Abstract
In this study, we examined the sociological characteristics, health fitness, and physical activity levels of 372 patients with lung cancer who received their first chemotherapy in a tertiary hospital in Wuxi, China, and analyzed their associations with quality of life (QoL). Standardized measures were used to assess body composition, muscular function, cardiorespiratory fitness, and flexibility. Physical activity was measured using the International Physical Activity Questionnaire, and QoL was evaluated using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Lung Cancer 43. Higher physical activity levels were positively correlated with global health status but also linked to greater symptom burden and reduced functional limitations. Muscle mass, grip strength, and 6-min walk distance were positively associated with global health and inversely associated with symptom severity and functional scales. Females tended to report higher symptom burdens and lower functional scores. Multivariate analysis indicated that sex, educational level, comorbidities of chronic diseases, disease stage, and activity levels were associated with QoL measures. These findings suggest that better overall health and physical fitness, along with higher levels of physical activity, may be associated with improved QoL among patients with lung cancer.
Similar content being viewed by others
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide, despite recent advances in treatment that have improved 5-year survival rates1,2. Over 70% of patients are diagnosed with advanced3, inoperable lung cancer, wherein chemotherapy is a primary treatment modality. However, chemotherapy often induces side effects, such as fatigue, muscle weakness, and dyspnea, which can significantly impair the patients’ physical activity levels and overall quality of life (QoL)4,5. Furthermore, patients with lung cancer experience a more severe symptom burden than patients with other types of cancers6. Studies have shown that physical activity can mitigate the toxicity of anti-tumor therapies7, improve patient tolerance, and enhance the efficacy of traditional treatments. The American College of Sports Medicine and the American Cancer Society8,9 recommend that patients with cancer engage in at least 150 min of moderate-intensity or 75 min of high-intensity physical activity per week, along with resistance training twice weekly10. Despite these guidelines, a significant proportion of patients with lung cancer undergoing chemotherapy fail to meet these recommendations, with 74% reporting insufficient physical activity levels11,12,13.
Physical fitness is an indicator of the overall assessment of physical functioning during daily physical activities. It is an important component of QoL, and its recovery contributes to the improvement of functional status and social activities in patients with tumor14. Key assessment indicators include body composition, cardiorespiratory fitness, muscular function, and flexibility. Patients with lung cancer may experience a decline in cardiopulmonary function and metabolic disturbances due to the disease itself15,16. Furthermore, tumor treatments and a lack of physical activity can lead to impaired muscle function, subsequently affecting the overall health and fitness levels17,18. Individuals who transition from a sedentary lifestyle to achieving optimal physical fitness can reduce their mortality rate by 44%19. In contrast, patients with poor physical fitness without any intervention measures face the highest mortality rates20. The predominant demographic of patients with lung cancer consists of individuals who are inactive or lead sedentary lifestyles21. Assessment of physical function in patients receiving chemotherapy for malignant lymphoma indicated that while right grip strength diminished post-chemotherapy, other physical performance metrics (e.g., left grip strength, bilateral knee extension, and 6-min walk test) exhibited no significant changes22. This observation suggests that exercise interventions could play a crucial role in mitigating the decline of specific physical function parameters during chemotherapy22. A cohort study involving 38,000 men found that lung cancer mortality was inversely proportional to the cardiopulmonary fitness indicators, suggesting that improving cardiopulmonary fitness through appropriate interventions may reduce lung cancer-related mortality in men23. In patients with breast cancer and hematologic malignancies, exercise programs focusing on muscle strength, cardiorespiratory fitness, and body composition have been shown to significantly improve QoL24. These programs help patients navigate treatment and recovery more effectively25,26.
In this study, we evaluated the health and fitness status of patients with lung cancer undergoing chemotherapy and identified determinants of their physical activity levels, providing a scientific foundation for designing personalized exercise interventions tailored to their needs. By exploring how such programs can enhance QoL, we aim to support strategies that enhance their well-being, QoL, and overall survivorship experience.
