Abstract
Cocaine self-administration behavior in rats is explained by a pharmacokinetic/pharmacodynamic interaction model. This self-administration model represents a pharmacological bioassay system that can measure the pharmacokinetics and the pharmacodynamic potency of self-administered stimulants, and also dopamine receptor antagonists. However, the time course of effect of antagonists reflects in part their pharmacodynamic potency, complicating conclusions about their pharmacokinetics. The time course of absorption of drugs is typically different by different routes of administration. This study investigated the time course and magnitude of effect of the D1- and D2- selective antagonists, SCH23390 and (-)Eticlopride respectively, on cocaine and apomorphine self-administration when administered via intravenous, subcutaneous, and intraperitoneal routes. The time course of antagonist effects on both cocaine and apomorphine self-administration were different by the different routes of administration, consistent with different rates of absorption especially by the IP route. However, the area under the time-effect curve were relatively similar indicating that antagonist bioavailability was similar via these different routes. Cocaine and apomorphine self-administration in rats can be used as a pharmacological bioassay system to measure the pharmacokinetics of dopamine receptor antagonists, presumably in the brain, in real time in-vivo. This assay system may provide pharmacokinetic information complimentary to standard timed plasma concentration methods.
Similar content being viewed by others
Introduction
Cocaine self-administration in rats is a model of cocaine use disorder. The regulation of drug self-administration by animals has been explained by pharmacokinetic/pharmacodynamic interaction models1,2,3,4,5. It has been demonstrated that cocaine induces lever pressing behavior only when cocaine levels are above the priming/remission threshold and below the satiety threshold, a range of cocaine levels termed the compulsion zone2. The regularity and the non-linear dose-dependency of inter-injection intervals during cocaine self-administration on the FR1 schedule is explained by the time required for higher cocaine levels to fall back to the satiety threshold3. The satiety threshold is assumed to be constant and represents an equiactive cocaine concentration2,3.
Based on receptor occupancy theory, the magnitude of competitive antagonist-induced increase in the equiactive agonist concentration (concentration ratio) should be directly proportional to the antagonist concentration6,7,8,9. Because the satiety threshold represents an equiactive cocaine concentration3 the Schild method can be applied to cocaine and apomorphine self-administration behavior10. Several D1-like11 and D2-like12 dopamine receptor antagonists increase the rate of self-administration of cocaine and related stimulants, and the D1-like dopamine receptor antagonist SCH23390 increases the cocaine satiety threshold10,13,14,15. This antagonist-induced increase in the cocaine satiety threshold explains the increase in the rate of cocaine self-administration10. Additionally, it has been demonstrated that Schild analysis of antagonist pharmacodynamic potencies can be applied to data obtained from cocaine and apomorphine self-administration10. Interestingly, although the Schild method would be expected to apply to the direct dopamine receptor agonist apomorphine, it also provided similar results for cocaine, an indirect dopamine receptor agonist.
The time course of the antagonist-induced change in agonist satiety threshold could reflect the pharmacokinetics of the antagonist in the brain10. However, this interpretation was complicated by the finding that Tmax (time to maximum response) was proportional to antagonist affinity for D2 dopamine receptors, suggesting that the time course of antagonist-induced change in agonist satiety threshold was also influenced by the pharmacodynamic potency of the antagonist13.
It is known that the absorption of a drug into the body and its distribution to its site of action differs when the drug is injected via different routes. Therefore, the time course and magnitude of effect of the same antagonist on agonist self-administration is predicted to be different when injected intravenously (IV), subcutaneously (SC), and intraperitoneally (IP). This hypothesis was tested in rats that self-administered cocaine and apomorphine using SCH23390 and the D2-like dopamine receptor antagonist (-)Eticlopride.
Results
Agonist self-administration
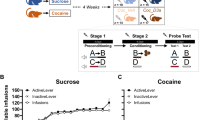
Rats maintained stable cocaine self-administration at 3 µmol/kg unit dose and apomorphine self-administration at the 0.15 µmol/kg unit dose. Inter-injection intervals were regular for 2 h before the antagonist injection (Fig. 1A). Similarly, calculated cocaine levels and calculated apomorphine levels at the time of self-administrations were consistent and maintained within a small range of levels during the first two hours of the session (Fig. 1B).
