiungue were unique and distinct from the sequences deposited in GenBank (Supplementary Data S3 online). The ranges of the uncorrected p-distances between M. cf. longiungue and sequences of other congeners are as follows (Supplementary Data S4 online):
18S rRNA: 0.0–3.8% (1.2% on average), with undetermined Milnesium. sp. PL. 247 from Poland (MK48409626).
28S rRNA: 1.4–14.0% (7.0% on average), with the most similar being Milnesium alpigenum Ehrenberg, 1853 from Italy (MH00038435).
ITS2: 8.7–31.8% (22.0% on average), with the most similar being M. alpigenum from Italy (MH00038235).
COI: 11.8–24.8% (19.0% on average), with the most similar being M. alpigenum from Italy (MH00038035).
-Remarks.
All morphometric characters of Milnesium cf. longiungue from this study fall within the range reported in the original description of M. longiungue. However, due to the considerable geographic distance between the type locality (Lahaul, India) and the current sampling site (Ella Island, Greenland; over 7,000 km apart) and the absence of DNA sequence data for the Indian population, we assign the Greenlandic specimens to Milnesium cf. longiungue pending further integrative comparison.
Milnesium grandicupula sp. nov.
urn: lsid: zoobasnk.org: act:4A9A6E31-FAF7-458D-B84B-3511BE8B6932.
- Examined material.
Forty-five animals on slides in Hoyer’s medium, forty-three animals mounted on stubs for SEM observation, and four animals for DNA extraction.
-Type repositories.
The holotype (slide code: KOPRIF 2019-Ella-Milnesium grandicupula 02), along with 42 paratype specimens on Hoyer’s medium (slide codes: KOPRIF 2019-Ella-Milnesium grandicupula 01–34, and KOPRIF2019-Ella-Milnesium H01–H11) and forty-three specimens on three SEM stubs (stub code: Milnesium Ella Ø Lake Sep 230121-1, Milnesium Ella Ø Lake Sep 230121-2, Milnesium Ella Ø Lake Sep 230214), are deposited in the KOPRI Paleontology collection (Division of Glacier & Earth Sciences, KOPRI, Korea). Additionally, two paratypes (slide codes: KOPRIF 2019-Ella-Milnesium grandicupula 09, 10) are deposited at the Department of Taxonomy and Ecology at Adam Mickiewicz University, Poznań, Poland.
-Type locality.
72°50’51.23"N, 25°5’8.71"W, 471 m a.s.l.: the limestone outcropping at the margin of the Lake September, Ella Island, Greenland.
-Etymology.
The name “grandicupula” derived from Latin, where grandis, means large and cupula means cup. It refers to a large, cup-shaped buccal tube of this species.
-General description.
Mature females (from the third instar upward; morphometrics in Supplementary Table S2 online and Supplementary Data S2 online).
Body yellowish to brownish in life, becoming slightly brownish in Hoyer’s medium (Fig. 3a, b). Eyes present and persist after fixation in Hoyer’s medium. Dorsal pseudoplates present with ten rows, but visible only under SEM (Fig. 3c, d). The arrangement of pseudoplates: row I and II situated anteriorly to legs I; row III situated in line with legs I; row IV situated between legs I and II; row V situated in line with legs II; row VI situated between II and III; row VII situated in line with legs III; row VIII–X situated between legs III and IV. Configuration of pseudoplates (CP): CP: I:2;II:5;III:4;IV:2;V:4;VI:2;VII:2;VIII:5;IX:8;X:4. Cuticle smooth, lacking evident pores and granulation. Six peribuccal papillae with longitudinal stripes present (Fig. 3e, f), all similar in size except for a smaller ventral one. Six triangular peribuccal lamellae present, arranged in a 4 + 2 configuration; two lateral lamellae slightly smaller than the others. Two lateral papillae present. Buccal-pharyngeal apparatus with a funnel-shaped buccal tube (Fig. 3f); valvular system of type 1 (tardigradum type28), with three relatively short caudal expansions and the thin flaps. Anterolateral sensory fields exhibit several scattered pores (Fig. 4a, b). Under FE-SEM observation, a cephalic sensory organ complex (CSC) present in the upper part of the anterolateral sensory field (Fig. 4c), consisting of a central organ (~ 250 nm in diameter) surrounded by smaller pores (< 100 nm), forming a complex ~ 1 μm in diameter. Due to its minute size, the CSC not clearly visible under DIC microscopy. Posterolateral sensory field also display scattered pores, although CSC is absent in this region (Fig. 4a, d). Surface texture of the posterolateral sensory field differs slightly different from adjacent cuticle (Fig. 4d). The median sensory field features a distinct mound structure, with CSC occurring at its anterior region (Fig. 4a, e, f). Claws typical of the genus Milnesium (Supplementary Fig. S2 online), with accessory points present on all primary branches. All secondary branches have three points, resulting in CC: [3-3]-[3-3]. Cuticular bars observed beneath claws I–III. Under both SEM (Fig. 5a) and TEM (Fig. 5b) observations, three distinct layers (epicuticle, inner trilaminate layer, and basal cuticle) distinguishable. Channels occur within the inner epicuticle (Fig. 5b). The inner trilaminate layer contains numerous nano-scale granules (Fig. 5b, c), which appear to integrate with the net-like surface of the basal cuticle (Fig. 5d). This structural organization evident throughout the body, including the dorsal (Fig. 5a, c) and ventral (Fig. 5e, f) parts of the trunk, as well as the legs (Fig. 5g). In the dorso-caudal region, numerous pores (50–200 nm in diameter) visible at the interface where the basal cuticle meets the epidermis (Fig. 5h).
Sensory fields and cephalic sensory organ complexes of Milnesium grandicupula sp. nov. (SEM). (a) Head part. (b) Anterolateral sensory field and lateral papilla. (c) Cephalic sensory organ complex on anterolateral sensory field. (d) Posterolateral sensory field. (e) Median sensory field. (f) Cephalic sensory organ complex on median sensory field with cover. (g) Cephalic sensory organ complex on median sensory field without cover. ASF: Anterolateral sensory field; CMAS: Cribriform muscle attachment site; MED: Median sensory field; PSF: Posterolateral sensory field.
Intrastructures of cuticle of Milnesium grandicupula sp. nov. SEM images (a, c–h) and TEM image (b). (a, b) Cross section of cuticle of dorsal trunk part. (c) Granules of inner trilaminate layer. (d) Interdigitation between granules of inner trilaminate layer and pits on net-like basal cuticle surface. (e) Surface of basal cuticle at ventral trunk part. (f) Pillar-like structures at the basal cuticle. (g) Cross section of leg IV. (h) Pores at the innermost surface of the basal cuticle. B: Basal cuticle; CMAS: Cribriform muscle attachment site; E: Epicuticle; IT: Inner trilaminate layer.
Mature males (from the third instar upward; Supplementary Fig. S3a, b online; morphometrics in Supplementary Table S3 online and Supplementary Data S2 online).
Three specimens in the sample. Body slightly slenderer than that in females. Buccal tube markedly narrower in males than in females, with the ratio of standard width to the length of buccal tube ranging 32.0–33.8% for males and 43.7–69.7% for females. Secondary branch of the claw I modified into rigid hooks with a spur. Although the morphology of claw I differs between males and females, their sizes do not vary. Cuticular bars present beneath claws I–III. No pronounced sex dimorphism in overall body size relative to females.
Juveniles (the second instar; Supplementary Fig. S3c online; morphometrics in Supplementary Table S4 online and Supplementary Data S2 online).
Eyes present in Hoyer’s medium. Body color similar to that of adults; CC identical to that of adults. Cuticular bars present beneath claws I–III.