Methods
Study design and participants
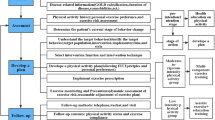
Methodological quality was evaluated using the checklist from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement27. In this cross-sectional study, we sampled patients with lung cancer undergoing chemotherapy from the oncology treatment center of a tertiary general hospital in Wuxi, China. Patients with lung cancer who started their first round of chemotherapy between June 2024 and December 2024 were selected using convenience sampling.
The inclusion criteria were: (1) age > 18 years; (2) pathologically confirmed diagnosis of lung cancer; (3) receiving chemotherapy for the first time; and (4) being conscious and able to communicate verbally. The exclusion criteria were: (1) presence of neurological, muscular, skeletal, or joint disorders that would prevent completion of the health fitness assessment; and (2) presence of other severe comorbidities, such as advanced heart failure, cerebral infarction, and cerebral hemorrhage. We obtained approval from the Ethics Committee (approval number: LS2023086) and ensured registration in the Chinese Clinical Trial Registry (registration number: ChiCTR2400081003). Prior to participation, each participant provided written informed consent.
Sample size
Based on correlation analyses in cross-sectional studies, the required sample size was estimated to be 10–20 times the number of variables28. With 18 independent variables and accounting for 20% of rate of invalid questionnaires, the target sample size was calculated as 225–450 participants. Ultimately, 372 patients with lung cancer receiving first-time chemotherapy were included in the questionnaire survey.
Measures
Socio-demographic and clinical data
Based on similar studies29,30, the research team independently designed the sociodemographic data collection form. The form included details on sex, age, education level, residence, marital status, primary caregiver, monthly household income (yuan), medical payment method, employment status, and comorbid chronic diseases, specifically the presence of hypertension and/or diabetes. Clinical data, including disease stage, surgical history, hemoglobin concentration, white blood cell count, platelet count, ultrasensitive C-reactive protein, and albumin levels, were extracted from medical records.
Health fitness assessment techniques
All measurement devices were standardized instruments and equipment mandated for the national physical fitness evaluation in China.
(1) Body composition: Evaluated using metrics, such as body weight, body mass index (BMI), and muscle mass.
We used the bioelectrical impedance analysis (BIA) technology of the clinical nutrition detection and analysis instrument (model: AiNST-CNDS20; Jiangsu Kangai Nutrition Technology Co., Ltd., China) to collect body composition data. The body composition analyzer is a high-precision model that adopts the BIA measurement technology. Using multi-frequency BIA tailored to different populations, it accurately measured body components, such as muscle mass and body fat percentage31.
(2) Muscular function: Assessed through upper limb muscle strength and lower limb muscle strength.
-
1.
Upper limb muscle strength: Muscle strength and composition (muscle mass or size) are the most accurate parameters for assessing muscle fitness32, with grip strength commonly used as proxy for upper limb muscle strength. Grip strength was measured in the ward using an electronic grip strength meter (EH201R). Patients were instructed to stand with their arm relaxed and hanging naturally, gripping the device firmly with their fingers positioned snugly on the handle. During the assessment, the grip strength gauge was maintained at a specified distance from the body, and patients were prohibited from bending their arm, waist, or moving their feet while applying force. A rest period of 2 min was allowed between each of the two tests, with the highest score recorded for evaluation.
-
2.
Lower limb muscle strength: Evaluated using a five-repetition sit-to-stand test. A 40 cm high back chair and a timer were used. Patients sat with legs shoulder-width apart and arms crossed. Upon the tester’s ‘start’ command, patients performed five 'stand up-sit down’ actions as quickly as possible. The timer stopped after the fifth repetition31.
-
3.
Cardiorespiratory fitness: Cardiopulmonary fitness is widely regarded as the best indicator of overall health and function33. It was assessed using the 6-min walking distance test, which is a low-difficulty, cost-effective, and safe method for assessing the exercise tolerance and cardiopulmonary function34. Patients stood at the starting point of a 50-m course to prepare. Upon the tester’s ‘start’ command, patients began walking. When the ‘stop’ command was signaled, patients remained in place, and the tester measured and recorded the distance in meters.
-
4.