Effect of antagonist on inter-injection intervals during agonist self-administration
When the antagonist (-)Eticlopride was injected (0.02 µmol/kg) after approximately 2 h of stable apomorphine self-administration, the rate of apomorphine self-administration immediately increased, and then slowly decreased (Fig. 1A). Inter-injection intervals first shortened after the (-)Eticlopride injection and gradually increased, eventually returning to the baseline values during the subsequent 6 h (Fig. 1A). This increase in rate of apomorphine self-administration was highest when (-)Eticlopride was injected intravenously, and lowest when (-)Eticlopride was injected intraperitoneally (Fig. 1A). However, the increased rate of apomorphine self-administration was sustained for a longer duration when (-)Eticlopride was injected subcutaneously (Fig. 1A). Additionally, the overall effect of (-)Eticlopride on the rate of apomorphine self-administration was least prominent for the intraperitoneal route (Fig. 1A). The effect of (-)Eticlopride injection IV, SC, and IP was similar to apomorphine on the rate of cocaine self-administration.
The same dose (0.02 µmol/kg) of SCH23390 administered either intravenously, subcutaneously, or intraperitoneally approximately 2 h after the start of a session, had a similar effect to (-)Eticlopride on inter-injection intervals during both apomorphine and cocaine self-administration as shown in Fig. 1A.
Effect of antagonist on calculated agonist levels at the time of agonist self-administrations
When the antagonist (-)Eticlopride (0.02 µmol/kg) was injected 2 h after stable apomorphine self-administration, apomorphine levels increased with each subsequent self-administration reaching peak concentration, and then gradually decreased returning to baseline levels during the subsequent 6 h (Fig. 1B). The route of (-)Eticlopride administration also had an effect on calculated apomorphine levels at the time of self-administrations, corresponding to its effect on the rate of apomorphine self-administration (Fig. 1). The magnitude of increase in apomorphine level was highest when (-)Eticlopride was injected intravenously, and lowest when (-)Eticlopride was injected intraperitoneally (Fig. 1B). Similarly, apomorphine levels at the time of self-administrations remained elevated for longer duration when (-)Eticlopride was injected subcutaneously (Fig. 1B). (-)Eticlopride had the least overall effect on apomorphine levels at the time of self-administration for the intraperitoneal route (Fig. 2B). The effect of (-)Eticlopride injection IV, SC, and IP was similar to apomorphine on the calculated cocaine levels at the time of cocaine self-administration.
The same dose (0.02 µmol/kg) of SCH23390 administered either intravenously, subcutaneously, or intraperitoneally approximately 2 h after the start of the session, had a similar effect on both apomorphine and cocaine levels as shown in Fig. 1B.
Cumulative number of apomorphine (A) or cocaine (C) self-administrations and the corresponding calculated level of apomorphine (B) or cocaine (D) at the time of each self-administration during three representative apomorphine or cocaine self-administration sessions. (-)Eticlopride (0.02 µmol/kg) was injected Intravenously (pink squares), Subcutaneously (yellow circles), or Intraperitoneally (blue triangles) approximately 2 h after starting the session. The unit doses of apomorphine and cocaine were 0.15 and 3 µmol/kg, respectively.
Effect of route of antagonist injection on agonist self-administration as reflected by agonist concentration ratio over time
It is assumed that each self-administration occurs at an equiactive concentration of agonist, that is the baseline agonist level. In the presence of a competitive antagonist, the degree to which the equiactive agonist concentration increases should be directly proportional to the effective antagonist concentration. Therefore, the effect of administration of the two antagonists (SCH23390 and (-)Eticlopride), using the three routes of administration (IV, SC and IP) on the self-administration of the two agonists (apomorphine and cocaine) were compared directly by determining the concentration ratio of calculated agonist levels after administration of the antagonist relative to the calculated agonist levels during baseline self-administration (Fig. 2). The concentration ratio was plotted as concentration ratio minus one so that the baseline agonist concentrations, or absence of antagonist, is represented as 0.
Both SCH23390 and (-)Eticlopride showed maximal effect on both apomorphine and cocaine concentration ratios when injected intravenously, followed closely by the subcutaneous route, and had minimal effect when injected intraperitoneally (Fig. 2). Additionally, it took less time for the antagonists to cause a maximum agonist concentration ratio when the antagonist was injected intravenously while intraperitoneal antagonist injection took the most time to cause a maximum agonist concentration ratio. (Fig. 2).