Hatchlings (the first instar; Supplementary Fig. S3d online; morphometrics in Supplementary Table S5 online and Supplementary Data S2 online).
The hatchlings are offspring from two females that had been identified as M. grandicupula. Eyes present in living animals, but absent in Hoyer’s medium. Body color in Hoyer’s medium is considerably transparent. CC identical to that of adults and juveniles. Cuticular bars present beneath claws I–III, as juveniles and adults.
-Morphological differential diagnosis.
Milnesium grandicupula sp. nov. is similar to sixteen previously reported Milnesium species characterized by a smooth cuticle with pseudoplates, [3-3]-[3-3] CC, six lamellae with 4 + 2 configuration, six papillae around the mouth opening and presence of accessory points on primary branches. M. grandicupula sp. nov. differs specifically from
-
Milnesium alpigenum36 known from Monte Rosa massif, Italy (1370 m a.s.l.)35 found in moss on roof by: the pt indices of the anterior width (29.6–44.8 in M. alpigenum vs. 59.1–79.1 in M. grandicupula sp. nov.) and the standard width (21.0–38.4 in M. alpigenum vs. 45.0–69.9 in M. grandicupula sp. nov.);
-
Milnesium antarcticum Tumanov, 200634 King George Island, Antarctica found in moss in a little stream by: the different valvular system type (type 2 (shilohae type) in M. antarcticum vs. type 1 (tardigradum type) in M. grandicupula sp. nov.) and the pt indices of the stylet support insertion point (70.0–73.7 in M. antarcticum vs. 57.7–69.7 in M. grandicupula sp. nov.) and standard width (35.4–43.9 in M. antarcticum vs. 45.0–69.9 in M. grandicupula sp. nov.);
-
Milnesium argentinum Roszkowska, Ostrowska & Kaczmarek, 201537 Río Negro Province, Argentina (ca. 1,200 m a.s.l.) found in moss on trees and rocks by: the pt indices of the stylet support insertion point (70.0–73.7 in M. argentinum vs. 57.7–69.7 in M. grandicupula sp. nov.), the anterior width (30.3–39.8 in M. argentinum vs. 59.1–79.1 in M. grandicupula sp. nov.), the standard width (24.2–32.3 in M. argentinum vs. 45.0–69.9 in M. grandicupula sp. nov.), the posterior width (23.1–33.6 in M. argentinum vs. 35.2–56.1 in M. grandicupula sp. nov.), and the pt indices of claw I to III (the external primary branch, the external base + secondary branch, the external spur, the internal primary branch, the internal base + secondary branch, the internal spur);
-
Milnesium asiaticum Tumanov, 200634 Kirghizian ridge, Kirghizia (2000 m a.s.l.) found in moss from soil by: the pt indices of the standard width (30.0–41.6 in M. asiaticum vs. 45.0–69.9 in M. grandicupula sp. nov.), and primary branch of claw IV (63.9–76.0 in M. asiaticum vs. 46.2–60.8 in M. grandicupula sp. nov.);
-
Milnesium beatae Roszkowska, Ostrowska & Kaczmarek, 201537 known from Río Negro Province, Argentina (ca. 1,000 m a.s.l.) found in moss on rocks by: the different length between the dorsal and ventral peribuccal papillae (similar length in M. beatae vs. evidently longer dorsal peribuccal papilla in M. grandicupula sp. nov.) and development degree of pseudopores (well-developed in M. beatae vs. invisible in M. grandicupula sp. nov.);
-
Milnesium brachyungue Binda & Pilato, 199038 Chile and Río Negro Province, Argentina found in moss on trees and rocks by: different valvular system type (type 2 (shilohae type) in M. brachyungue vs. type 1 (tardigradum type) in M. grandicupula sp. nov.) and the pt indices of primary branches of claws I–III (22.9–27.1 in M. brachyungue vs. 39.1–52.0 in M. grandicupula sp. nov.) and primary branches of claws IV (33.1 in M. brachyungue vs. 46.2–60.8 in M. grandicupula sp. nov.);
-
Milnesium burgessi Schlabach, Donaldson, Hobelman, Miller & Lowman, 201839 eastern Kansas, USA, found in moss and lichen on canopy by: different valvular system type (type 2 (shilohae type) in M. burgessi vs. type 1 (tardigradum type) in M. grandicupula sp. nov.). and the pt indices of the body length (2409–2024 in M. burgessi vs. 1082.3–1503.4 in M. grandicupula sp. nov.), and the external primary branches of the claws I–III (52.2–79.5 in M. burgessi vs. 39.1–52.0 in M. grandicupula sp. nov.), the external primary branches of the claws IV (70.7–89.6 in M. burgessi vs. 46.2–60.8 in M. grandicupula sp. nov.) and posterior/anterior width ratio (85.19–100.00% in M. burgessi vs. 58.8–77.2% in M. grandicupula sp. nov.);
-
Milnesium cassandrae Moreno-Talamantes, Roszkowsak, García-Aranda, Flores-Maldonado & Kaczmarek, 201940 known from Nuevo Leon, Mexico (632 m a.s.l.) found in lichen on trees by: several buccal tube characters, including buccal tube length (26.2–42.5 μm in M. cassandrae vs. 56.2–70.2 μm in M. grandicupula sp. nov.), anterior width (12.3–24.8 in M. cassandrae vs. 25.9–50.2 μm in M. grandicupula sp. nov.), posterior/anterior width ratio (81–96% in M. cassandrae vs. 58.7–79.3% in M. grandicupula sp. nov.), and pseudoplate arrangement pattern (nine rows in M. cassandrae vs. ten rows in M. grandicupula sp. nov.);
-
Milnesium eurystomum Maucci, 199141 in ref25. known from Greenland, Svalbard, and Scotland found in lichen and moss on rock and soil by: pseudopore (visible in M. eurystomum vs. invisible under light microscope in M. grandicupula sp. nov.; see remarks) and pseudoplate arrangement pattern (nine rows in M. eurystomum vs. ten rows in M. grandicupula. sp. nov.);
-
Milnesium fridae Moreno-Talamantes, León-Espinosa, García-Aranda, Flores-Maldonado & Kaczmarek, 202042 known from Nuevo Leon, Mexico (700 m a.s.l.) found in lichen on trees by: different valvular system type (type 2 (shilohae type) in M. fridae vs. type 1 (tardigradum type) in M. grandicupula sp. nov.);
-
Milnesium inceptum Morek, Suzuki, Schill, Georgiev, Yankova, Marley & Michalczyk, 201935 known from Tübingen, Germany (377 m a.s.l.) found in moss on soil by: the pt indices of the anterior width (30.8–42.9 in M. inceptum vs. 59.1–79.1 in M. grandicupula sp. nov.) and the standard width (23.1–37.8 in M. inceptum vs. 45.0–69.9 in M. grandicupula sp. nov.), and posterior/anterior width ratio (81–99% in M. inceptum vs. 58.8–77.2% in M. grandicupula sp. nov.);
-
Milnesium iniquum Brotto-Guidetti, Morek & Garraffoni, 202443 known from Campinas City, Brazil (606 m a.s.l.) found in moss on tree trunks by: the pt indices of the anterior width (42.2–52.8 in M. iniquum vs. 59.1–79.1 in M. grandicupula sp. nov.) and the primary branches of claw I–III (28.7–34.8 in M. iniquum vs. 39.1–52.0 in M. grandicupula sp. nov.), pseudoplate arrangement pattern (CP: I:?;II:?;III:?;IV:4;V:6;VI:2;VII:8;VIII:6;IX:10;X:4 in M. iniquum vs. CP: I:2;II:5;III:4;IV:2;V:4;VI:2;VII:2;VIII:5;IX:8;X:4 in M. grandicupula sp. nov.), and posterior/anterior width ratio (83–100% in M. iniquum vs. 58.8–77.2% in M. grandicupula sp. nov.);
-
Milnesium minutum Pilato & Lisi, 201644 known from Sicily, Italy found in moss on rock by: the absolute body size (284–288 μm in M. minutum vs. 706.4–875.4 μm in M. grandicupula sp. nov.) and the pt index of the standard width (38.6–42.4 in M. minutum vs. 45.0–69.9 in M. grandicupula sp. nov.);
-
Milnesium sandrae Pilato & Lisi, 201644 known from Hawai’i Island by: the shape of the buccal tube (cylindrical in M. sandrae vs. funnel-shaped in M. grandicupula sp. nov.);
-
Milnesium shilohae Meyer, 201545 known from Hawai’i (30 m a.s.l.) found in moss on tree by: the pt indices of the stylet support insertion point (75.5–77.5 in M. shilohae vs. 57.7–69.9 in M. grandicupula sp. nov.), the anterior width (47.5–58.3 in M. shilohae vs. 59.1–79.1 in M. grandicupula sp. nov.), the external spur of claw I–III (1.9–7.5 in M. shilohae vs. 39.1–52.0 in M. grandicupula sp. nov.), the posterior spur of claw IV (3.1–5.9 in M. shilohae vs. 46.2–60.8 in M. grandicupula sp. nov.), and posterior/anterior width ratio (90–107% in M. shilohae vs. 58.8–77.2% in M. grandicupula sp. nov.);
-
Milnesium tumanovi Pilato, Sabella & Lisi, 201646 known from Yalta, Crimea (40 m a.s.l.) found in moss on rock by: different valvular system type (type 2 (shilohae type) in M. tumanovi vs. type 1 (tardigradum type) in M. grandicupula sp. nov.), the shape of the buccal tube (cylindrical in M. tumanovi vs. funnel-shaped in M. grandicupula sp. nov.), and the pt index of the stylet support insertion point (52.3 in M. tumanovi vs. 57.7–69.9 in M. grandicupula sp. nov.).