Flexibility: Hip flexibility was used for assessment, measured using a seated forward flexion test. Flexibility can be assessed using either angle-based or length-based measurement methods. The length measurement method indirectly evaluates flexibility by measuring the distance reached during specific movements. Since joint flexibility refers to the range of motion in various directions, the sit-and-reach instrument estimates joint movement indirectly by measuring the length reached during forward flexion35. Patients sat on a cushion with legs together and knees straight. The patients slowly pushed a block forward with their longest finger along a scale. When it was impossible to continue to push forward, the tester measured the distance in cm, using the toes as the starting point.
International physical activity questionnaire (IPAQ)
The International Physical Activity Questionnaire (IPAQ) is a prominent tool utilized globally for assessing physical activity levels, recognized for its validity. In this study, we used the Chinese version of IPAQ, translated and adapted by Wang Jie36. It comprises 27 items that prompt respondents to indicate the frequency and duration of daily exercise, as well as to describe their physical activity over the past week across various domains, including leisure and recreation, household tasks, transportation methods, and occupational activities37. The duration (in minutes) spent at each intensity of physical activity was multiplied by its corresponding metabolic equivalent of task (MET) value (walking = 3.3, moderate intensity = 4, high intensity = 8). The MET-minutes from each intensity level were aggregated to calculate the total physical activity energy expenditure (in MET-minutes) for the previous week. A weekly total physical activity level of ≥ 1500 MET-min, 600–1499 MET-min, and < 600 MET-min was classified as high, moderate, and low, respectively38.
European organization for research and treatment of cancer quality of life questionnaire core 43 (EORTC QLQ-LC43)
The EORTC QLQ-LC43 was developed by the European Organization for Research and Treatment of Cancer (EORTC) and comprises the core QoL scale (EORTC QLQ-C30), and the lung cancer-specific subscale (EORTC QLQ-LC13). In this study, we used the Chinese version of EORTC QLQ-LC43, which was translated and adapted by Wan Chonghua39. The EORTC QLQ-C30 consists of 30 items divided into three components40: (1) functional subscales (physical function, social function, emotional function, cognitive function, role function); (2) symptom subscale; and (3) global health status/QoL scale. Items 29 and 30 are scored on a 7-point scale, ranging from 1 (very poor) to 7 (very good), while the remaining items use a 4-point scale from 1 (none) to 4 (very many). Each domain score is calculated by summing the relevant item scores and dividing by the number of items. The scale demonstrated good internal consistency, with a Cronbach’s alpha of 0.83.
The EORTC QLQ-LC13 tool consists of 13 items, specifically designed to assess lung cancer-related symptoms and treatment side effects40. It covers 13 common symptoms, such as coughing, hemoptysis, shortness of breath, breathing difficulties, pain, and hair loss. Dyspnea, which forms a sub-scale, was not scored while the remaining items are reported individually. The scale demonstrated reliability and applicability among Chinese patients with lung cancer, with a Cronbach’s alpha coefficient exceeding 0.7041.
Statistical analysis
We utilized Excel 2010 (Microsoft Corporation, Redmond, WA, USA) for data entry and aggregation, while statistical analysis was performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA). To characterize the demographic, sociological attributes, and disease-related factors (e.g., disease stage, total white blood cell count, platelet count, ultrasensitive C-reactive protein, and albumin levels) of the respondents, categorical data were represented as rates or proportions. For continuous data, those conforming to a normal distribution were presented as mean ± standard deviation, while non-normally distributed data were expressed as interquartile range (IQR: P25-P75).
Factors affecting health fitness status, physical activity levels, and QoL among patients with lung cancer were analyzed using independent samples t-tests, analysis of variance, and non-parametric methods (Mann–Whitney U-test and Kruskal–Wallis H-test). Non-parametric tests were specifically employed for data exhibiting heterogeneity. Multivariate linear regression was used to perform a multifactorial analysis of the determinants affecting physical activity levels and QoL among patients with lung cancer. Two-tailed tests were conducted, with a p value of 0.05 set as the significance level.