Consistent with these observations, average data for effect of antagonist on cocaine and apomorphine self-administration showed that the effect of IV injection was quickest (Fig. 3A, B) and highest (Fig. 3C, D), while the effect of IP injection was slowest (Fig. 3A, B) and lowest (Fig. 3C, D). Additionally, the area under the curve was highest for the IV route and lowest for the IP route (Fig. 3E, F).
The effect of route of administration on the time course and magnitude of antagonist effect on equiactive cocaine or apomorphine concentrations depicted as agonist concentration ratio − 1. Each point represents cocaine or apomorphine concentration ratio − 1 at the time of each self-administration after the injection of antagonist. The concentration ratios were calculated by dividing the agonist concentration at the time of each self-administration after the antagonist injection by the mean baseline agonist concentration in each session. The curve through each data set represents the best fit generated by a pharmacokinetic model using WinNonlin. The vertical line represents the time of maximum effect.
The mean ± SEM T-max values for SCH23390 (A) and (-)Eticlopride (B), Cmax values for SCH23390 (C) and (-)Eticlopride (D), and area under the curve (AUC) values for SCH23390 (E) and (-)Eticlopride (F). The data was pooled between the cocaine and apomorphine sessions for SH23390 and Eticlopride injection through different routes (IV, SC, IP). Bar graphs with standard error bars have been made for visual representation. * P < 0.05, ** P < 0.01, *** P < 0.001 when compared with corresponding IV data.
Methods
Cocaine self-administration training
Rats were trained to self-administer cocaine as previously reported in Norman et al. 201110. Male Sprague Dawley rats (from SASCO, Wilmington, MA, initial weight 180–200 g and 400–500 g over the duration of these studies) were housed individually on a 12 h light-dark cycle (lights on at 6:00 a.m.) and food and water were available ad libitum. All studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati, and reported in accordance with ARRIVE guidelines. Surgical implantation and maintenance of IV catheters and cocaine self-administration training procedures were completed as described previously2 for all rats. These same rats that stably self-administered cocaine also stably self-administered the direct dopamine receptor agonist apomorphine. Antagonist effects were typically first tested on apomorphine self-administration and then were tested again on cocaine self-administration. There was no evidence that the order of apomorphine or cocaine sessions or the order of antagonists influenced the magnitude or duration of antagonist effects16.
During daily sessions, run 5 days per week during the light cycle, rats self-administered a unit dose of 0.15 or 3 µmol/kg of apomorphine or cocaine, respectively. In every session the rats self-administered the set dose of apomorphine or cocaine for approximately 120 min to determine if the rate of self-administration was stable and similar to previous sessions. Then a single IV dose (0.02 µmol/kg) of either the selective D1-like dopamine receptor antagonist SCH23390or the selective D2-like dopamine receptor antagonist (-)Eticlopride were rapidly administered (IV, SC or IP) and the session continued. The dose of antagonist was the same for all routes of administration.
Real-time calculation of agonist levels in the body
The agonist levels in the body were calculated by monitoring the amount of agonist that was administered to the animals and then using predetermined pharmacokinetic values to estimate the resulting agonist levels in the animals over time. The real time cocaine and apomorphine levels were calculated as described in Norman et al. 201110, and is based on the method described in Tsibulsky and Norman 200517.
Agonist concentration ratios
Agonist concentration ratios were determined as previously described in Norman et al. 201110. The mean of the values of apomorphine or cocaine level at the time of each lever press during the maintenance phase (not including the initial loading phase) prior to the antagonist injection represented the baseline agonist level. The calculated agonist levels (L) were assumed to be directly proportional to the agonist concentrations (C) according to the equation C = L/Vd, where Vd, is the volume of distribution. The level of agonist at the time of each lever press after the injection of antagonist was divided by the average baseline value for that session and the resulting value represented the agonist concentration ratio. These agonist concentration ratios minus one were plotted as a function of time after the injection of antagonist. Any negative values were excluded from the plot and the data analysis.
Pharmacokinetic modeling and statistical analysis
Agonist concentration ratios were assumed to be proportional to the antagonist fractional occupancy of the receptor population underlying the agonist-induced satiety response.
SigmaPlot 15 was used to apply best fit curves over the data points to create pharmacokinetic models for each session. The pharmacokinetic models provided visuals for the maximum concentration ratio, the corresponding time for the maximum concentration ratio, and the area under the curves for each session.