-Genetic comparison
All sequences obtained for M. grandicupula sp. nov. were unique and distinct from the sequences deposited in GenBank (Supplementary Data S3 online). The ranges of the uncorrected p-distances between M. grandicupula sp. nov. and sequences of other congeners are as follows (Supplementary Data S4 online):
18S rRNA: 0.0–3.5% (1.4% on average), with the most similar being Milnesium cf. eurystomum from Greenland (MW53812547).
28S rRNA: 0.1–14.3% (7.6% on average), with the most similar being Milnesium cf. eurystomum from Norway (MK48398025).
ITS2: 5.9–30.6% (20.8% on average), with the most similar being Milnesium cf. eurystomum from Norway (MK48400725).
COI: 10.9–23.8% (18.3% on average), with the most similar being Milnesium eurystomum from Greenland (MT27619125).
-Phylogenetic analysis (Fig. 6; Supplementary Fig. S4 online).
Phylogeny of the genus Milnesium. With dataset of ref47, two newly gathered species in this study and two previously reported species were added. A concatenated 18 S rRNA + 28 S rRNA + ITS2 + COI consensus tree was obtained based on Bayesian inference analysis. Node values are given as BI posterior probability (PP) values. The scale bar represents 0.1 substitutions per nucleotide position. Supplementary Fig. S4 online shows the entire tree.
The concatenated 18S rRNA + 28S rRNA + ITS2 + COI phylogenetic reconstruction, based on the Bayesian inference analysis, positions both Milnesium cf. longiungue and Milnesium grandicupula sp. nov. within clade B as identified by ref47. Specifically, Milnesium cf. longiungue is placed as a sister group of M. alpigenum, while M. grandicupula sp. nov. is identified as a sister group of (M. eurystomum + Milnesium sp. MY.025).
-Remarks.
In one specimen of Milnesium grandicupula sp. nov., a portion of the basal cuticle exhibits a pillar-like structure (Fig. 5f). Although this structure shares a similar height with the pillar of Murrayon48, their locations differ: the basal cuticle in M. grandicupula sp. nov. and the inner epicuticle in Murrayon. Instead, the position of pillar-like structures of M. grandicupula sp. nov. resembles the strut in the medial zone of nematode Caenorhabditis elegans49. However, the presence of pillar-like structures of M. grandicupula sp. nov. has not been confirmed through TEM observation of this study. Therefore, it remains uncertain whether this structure is genuine or an artefact introduced during SEM preparation.
A specimen exhibits a cover over the central hole of the CSC (Fig. 4f), while other specimens analyzed do not (Fig. 4g). It remains unclear whether the central organs of the CSC in the specimens lacking a cover originally possessed one that was lost during SEM preparation. Similarly, due to its association with a thin and flexible cuticle, the central gland of crustacean SDO frequently collapses during SEM preparation. This aspect is also reminiscent of the cover of the median sensory field CSC of Milnesium.
TEM observation in this study identified several channel structures within the inner epicuticular layer (arrow in Fig. 5b), as previously reported in M. tardigradum50. These channels are located exclusively in the inner epicuticular layer and are covered by the outermost layer, making them undetectable via SEM. These channels may be pseudopores of M. grandicupula sp. nov. However, due to their minute size (diameter is less than 50 nm), they are difficult to observe under DIC microscopy.
While observations using TEM have not confirmed their presence, numerous pores have been identified using FE-SEM (Fig. 5h). These pores are located on the innermost side of the basal cuticle. Consequently, the basal cuticle exhibits pits and pores at the outermost (Fig. 5d) and the innermost sides (Fig. 5h), respectively. The diameter of the pits of the outermost surface (ca. 50 nm) is smaller than that of pores of the innermost surface (ca. 50–200 nm). The epidermis, which directly contacts the innermost surface of the basal cuticle, also contains numerous pores on its surface. These pores on the epidermis surface likely correspond to the distribution of those on the innermost side of the basal cuticle. However, since the channel structures were not observed within the basal cuticle under TEM examination in this study, the function of these pores remains unclear.
Discussion
- Head sensory organs in Tardigrada.
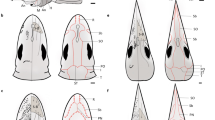
Using neuroanatomical comparative data, several studies have proposed the possibility of homology between head sensory organs of heterotardigrades and head sensory fields of eutardigrades20,51,52,53,54,55. Notably, several eutardigrade species exhibit external sensory organs on the anterolateral sensory field, such as frontal lobes or cephalic papillae reported in some isohypsibioid species (e.g. Ursulinius, Halobiotus, and Apodibius)56 and on the posterolateral sensory field, such as elliptical organs in Ramazzotius, Cryoconicus, Fractonotus, and Calohypsibius57,58,59. The recent integrative description of the Arctic, parachelan species Ramazzottius groenlandensis revealed a set of external head sensory organs on the head sensory fields, potentially corresponding to the head sensory organs of heterotardigrades. This finding supports the hypothesis of homology for cephalic sensory structures between eutardigrades and heterotardigrades22. However, except for the mouth part and the lateral papillae, no external sensory organs on the head have been reported in apochelans. Through detailed SEM observations, this study reveals that apochelan tardigrades also possess external sensory organs, consisting of several scattered pores with slightly different surface textures on each sensory field. Along with a pair of lateral papillae, these organs in the head sensory fields of Milnesium correspond to the head sensory organs of heterotardigrades and a set of external sensory organs of R. groenlandensis (Fig. 7). Additionally, the characteristic cephalic sensory organ complex (CSC), are also present in the anterolateral sensory fields and the median sensory field. This discovery indicates that all major phylogenetic lines within Tardigrada possess external sensory organs in homologous locations, further supporting the hypothesis of homology of cephalic sensory structures across tardigrades (Fig. 7).