Results
Participant characteristics
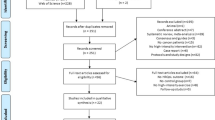
Out of 438 eligible patients, 405 were included in the study (response rate: 92.47%). Among these, 33 were excluded due to incomplete responses, resulting in a final sample size of 372 patients.
The mean age of participants was 64.1 years, with 77.96% being male. The most frequent cancer types were adenocarcinoma (48.66%) and squamous cell carcinoma (23.12%). Detailed sociodemographic characteristics of the participants are described in Table 1.
Factors that influence patients’ quality of life
The survey indicates that QoL significantly varies by primary caregivers, smoking status, and physical activity levels (all P < 0.05). Among the covariates analyzed, age was significantly associated with EORTC QLQ-LC13 scores (P = 0.005), with the greatest impact observed in patients aged 18–45 years. Sex-specific analysis indicated that female patients scored lower on global health status and functioning scales but reported higher symptom scale scores than those of their male counterparts (P = 0.001). Educational attainment emerged as a significant factor, with patients holding bachelor’s degrees or higher qualifications demonstrating superior functional outcomes (P = 0.011), albeit with increased symptom burden and lung cancer-specific symptoms. While no significant differences were observed in global health status, functional scales, or symptom scales (P > 0.05), residential status significantly impacted EORTC QLQ-LC13 scores (P < 0.001), with rural residents reporting elevated symptom burden compared with urban and town dwellers. Socioeconomic analysis revealed that income level significantly correlated with global health status (P < 0.001), with higher income brackets associated with improved global health outcomes. However, no significant associations were found with functional scales, symptom scales, or EORTC QLQ-LC13 scores (P > 0.05). Multiple factors, including medical payment method, employment status, occupational history, alcohol consumption patterns, comorbid chronic diseases, and living arrangements, demonstrated significant effects across functional scales, symptom scales, and EORTC QLQ-LC13 scores (all P < 0.05). Clinical characteristics, particularly diagnosis type and lung cancer staging, significantly influenced global health status (P < 0.05). Notably, marital status showed no statistical significance in relation to surgical status across all QoL domains (all P > 0.05) (Table 2).
Health fitness, physical activity levels, and QoL
Patients with lung cancer receiving chemotherapy for the first time had a BMI of 23.51 (IQR 21.32, 24.97), and muscle mass score of 27.40 (IQR 21.43, 34.90). The overall physical activity level of this cohort was low to moderate, with 90 (24.19%), 236 (63.44%), and 46 (12.37%) classified as having high, moderate, and low physical activity levels, respectively. On the QLQ-C3, the lowest median score was observed in the functioning domain at 35.33 (IQR 25.67, 51.33) while the highest was for global health status at 66.67 (IQR 50, 75). For the QLQ-LC13, the highest symptom scores were recorded for dyspnea (33.33; IQR 11.11–44.44), coughing (33.33; IQR 0.00–33.33), and alopecia (33.33; IQR 0.00–33.33), as detailed in Table S1.
Multiple linear regression analysis of factors influencing patients’ QoL
Influencing factors with statistically significant differences in univariate analyses of variance, along with age, were included as independent variables, while scale scores were included as dependent variables in multiple linear regression analyses. The influencing factors of global health status in the QLQ-C30 scale included sex, education level, primary caregiver, employment status, smoking status, comorbid chronic diseases, living arrangement, disease stage, physical activity level, and 6-min walk distance (P < 0.05). Female patients exhibited better functional scales (B = 11.405, P < 0.001). Patients with employee medical insurance (B = 7.307, P = 0.002) and other payment methods (B = 5.487, P = 0.042) also had better functional scales. The absence of comorbid chronic diseases was associated with better functional scales (B = 10.251, P < 0.001). Patients in Stage II had slightly lower functional scales (B = − 7.674, P = 0.038). Higher physical activity levels were associated with better functional scales (B = 11.066, P = 0.013), while lower muscle mass was associated with worse functional scales (B = − 0.377, P = 0.002). Multivariate analysis identified several significant predictors of symptom burden (all P < 0.05) in chemotherapy-naïve patients with lung cancer, including sex, educational attainment, Monthly Household Income (yuan), primary caregiver status, disease stage, comorbidity index, physical activity level, 6-min walk distance and seated forward bend test performance. The EORTC QLQ-LC13 scores demonstrated significant associations with multiple clinical and demographic variables, including chronological age, educational attainment, primary caregiver status, occupational history (including pre-retirement occupation), and diagnostic characteristics (all P < 0.05). Only statistically significant factors are listed in the table (Table 3).