The maximum concentration ratio (Cmax) was determined as the average of the highest values, and the range of these values widened with respect to the different routes of antagonist administration. The times for the maximum concentration ratio (Tmax) were determined as the averages of the time values corresponding to the same highest Cmax values. SigmaPlot features were used to obtain measurements for the area under the curves (AUC) for each session. The Cmax, Tmax, and AUC were noted for each session with one of the three different routes of administration (IV, SC, IP).
A two-way analysis of variance (ANOVA) was performed for the Tmax, Cmax, and AUC. The two factors were the agonists, apomorphine and cocaine, and the routes of administration, IV, SC, and IP and we observed the effects of the interactions of the two factors on the antagonists, SCH23390 and (-)Eticlopride. The analysis for Tmax yields a large p-value for both antagonists meaning the effect of the agonists is significant. However, the analysis results for Cmax and AUC indicate that the agonist factor is insignificant for SCH23390 and (-)Eticlopride. Combining the data between the two self-administered agonists allows a comparison of the different routes of administration on the time course and magnitude of effects of the antagonists.
Discussion
The antagonist-induced increase in the rate of self-administration is the result of the antagonist-induced increase in the cocaine satiety threshold10,13,14,15,16. It was previously suggested that the time course of the antagonist-induced changes in the level of the satiety threshold reflects the change in antagonist levels in the brain and, therefore, the pharmacokinetics of antagonists in the body13. However, the observation that the time to maximal effect of antagonists on cocaine self-administration behavior was dependent on the affinity of a series of antagonists for D2-like dopamine receptors indicated that the time course of effect may also reflect a pharmacodynamic interaction with dopamine receptors, complicating the interpretation of the time course reflecting the pharmacokinetics of the antagonists13. The different time course of the effects of SCH23390 and (-)Eticlopride by different routes of administration are consistent with the expected delays due to absorption and distribution from the injection site to the body, and to the brain. This is especially evident by the IP route for both antagonists, where the slow onset of effect was clear. Furthermore, the magnitude of effect relative to the IV route may reflect the slower adsorption of the antagonists by the IP route.
The AUC typically reflects the bioavailability of a drug, and the lower AUC from the IP route may reflect first-pass metabolism of the antagonists when absorbed and delivered to the body via the portal vein through the liver. This is more obvious for SCH23390 than for (-)Eticlopride. It should be noted that the measurement of the AUC for the IP route for these antagonists was complicated by the long time-course and the low magnitude of response. This low magnitude of effect likely reflects the low levels of antagonist being absorbed at any particular time and the use of the IP route of administration should be viewed with caution for these, and potentially other, drugs in animal research. In contrast, the SC route provided AUC values similar to the IV route and may be the preferred non-IV route for these and similar drugs. Indeed, the first study to demonstrate that SCH23390 increases the rate of cocaine self-administration had used the SC route to administer SCH2339011.
The time course of effects of these antagonists by the IV and SC routes were notably rapid increase followed by a rapid decrease in response magnitude. This prominent biphasic response pattern should be considered when measuring the effects of antagonists on self-administration behavior. If the test drug is administered before a session is initiated, the measured magnitude of effect will be highly dependent on the time from the injection that the response is measured.
Cocaine and apomorphine self-administration behavior appear to serve as a pharmacological bioassay system that can measure not just the pharmacodynamic potency of dopamine receptor antagonists, but also the pharmacokinetics of these antagonists. It can measure the absolute bioavailability of a dopamine receptor antagonist by reference to the IV route of administration and can measure the time for the antagonist to be absorbed and reach its site of action. Consequently, the self-administration paradigm appears to represent a technique that has utility for investigating the pharmacokinetic/pharmacodynamic interactions of dopamine receptor antagonists at their site of action in the brain in real time. This may provide information that is complimentary to the standard pharmacokinetic technique of measuring antagonist concentrations in plasma over time.
Data availability
The original data files can be provided by the corresponding author on reasonable request.
References
Ahmed, S. H. & Koob, G. F. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology 180, 473–490. https://doi.org/10.1007/s00213-005-2180-z (2005).
Norman, A. B. & Tsibulsky, V. L. The compulsion zone: a Pharmacological theory of acquired cocaine self-administration. Brain Res. 1116, 143–152. https://doi.org/10.1016/j.brainres.2006.07.092 (2006).
Tsibulsky, V. L. & Norman, A. B. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 839, 85–93 https://doi.org/10.1016/s0006-8993(99)01717-5 (2013).