False-coloured scanning electron microscopic images of representatives of four orders within Tardigrada. This figure follows the hypothesis for the homology of head sensory organs and head sensory fields by ref20. (SEM). (a) Marine heterotardigrade (arthrotardigrade) Styraconyxid species. (b) Echiniscoidean Echiniscus testudo (Doyère, 1840). (c) apochelan Milnesium grandicupula sp. nov. (d) Parachelan Ramazzottius groenlandensis Kihm, Zawierucha, Rho & Park, 2023. Asterisks indicate the locations of cephalic sensory organ complexes.
Although this study did not detect the presence of CSC in the posterolateral sensory fields, it is noteworthy that the CSC was detected in a limited number of specimens (two specimens of M. cf. longiungue and five specimens of M. grandicupula sp. nov.). The absence of visible CSC in the posterolateral sensory field may result from damage or loss during SEM preparation, given their significantly small size and fragility. However, the cuticular surface of the posterolateral sensory field can still be distinguished from the surrounding area with small pores (Figs. 2g and 4d). Further investigation is required to confirm whether the CSC is present or absent in the posterolateral sensory fields in Apochela. Additionally, several factors could potentially influence the detection of CSC in SEM specimens, including the thickness of the coating. Notably, specimens coated with a relatively thicker layer of platinum particles did not exhibit visible CSC.
-Comparison of cephalic median sensory structures within tardigrades.
Several lines of evidence, including the higher genetic divergence in both 18 S and 28 S sequences60, remarkable morphological diversity, and their exclusive habitation in marine environments61, suggests that marine heterotardigrades represent an ancient lineage within tardigrades62. Consequently, marine heterotardigrades are hypothesized to retain ancestral morphological traits. Based on this notion, the hypothetical ancestral tardigrade proposed by ref63, is characterized by marine heterotardigrade-like features, such as digits on the feet, telescopic legs, cirri-form sensory structures on the head and trunk, and clavae on the head63. Furthermore, the hypothesis that cirri-form sensory structures are plesiomorphic traits in tardigrades is supported by the ultrastructural comparison between the cirri of marine heterotardigrades and the sensilla of arthropods64. In contrast, the terrestrialization event in eutardigrades appears to have driven the reduction of head sensory organs, with cirri replaced by sensory fields, whereas the limno-terrestrial heterotardigrades, such as echiniscids, retain marine-heterotardigrade-like cirri. In this evolutionary context, the median cirrus of marine heterotardigrades may represent a plesiomorphic morphological character, while the reduced structures on the median sensory fields of eutardigrades, such as CSC of M. grandicupula sp. nov. and centrodorsal organ of R. groenlandensis, are likely derived features.
On the other hand, a previous morphological comparison within Panarthropoda, including Cambrian lobopodians, failed to support marine heterotardigrades as the basal group within tardigrades14. Similarly, multiple molecular phylogenetic analyses of Tardigrada consistently recover a sister-group relationship between Eutardigrada and Heterotardigrada15,60,62,65,66,67,68,69,70,71. Additionally, a recent molecular clock study on tardigrades estimated that the divergence time of crown heterotardigrades is not significantly earlier than that of crown eutardigrades (mean split times: 370.94 Ma and 315.69 Ma, respectively)15. This suggests that much higher genetic divergence observed in the 18 S and 28 S sequences of marine heterotardigrades may be attributed to a higher substitution rate, as previously suggested60,62. Supporting this hypothesis, the average genetic distance in 18 S sequences is greater in echiniscoideans (an order of heterotardigrades) than in eutardigrades (9.7–18.1% vs. 1.0–12.6%, respectively)60 despite the later estimated divergence time of crown echiniscoideans (mean: 270.26 Ma). Therefore, the hypothesis that marine heterotardigrades represent the plesiomorphic condition remains unresolved.
-Comparison of the CSC of Milnesium to sensory organs of arthropods.
The cephalic sensory organ complex (CSC) observed in Milnesium cf. longiungue and M. grandicupula sp. nov. shares the distinctive morphology, characterized by a relatively large central organ surrounded by small pores. Interestingly, the overall structure and location of the median sensory field CSC resemble the sensory dorsal organs (SDO) found in many crustaceans (Fig. 8a, c). In crustaceans, this minute organ complements major head sensory organs such as the eyes or antennae72. A typical SDO consists of a central gland and four surrounding structures, often arranged in a quincunx pattern73. The surrounding elements of SDOs are usually circular plates, domes or pits, and SDOs are believed to function as mechanoreceptors, particularly for detecting pressure73,74. The median sensory field CSC of Milnesium cf. longiungue and M. grandicupula sp. nov. shares similarities with crustacean SDOs, as both structures feature a central organ surrounded by smaller structures, forming a complex (Fig. 8b, d). These complexes are situated on the mound structures distinguishable from the surrounding regions and are positioned along the sagittal line of the anterior half of the head or carapace. The median sensory field CSC of Milnesium could play a function as a sensory organ, akin to the SDO in crustaceans. However, while four surrounding elements of the SDO are comparable in size to the central gland, the CSC exhibits significantly smaller pores (likely eight).
Morphological comparison of cephalic sensory organ complex of tardigrade Milnesium grandicupula sp. nov. to sensory dorsal organ of crustaceans (SEM). (a, b) cephalic sensory organ complex on the median sensory field of M. grandicupula sp. nov. (c, d) Sensory dorsal organ of Caridina multidentata Stimpson, 1860.
Additionally, many trilobite groups show a structure similar to the crustacean SDO: the cephalic median organ (CMO). The CMO is a small complex which consists of four small pits on the sagittal line of the head, usually on the occipital ring, though in some groups, it is found on the glabella75. Several trilobites have an additional pit on the center of the CMO (e.g. see Figs. 5–20 in ref76.), and this arrangement of five pits considerably resembles the SDO. The Cambrian agnostoid Agnostus (Agnostus) pisiformis77 also shows a similar complex, with six pores encircling the central pore at the glabellar node on the sagittal line of the head78. The CMO in many trilobites and the complex of A. pisiformis are both located on the node, similar to the CSC of Milnesium and the SDO of crustaceans on the mound structures. It is intriguing that the position and the overall morphology of these sensory organs are similar in distinct phylogenetic groups, despite the different lifestyles of these organisms: shrimps and trilobites are nektonic and benthic, while Milnesium are limno-terrestrial. It could be inferred that the presence of the complex with a central organ surrounded by several structures on the sagittal line of the head node or mound may represent a plesiomorphic trait of panarthropods. Alternatively, however, given the absence of similar records in other panarthropod relatives, it cannot be ruled out that the presence of a complex with similar morphology and function across distantly related groups is a result of convergent evolution for shared function.
Conclusion
Although Milnesium is one of the earliest established groups among tardigrades, its detailed morphology remains to be further explored, with the identification of several species still relying solely on optical microscopy observations. This study utilized Field Emission SEM (FE-SEM) to uncover nano-scale level organs, enabling a more in-depth examination of their forms and functions, including the cephalic sensory organ complex (CSC) and the internal structures of the cuticle. The identification of the CSC indicates the presence of a set of external head sensory organs in apochelans, further corroborating the hypothesis of homology of the head sensory organs of tardigrades. The median sensory field CSC bears resemblance to the sensory dorsal organ (SDO) of crustaceans in terms of their shape, position, and likely function, suggesting potential homology between the two structures. Similarly, the cephalic median organ (CMO) of polymerid trilobites and agnostoid Agnostus also show comparable morphology and positioning. These similarities may suggest that a small complex on the mound or node, characterized by a central organ surrounded by several pores on the sagittal line of the head represents a plesiomorphic trait shared across Panarthropoda. However, the possibility of convergent evolution among the distant phylogenetic groups also cannot be ruled out. More studies are needed to test the hypotheses on the homology of head sensory organs among distant phyla in panarthropods, including the detailed microscopic observations, paleontological evidence, and evo-devo approaches.
Material and methods
-Sample processing.
During the 2019 summer season, the Korea Polar Research Institute (KOPRI) palaeontology team collected a 15 cm × 15 cm plastic bag of mixed bryophyte and lichen samples from the limestone outcropping at the margin of the Lake September (72°50’51.23"N, 25°5’8.71"W, 471 m above sea level (a.s.l.), August 14, 2019) (Supplementary Fig. S5 online). The obtained sample was dried and kept in a plastic bag, brought to KOPRI (Incheon, Korea), and stored at 4℃ for two months. Subsequently, the sample was placed on a dish filled with Volvic water and squeezed over the Petri dish. Tardigrades (Milnesium spp., Echiniscus testudo of Fig. 7b, and Ramazzottius groenlandensis of Fig. 7d) were picked up under a stereomicroscope (Leica M205C) from the supernatant.
The styraconyxid specimen (Fig. 7a) was collected from a brown algae sample from the uppermost intertidal zone of Bugu area, Uljin, Korea (37°6.35’N, 129°22.65’E) on June 15, 2023. The specimen was picked up under a steromicroscope (Leica M205C) from the brown algae.
Specimens of Caridina multidentata Stimpson, 186079 (Yamato shrimp) presented on the Fig. 8c and d were purchased from a pet shop. They were euthanized and fixed using 5% formalin.
-Microscopy and imaging.
For light microscopic observation, specimens were prepared following a previously reported method80. Tardigrades were relaxed at 60 ℃ for thirty minutes and were mounted on a microscope slide in Hoyer’s medium. Subsequently, the slides were dried seven days at 60 ℃, sealed with nail polish, and examined under a Differential Interference Contrast (DIC) microscope (Carl Zeiss Axio Imager 2), with the camera AxioCam HRc. For deep-focus structures, a series of numerous DIC images were merged into an image using Zerene Stacker ver 1.04.
For SEM observation, specimens were prepared following a previously reported method81. First, the tardigrades were incubated at 60 ℃ for 30 min. After fixation in 5% formalin, individuals were washed three times with distilled water. Afterwards, specimens were dried using a freeze dryer Eyela FDU-1200, at East Sea Research Institute, Korea. Dried animals were then mounted on SEM stubs using an eyebrow and coated with a thin layer of platinum. Due to an accidental destroy of specimens on the SEM stub, some specimens were damaged, unexpectedly exposing internal structures of cuticle. SEM observations were made using a Field Emission SEM JSM-7200 F, at KOPRI. TEM observation were conducted at the Korea Research Institute of Bioscience and Biotechnology (KRIBB). For TEM analysis, animals were fixed in 5% formalin and post-fixed with 2% osmium tetroxide. After transition with 100% propylene oxide, individuals were polymerized in Spurr’s resin. TEM observations were made using a Philips CM20.
-Terminology of cuticular sub-layers.
The terminology for cuticular sub-layers follows ref82, and is as follows, from the outermost to the innermost layer: the epicuticle, the inner trilaminate layer, and the basal cuticle.
-Morphometrics.
Selection of characters for morphometry and the morphological terminology follows a previous ref34. All measurements are given in micrometres (µm) and were conducted under a DIC microscope. Characters were measured when the specimens were mounted in a suitable orientation on the slide (characters were not broken or unnaturally bended). Body length was measured from the anterior tip to the posterior end of the body, excluding the legs IV. The pt index represents the percentage ratio of the length of a character to the length of buccal tube83. For measurements of the buccal-pharyngeal apparatus and claws the scheme by ref34. was used. The morphometric data were handled using the Apochela spreadsheet ver. 1.3. available from Tardigrada Register84www.tardigrada.net.
Ontogenetic clusters are clearly grouped based on the buccal tube length-body length graph (Supplementary Fig. S6) as follows: first instar (hatchlings), second instar (juveniles), and third and fourth + instars (adult females).
-Microscopic identification.
Milnesium species were identified according to descriptions and keys provided in ref25,28,29,34,39. The key morphological traits used for Milnesium species identification include dorsal cuticle sculpturing (sculptured or smooth), peribuccal lamellae configuration (either 6 identical-sized lamellae or 4+2 with larger dorsal and ventral lamellae and smaller lateral lamellae), valvular system type (type 1 (tardigradum type) or type 2 (shilohae type)), claw configuration (the number of points on the secondary branches of external/internal claws I–III or anterior/posterior claw IV, CC), and the presence or absence of pseudoplates.
While ref28 classified the valvular system of M. eurystomum as type 2, figures from previous studies showed a type 1 valvular system in M. eurystomum25,29. Therefore, we have followed the classification of M. eurystomum as having a type 2 valvular system. Additionally, although ref25. classified the peribuccal lamellae configuration as 6 (six equal lamellae), the structure of M. eurystomum still show a size difference between 4 larger and 2 smaller lamellae, as mentioned by ref25. Consequently, we have classified the peribuccal lamellae configuration of M. eurystomum as 4+2, consistent with that of M. grandicupula sp. nov.
-Genotyping.
After DIC microscopy observation and sorting into two groups, DNA was extracted from six hologenophore individuals using QIAamp DNA Micro Kit: two females identified as M. cf. longiungue and four females as M. grandicupula sp. nov. A PCR mixture was prepared with a total volume 25 µl, containing 12.5 µl Takara EmeraldAmp PCR Master Mix, 2 µl of DNA template, 0.25 µl of each primer and 10 µl of ddH2O. Four DNA fragments were sequenced, namely, the small ribosome subunit (18 S rRNA), the large ribosome subunit (28 S rRNA), the internal transcribed spacer 2 (ITS2), and the cytochrome oxidase c subunit I (COI). The PCR settings followed a previous method85; primers and original references for PCR settings of all partial genes are listed in Supplementary Table S6 online. The PCR products were sent to the commercial company for sequencing (Macrogen, Korea). The sequences were processed in Geneious v. 9.0.5 (https://www.geneious.com). The data underlying this article are available in the GenBank Nucleotide Database at https://www.ncbi.nlm.nih.gov/, and can be accessed with accession numbers: Milnesium cf. longiungue 18 S (PV590764), 28 S (PV590766), ITS2 (PV590768), and COI (PV590579), and Milnesium grandicupula sp. nov. 18S (PV590765), 28S (PV590767), ITS2 (PV590769), and COI (PV590580).
-Genetic distance and phylogenetics.
The phylogenetic analysis was performed using concatenated sequences of 18S rRNA + 28S rRNA + ITS2 + COI from Milnesiumspecies, with the taxa list sourced from ref47. This analysis was expanded to include M. pacificum Sugiura, Minato, Matsumoto & Suzuki, 202086, and M. iniquum Brotto-Guidetti, Morek & Garraffoni, 202443. Sequences were downloaded from GenBank, a full list of accession number is provided in Supplementary Data S3. The 18S rRNA and 28S rRNA sequences were aligned using the Q-INS-I method, and ITS2 and COI sequences were aligned using the Auto strategy in MAFFT online service87 and checked manually in MEGA X88. The sequences were concatenated using FASconCAT-G89 in the following order: 18S rRNA, 28S rRNA, ITS2, and COI.