WiseRA Cognitive factors in addiction and nucleus accumbens function: some hints from rodent models. Psychobiology 27, 300–310 (1999).
Yokel, R. A. & Pickens, R. Drug level of d- and l-amphetamine during intravenous self-administration. Psychopharmacologia 34, 255–264. https://doi.org/10.1007/BF00421966 (1974).
Arunlakshana, O. & Schild, H. O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 14, 48–58. https://doi.org/10.1111/j.1476-5381.1959.tb00928.x (1959).
Colquhoun, D. Why the Schild method is better than Schild realised. Trends Pharmacol. Sci. 28, 608–614. https://doi.org/10.1016/j.tips.2007.09.011 (2007).
Schild, H. O. Drug antagonism and pAx. Pharmacol. Rev. 9, 242–246 (1957).
Schild, H. O. pA, a new scale for the measurement of drug antagonism. Br. J. Pharmacol. 120, 29–46. https://doi.org/10.1111/j.1476-5381.1997.tb06773.x (1997).
Norman, A. B., Tabet, M. R., Norman, M. K. & Tsibulsky, V. L. Using the self-administration of apomorphine and cocaine to measure the pharmacodynamic potencies and pharmacokinetics of competitive dopamine receptor antagonists. J. Neurosci. Methods. 194, 252–258. https://doi.org/10.1016/j.jneumeth.2010.10.017 (2011).
Koob, G. F., Le, H. T. & Creese, I. The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neurosci. Lett. 79, 315–320. https://doi.org/10.1016/0304-3940(87)90451-4 (1987).
Yokel, R. A. & Wise, R. A. Increased lever pressing for amphetamine after Pimozide in rats: implications for a dopamine theory of reward. Science 187, 547–549. https://doi.org/10.1126/science.1114313 (1975).
Norman, A. B. et al. The affinity of D2-like dopamine receptor antagonists determines the time to maximal effect on cocaine self-administration. J. Pharmacol. Exp. Ther. 338, 724–728. https://doi.org/10.1124/jpet.111.183244 (2011).
Wetzel, H. N., Tsibulsky, V. L. & Norman, A. B. Differential effects of acute and chronic antagonist and an irreversible antagonist treatment on cocaine self-administration behavior in rats. Sci. Rep. 12, 8782 https://doi.org/10.1038/s41598-022-12798-x (2022).
Zinani, D. B., Desai, J. N. & Norman, A. B. SCH23390 and a humanized anti-cocaine mAb decrease the latency to cocaine-induced reinstatement of lever pressing behavior in rats that self-administer cocaine. Sci. Rep. 13, 14566. https://doi.org/10.1038/s41598-023-41284-1 (2023).
Norman, A. B., Norman, M. K., Tabet, M. R., Tsibulsky, V. L. & Pesce, A. J. Competitive dopamine receptor antagonists increase the equiactive cocaine concentration during self-administration. Synapse 65, 404–411. https://doi.org/10.1002/syn.20858 (2011).
Tsibulsky, V. L. & Norman, A. B. Real time computation of in vivo drug levels during drug self-administration experiments. Brain Res. Protoc. 15, 38–45. https://doi.org/10.1016/j.brainresprot.2005.03.003 (2005).
Acknowledgements
The authors would like to thank William Buesing for technical assistance, Dr. Vladimir L. Tsibulsky and Dr. Rose Webster for helpful discussions and Dr. Jeffrey A. Welge for advice on statistical analysis. We are grateful to Dr. Jo El Schultz and the ASPET SURF Program at the University of Cincinnati, and the Dalton/Zannoni Fund for the support of Heather J Song. This work was supported by the National Institute on Drug Abuse grants DP1DA031386 and U01DA050330 to ABN.
Author information
Authors and Affiliations
Contributions
HJS: Analyzed data, prepared graphs, statistical analysis, wrote initial drafts and edited all subsequent drafts.JND: Supervision of data analysis, edited all drafts of the manuscript. MKN: Generated all data, supervised the experiments.ABN: Study design, supervision, data interpretation, edited final drafts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, H.J., Desai, J.N., Norman, M.K. et al. Using cocaine and apomorphine self-administration in rats to measure the pharmacokinetics of competitive dopamine receptor antagonists administered by different routes. Sci Rep 15, 24087 (2025). https://doi.org/10.1038/s41598-025-06797-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06797-x