Partitionfinder v. 2.1.190, under Bayesian Information Criterion, was utilized to determine the best scheme of partitioning and substitution models. The following models were suggested: SYM+I+G for 18S rRNA, GTR+I+G for 28S rRNA, GTR+I+G for ITS2, and K81UF+G, GTR+I+G, GTR+G for the first, second, and third codon position of COI, respectively. Bayesian inference (BI) posterior probabilities (PP) were calculated using MrBayes v. 3.2.691. Two random starting trees were initiated, each using four Metropolis coupled Markov chains Monte Carlo method, running for 107 generations. Trees were sampled every 1,000 generations, and the initial 10% trees were discarded as burn-in. Convergence was assessed by checking the standard MrBayes convergence diagnostics: estimated sample size scores > 200, average standard deviation of split frequencies values < 0.01, and potential scale reduction factor values ~ 1.00. The obtained tree samples were summarized as a majority rule consensus tree, and the final consensus tree was visualized using FigTree v. 1.4.4.
Uncorrected pairwise distances (p-distance) between nucleotide sequences were computed using a distance model for all codon position, as implemented in MEGA X88. Additionally, using data sets for COI, we performed a genetic species delimitation analysis by Assemble Species Automatic Partitioning (ASAP)92 Poisson tree processes (bPTP) analysis93 and Automatic Barcode Gap Discovery analysis (ABGD)94. Analyses were conducted on the online website of ASAP, https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html, with default settings, bPTP online website (https://species.h-its.org/ptp), with 500,000 MCMC generations, thinning the set to 100, burning at 10% and performing a search for Maximum likelihood and Bayesian inference solutions, following ref25. and ABGD online website (https://spartexplorer.mnhn.fr/delimitation), with 1.0 relative gap width. The results of these analyses are given in Supplementary Data S4 online.
Data availability
Morphometric data is provided within the supplementary information files. The datasets generated and/or anlayzed during the current study are available in the NCBI GenBank repository, with accession numbers: Milnesium cf. longiungue 18S (PV590764), 28S (PV590766), ITS2 (PV590768), and COI (PV590579), and Milnesium grandicupula sp. nov. 18S (PV590765), 28S (PV590767), ITS2 (PV590769), and COI (PV590580).
References
Nelson, D. R., Bartels, P. J. & Guil, N. in Water bears: the biology of tardigrades Zoological Monograph (ed Ralph O Schill) Ch. 7, 163–210 (Springer Cham, 2018).
Hallas, T. E. & Yeates, G. W. Tardigrada of the soil and litter of a Danish Beech forest. Pedobiologia 12, 287–304 (1972).
Guil, N. & Sanchez-Moreno, S. Fine-scale patterns in micrometazoans: tardigrade diversity, community composition and trophic dynamics in leaf litter. Syst. Biodivers. 11, 181–193 (2013).
Zawierucha, K., Buda, J., Jaromerska, T. N., Janko, K. & Gąsiorek, P. Integrative approach reveals new species of water bears (Pilatobius, Grevenius, and Acutuncus) from Arctic cryoconite holes, with the discovery of hidden lineages of Hypsibius. Zool. Anz. 289, 141–165 (2020).
Hagelbäck, P. & Jönsson, K. I. An experimental study on tolerance to hypoxia in tardigrades. Front. Physiol. 14, 1249773 (2023).
Møbjerg, N. & Neves, R. C. New insights into survival strategies of tardigrades. Comp. Biochem. Physiol. A. 254, 110890 (2021).
Wilanowska, P. A., Rzymski, P. & Kaczmarek, Ł. Long-term survivability of tardigrade Paramacrobiotus experimentalis (Eutardigrada) at increased magnesium perchlorate levels: implications for Astrobiological research. Life 14, 335 (2024).
Horikawa, D. D. et al. Establishment of a rearing system of the extremotolerant tardigrade Ramazzottius varieornatus: a new model animal for astrobiology. Astrobiology 8, 549–556 (2008).
Jönsson, K. I. Radiation tolerance in tardigrades: current knowledge and potential applications in medicine. Cancers 11, 1333 (2019).
Hvidepil, L. K. & Møbjerg, N. New insights into osmobiosis and chemobiosis in tardigrades. Front. Physiol. 14, 1274522 (2023).
Smythers, A. L. et al. Chemobiosis reveals tardigrade Tun formation is dependent on reversible cysteine oxidation. PLoS ONE. 19, e0295062 (2024).
Kasianchuk, N., Rzymski, P. & Kaczmarek, Ł. The biomedical potential of tardigrade proteins: A review. Biomed. Pharmacothe. 158, 114063 (2023).
Ortega-Hernández, J. & Lobopodians Curr. Biol. 25, R873–R875 (2015).
Kihm, J. H. et al. Cambrian lobopodians shed light on the origin of the tardigrade body plan. Proc. Natl. Acad. Sci. 120, e2211251120 (2023).
Mapalo, M. A. & Wolfe, J. M. Ortega-Hernández, J. Cretaceous amber inclusions illuminate the evolutionary origin of tardigrades. Comm. Biol. 7, 953 (2024).
Tweedt, S. M. Gene regulatory networks, homology, and the early Panarthropod fossil record. Integr. Comp. Biol. 57, 477–487 (2017).
Degma, P. & Guidetti, R. Actual checklist of Tardigrada species (2009–2025, 44th Edition: 10-06-2025). https://iris.unimore.it/handle/11380/1178608 (2025).
Guidetti, R. & Bertolani, R. Tardigrade taxonomy: an updated check list of the taxa and a list of characters for their identification. Zootaxa 845, 1–46 (2005).
Degma, P. & Guidetti, R. Notes to the current checklist of Tardigrada. Zootaxa 1579, 41–53 (2007).
Gross, V., Epple, L. & Mayer, G. Organization of the central nervous system and innervation of cephalic sensory structures in the water bear Echiniscus testudo (Tardigrada: Heterotardigrada) revisited. J. Morphol. 282, 1298–1312 (2021).
Biserova, N. & Kuznetsova, K. Head sensory organs of Halobiotus stenostomus (Eutardigrada, Hypsibiidae). Biol. Bull. 39, 579–589 (2012).
Kihm, J. H., Zawierucha, K., Rho, H. S. & Park, T. Y. Homology of the head sensory structures between Heterotardigrada and Eutardigrada supported in a new species of water bear (Ramazzottiidae: Ramazzottius). Zool. Lett. 9, 22 (2023).
Morek, W., Wałach, K. & Michalczyk, Ł. Rough backs: taxonomic value of epicuticular sculpturing in the genus Milnesium doyère, 1840 (Tardigrada: Apochela). Sci. Rep. 12, 9857 (2022).
Bryndová, M., Stec, D., Schill, R. O., Michalczyk, Ł. & Devetter, M. Dietary preferences and diet effects on life-history traits of tardigrades. Zool. J. Linn. Soc. 188, 865–877 (2020).
Morek, W., Blagden, B., Kristensen, R. M. & Michalczyk, Ł. The analysis of inter-and intrapopulation variability of Milnesium eurystomum maucci, 1991 reveals high genetic divergence and a novel type of ontogenetic variation in the order Apochela. Syst. Biodivers. 18, 614–632 (2020).
Morek, W. & Michalczyk, Ł. First extensive multilocus phylogeny of the genus Milnesium (Tardigrada) reveals no congruence between genetic markers and morphological traits. Zool. J. Linn. Soc. 188, 681–693 (2020).
Morek, W., Ciosek, J. A. & Michalczyk, Ł. Description of Milnesium pentapapillatum sp. nov., with an amendment of the diagnosis of the order Apochela and abolition of the class apotardigrada (Tardigrada). Zool. Anz. 288, 107–117 (2020).
Camarda, D., Pilato, G. & Lisi, O. Considerations on the Claws of the Apochela and a novel detail of the bucco-pharyngeal apparatus of the genus Milnesium (Tardigrada: apochela: Milnesiidae). Eur. Zool. J. 89, 263–284 (2022).
Michalczyk, Ł., Wełnicz, W., Frohme, M. & Kaczmarek, Ł. Redescriptions of three Milnesium doyère, 1840 taxa (Tardigrada: eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3154, 1–20 (2012).
Doyère, L. M. F. Mémoire Sur les tardigrades. Ann. Des. Sci. Nat. Paris Ser. 2, 269–362 (1840).
Richters, F. in In Handbuch Der Zoologie. Vol. 3, 58–61 (eds Kükenthal, W. & Krumbach, T.) (Walter de Gruyter & Co., 1926).
Schuster, R., Nelson, D., Grigarick, A. & Christenberry, D. Systematic criteria of the Eutardigrada. Trans. Am. Microsc Soc. 99, 284–303 (1980).
Ramazzotti, G. Tardigradi Del Cile - con Descrizione Di quattro Nuove specifiche Di Una Nuova varietà. Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor Nat. Milano. 101, 275–287 (1962).
Tumanov, D. V. Five new species of the genus Milnesium (Tardigrada, eutardigrada, Milnesiidae). Zootaxa 1122, 1–23 (2006).
Morek, W. et al. Redescription of Milnesium alpigenum ehrenberg, 1853 (Tardigrada: Apochela) and a description of Milnesium inceptum sp. nov., a tardigrade laboratory model. Zootaxa 4586, 35–64 (2019).
Ehrenberg, C. G. Diagnoses novarum formarum. Ber Akad. Wiss Berlin. 8, 526–533 (1853).
Roszkowska, M., Ostrowska, M. & Kaczmarek, Ł. The genus Milnesium doyère, 1840 (Tardigrada) in South America with descriptions of two new species from Argentina and discussion of the feeding behaviour in the family Milnesiidae. Zool. Stud. 54, 1–17 (2015).
Binda, M. & Pilato, G. Tardigradi Di Terra Del Fuoco e magallanes. I. Milnesium brachyungue, Nuova specie Di Tardigrado Milnesiidae. Animalia 17, 105–110 (1990).
Schlabach, S., Donaldson, E., Hobelman, K., Miller, W. R. & Lowman, M. D. Tardigrades of the canopy: Milnesium burgessi nov. Sp. (Eutardigrada: apochela: Milnesiidae) a new Sp.cies from kansas, USA. Trans. Kans. Acad. Sci. 121, 39–48 (2018).
Moreno-Talamantes, A., Roszkowska, M., García-Aranda, M. A., Flores-Maldonado, J. J. & Kaczmarek, Ł. Current knowledge on Mexican tardigrades with a description of Milnesium Cassandrae sp. nov.(Eutardigrada: Milnesiidae) and discussion on the taxonomic value of dorsal pseudoplates in the genus Milnesium doyère, 1840. Zootaxa 4691, 501–524 (2019).
Maucci, W. Tre Nuove specie Di eutardigradi Della Groenlandia Meridionale. Boll Mus. Civ. Stor Nat. Verona. 15, 279–289 (1991).
Moreno-Talamantes, A., León-Espinosa, G. A., García-Aranda, M. A., Flores-Maldonado, J. J. & Kaczmarek, Ł. The genus Milnesium doyère, 1840 in Mexico with description of a new species. Ann. Zool. 70, 467–486 (2020).
Brotto-Guidetti, E., Morek, W. & Garraffoni, A. R. Morphological and molecular evidence for a new species of the genus Milnesium doyère, 1840 (Tardigrada: Apochela) from South America. Zool. Anz. 309, 55–65 (2024).
Pilato, G. & Lisi, O. Milnesium minutum and Milnesium sandrae, two new species of Milnesiidae (Tardigrada, Eutardigrada, Apochela). ZooKeys 580, 1–12 (2016).
Meyer, H. Water bears (Phylum Tardigrada) of oceania, with the description of a new species of Milnesium. N Z. J. Zool. 42, 173–186 (2015).
Pilato, G., Sabella, G. & Lisi, O. Two new species of Milnesium (Tardigrada: Milnesiidae). Zootaxa 4132, 575–587 (2016).
Morek, W., Surmacz, B., López-López, A. & Michalczyk, Ł. Everything is not everywhere: Time‐calibrated phylogeography of the genus Milnesium (Tardigrada). Mol. Ecol. 30, 3590–3609 (2021).
Guidetti, R., Rebecchi, L. & Bertolani, R. Cuticle structure and systematics of the Macrobiotidae (Tardigrada, Eutardigrada). Acta Zool. 81, 27–36 (2000).
Lažetić, V. & Fay, D. S. Molting in C. elegans. Worm 6, e1330246 (2017).
Greven, H. Vergleichende untersuchungen am integument von Hetero-und eutardigraden. Cell. Tissue Res. 135, 517–538 (1972).
Dewel, R., Nelson, D. R., Dewel, W. C. & Harrison, F. in Microscopic Anatomy of Invertebrates Vol. 12 (eds FW Harrison & ME Rice) 143–183 (1993).
Wiederhöft, H. & Greven, H. Notes on head sensory organs of Milnesium tardigradum doyre, 1840 (Apochela, Eutardigrada). Zool. Anz. 238, 338–346 (1999).
Zantke, J., Wolff, C. & Scholtz, G. Three-dimensional reconstruction of the central nervous system of Macrobiotus hufelandi (Eutardigrada, Parachela): implications for the phylogenetic position of Tardigrada. Zoomorphology 127, 21–36 (2008).
Persson, D. K., Halberg, K. A., Jørgensen, A., Møbjerg, N. & Kristensen, R. M. Neuroanatomy of Halobiotus crispae (Eutardigrada: Hypsibiidae): Tardigrade brain structure supports the clade Panarthropoda. J. Morphol. 273, 1227–1245 (2012).
Persson, D. K., Halberg, K. A., Jørgensen, A., Møbjerg, N. & Kristensen, R. M. Brain anatomy of the marine tardigrade Actinarctus doryphorus (Arthrotardigrada). J. Morphol. 275, 173–190 (2014).
Gąsiorek, P., Stec, D., Morek, W. & Michalczyk, Ł. Deceptive conservatism of claws: distinct phyletic lineages concealed within isohypsibioidea (Eutardigrada) revealed by molecular and morphological evidence. Contrib. Zool. 88, 78–132 (2019).
Marley, N. J., Mcinnes, S. J. & Sands, C. J. Phylum tardigrada: A re-evaluation of the Parachela. Zootaxa 2819, 51–64 (2011).
Zawierucha, K. et al. High mitochondrial diversity in a new water bear species (Tardigrada: Eutardigrada) from mountain glaciers in central asia, with the erection of a new genus Cryoconicus. Ann. Zool. 68, 179–201 (2018).
Gąsiorek, P., Morek, W., Stec, D., Blagden, B. & Michalczyk, Ł. Revisiting Calohypsibiidae and microhypsibiidae: Fractonotus pilato, 1998 and its phylogenetic position within isohypsibiidae (Eutardigrada: Parachela). Zoosystema 41, 71–89 (2019).
Jørgensen, A., Faurby, S., Hansen, J. G., Møbjerg, N. & Kristensen, R. M. Molecular phylogeny of Arthrotardigrada (Tardigrada). Mol. Phylogenet Evol. 54, 1006–1015 (2010).
Renaud-Mornant, J. in Proceedings of the Third International Symposium on Tardigrada (ed Diane R Nelson) 149–177East Tennessee State University Press, (1982).
Grollmann, M. M., Jørgensen, A. & Møbjerg, N. Actinarctus doryphorus (Tanarctidae) DNA barcodes and phylogenetic reinvestigation of Arthrotardigrada with new A. doryphorus and Echiniscoididae sequences. Zootaxa 5284, 351–363 (2023).
Møbjerg, N., Jørgensen, A., Kristensen, R. M. & Neves, R. C. in Water bears: the biology of tardigrades Zoological Monographs 2 (ed Ralph O Schill) Ch. 2, 57–94Springer Cham, (2018).
Kristensen, R. M. Sense organs of two marine arthrotardigrades (Heterotardigrada, Tardigrada). Acta Zool. 62, 27–41 (1981).
Jørgensen, A. & Kristensen, R. M. Molecular phylogeny of Tardigrada—investigation of the monophyly of Heterotardigrada. Mol. Phylogenet Evol. 32, 666–670 (2004).
Nichols, P. B., Nelson, D. R. & Garey, J. R. A family level analysis of tardigrade phylogeny. Hydrobiologia 558, 53–60 (2006).
Sands, C. J. et al. Phylum tardigrada: an individual approach. Cladistics 24, 861–871 (2008).
Guil, N. & Giribet, G. A comprehensive molecular phylogeny of tardigrades—adding genes and taxa to a poorly resolved phylum-level phylogeny. Cladistics 28, 21–49 (2012).
Bertolani, R. et al. Phylogeny of eutardigrada: new molecular data and their morphological support lead to the identification of new evolutionary lineages. Mol. Phylogenet Evol. 76, 110–126 (2014).
Fujimoto, S., Jørgensen, A. & Hansen, J. G. A molecular approach to arthrotardigrade phylogeny (Heterotardigrada, Tardigrada). Zool. Scr. 46, 496–505 (2017).
Guil, N., Jørgensen, A. & Kristensen, R. An upgraded comprehensive multilocus phylogeny of the Tardigrada tree of life. Zool. Scr. 48, 120–137 (2019).
Lerosey-Aubril, R. & Meyer, R. The sensory dorsal organs of crustaceans. Biol. Rev. 88, 406–426 (2013).
Laverack, M. S., Macmillan, D. L., Ritchie, G. & Sandow, S. L. The ultrastructure of the sensory dorsal organ of Crustacea. Crustaceana 69, 636–651 (1996).
Barrientos, Y. & Laverack, M. S. The larval crustacean dorsal organ and its relationship to the trilobite median tubercle. Lethaia 19, 309–313 (1986).
Lerosey-Aubril, R. & McNamara, K. J. in Advances in trilobite research (eds Isabel Rábano, Rodolfo Gozalo, & Diego C García-Bellido) 229–235 (Instituto Geológico y Minero de España, (2008).
Hong, P. S., Hughes, N. C. & Sheets, H. D. Size, shape, and systematics of the silurian trilobite Aulacopleura koninckii. J. Paleontol. 88, 1120–1138 (2014).
Wahlenberg, G. Petrificata telluris svecanae. Nova Acta Regiae Soc. Sci. Upsal. 8, 1–116 (1818).
Müller, K. J. & Walossek, D. in Morphology, ontogeny, and life habit of Agnostus pisiformis from the Upper Cambrian of Sweden Vol. 19 Fossils and Strata (ed Stefan Bengston) 1-124Universitetsforlaget, (1987).
Stimpson, W. & Crustacea Macrura Pars VIII of Prodromus descriptionis animalium evertebratorum, quae in Expeditione ad Oceanum Pacificum Septentrionalem, a Republica Federata missa, Cadwaladaro Ringgold et Johanne Rodgers Ducibus, observavit et descriptist. Proc. Acad. Nat. Sci. Phila, 22–47 (1860).
Morek, W. et al. An experimental test of eutardigrade Preparation methods for light microscopy. Zool. J. Linn. Soc. 178, 785–793 (2016).
Mitchell, C. & Miller, W. R. A simple SEM (Scanning Electron Microscope) Preparation protocol for tardigrades. J. Pa. Acad. Sci. 81, 86–90 (2008).
Kristensen, R. & Neuhaus, B. Special issue on Tardigrada-The ultrastructure of the tardigrade cuticle with special attention to marine species. Zool. Anz. 238, 261–282 (1999).
Pilato, G. Analisi Di Nuovi caratteri Nello studio Degli eutardigradi. Animalia 8, 51–57 (1981).
Michalczyk, Ł. & Kaczmarek, Ł. The Tardigrada register: a comprehensive online data repository for tardigrade taxonomy. J. Limnol. 72, s1.e22 (2013).
Stec, D., Morek, W., Gąsiorek, P. & Michalczyk, Ł. Unmasking hidden species diversity within the Ramazzottius oberhaeuseri complex, with an integrative redescription of the nominal species for the family Ramazzottiidae (Tardigrada: eutardigrada: Parachela). Syst. Biodivers. 16, 357–376 (2018).
Sugiura, K., Minato, H., Matsumoto, M. & Suzuki, A. C. Milnesium (Tardigrada: Apochela) in japan: the first confirmed record of Milnesium tardigradum ss and description of Milnesium Pacificum sp. nov. Zool. Sci. 37, 476–495 (2020).
Katoh, K., Rozewicki, J. & Yamada, K. D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166 (2019).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Kück, P. & Longo, G. C. FASconCAT-G: extensive functions for multiple sequence alignment preparations concerning phylogenetic studies. Front. Zool. 11, 81 (2014).
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T. & Calcott, B. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 34, 772–773 (2017).
Ronquist, F. et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Puillandre, N., Brouillet, S. & Achaz, G. ASAP: assemble species by automatic partitioning. Mol. Ecol. Resour. 21, 609–620 (2021).
Zhang, J., Kapli, P., Pavlidis, P. & Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29, 2869–2876 (2013).
Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 21, 1864–1877 (2012).
Acknowledgements
We thank to Hyun Woo Oh, Ji Ae Kim (Korea Research Institute of Bioscience and Biotechnology, KRIBB), and Sanghee Kim (KOPRI) for providing the TEM image (Fig. 5b). We also thank to Pilmo Kang (KOPRI) for assistance with SEM imaging and Junhyeok Jang (Philips Exeter Academy) for sorting tardigrades and aiding in specimen preparation. Sampling was carried out under the Greenlandic government permission number: SP-19-2019.
Funding
This work was supported by Korea Polar Research Institute (KOPRI) grant funded by the Ministry of Oceans and Fisheries (KOPRI project No. PE25060).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Author information
Ji-Hoon Kihm: Specimen collection, conceptualization, methodology, investigation, data acquisition, analysis, visualization, writing–original draft, review & editing; Krzysztof Zawierucha: Analysis, writing–review & editing; Hyun Soo Rho: Writing–review & editing; Tae-Yoon S. Park: Specimen collection, conceptualization, funding acquisition, writing–original draft, review & editing.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kihm, JH., Zawierucha, K., Rho, H.S. et al. Greenlandic water bears reveal a new morphological trait of external head sensory organs. Sci Rep 15, 22556 (2025). https://doi.org/10.1038/s41598-025-06766-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06766-4