Discussion
The analysis revealed several significant associations between demographic, socioeconomic, and clinical factors and the QoL of patients with lung cancer, as measured by the EORTC QLQ-C30 and QLQ-LC13 scales. Age emerged as a critical determinant, with younger patients (18–45 years) reporting a higher symptom burden compared with that of the older cohorts (P = 0.005). This finding aligns with that of previous studies suggesting that younger patients may experience more aggressive disease progression or heightened psychological distress, contributing to increased symptom severity. Epidemiological investigations consistently demonstrate a significant age-dependent disparity in psychological adaptation to illness42,43,44, with older patients exhibiting greater emotional resilience and lower disease-related distress scores than those of their younger counterparts. This phenomenon may be attributed to the differential psychosocial burden distribution, wherein younger patients frequently encounter substantial occupational and familial obligations. Sex-specific differences were also notable, with female patients scoring lower on global health status and functioning scales but reporting higher symptom scale scores than those of their male counterparts (P = 0.001), which aligns with the findings of Yifan et al.45. Educational attainment significantly influenced functional outcomes, with patients holding bachelor’s degrees or higher qualifications demonstrating superior functional scales (P = 0.011). However, this group also reported increased symptom burden, possibly due to greater awareness of their condition or higher expectations regarding health outcomes46. Residential status further impacted QoL, with rural residents experiencing elevated symptom burden compared to that of urban and town dwellers (P < 0.001). This could be linked to limited access to healthcare resources and delayed diagnosis in rural areas, exacerbating symptom severity. Socioeconomic factors, particularly income level, were significantly associated with global health status (P < 0.001), with higher income brackets correlating with improved health outcomes. This finding underscores the role of economic stability in accessing timely and effective medical care, which can significantly influence QoL. Furthermore, clinical characteristics, such as disease stage and diagnosis type significantly impacted global health status (P < 0.05), highlighting the importance of early detection and tailored treatment strategies in improving patient outcomes.
The QLQ-C30 and QLQ-LC13 scales revealed that global health status was relatively higher than functional and symptom scales, with dyspnea, coughing, and alopecia being the most prominent symptoms. This contradicts the observations of Yifan et al.45, who reported that chest pain was more pronounced in patients hospitalized after lung cancer surgery. This difference could be attributed to distinct characteristics of the population they studied, which included patients who experienced more pronounced postoperative pain in the first five days after lung cancer surgery. These findings suggests that while patients may perceive their overall health status as moderately good, they continue to experience significant functional limitations and symptom burden, particularly in domains related to respiratory and physical functioning. This pathophysiological phenomenon is primarily attributable to the tumor-mediated destruction of alveolar architecture and bronchial obstruction, which collectively contribute to impaired pulmonary function, manifested as compromised gas exchange efficiency and restricted airflow dynamics47.
This study highlights the health fitness, and QoL challenges faced by patients with lung cancer undergoing first-time chemotherapy. While most patients had a normal BMI, declines in muscle mass and grip strength suggest early sarcopenia and fatigue, consistent with prior findings48,49. Physical activity levels were predominantly low to moderate (87.81% overall), underscoring the need for interventions to improve activity levels and mitigate the adverse effects of chemotherapy. QoL assessments revealed moderate global health status but significant functional impairment, aligning with the physical and emotional burden of the treatment50. Dyspnea and coughing were the most prevalent symptoms, reflecting common respiratory complications. Alopecia and chest pain further emphasized the physiological toll of chemotherapy. Other symptoms were minimal, possibly due to early treatment stages or effective management. These findings advocate for integrated supportive care, including physical rehabilitation and symptom management, to enhance QoL and treatment outcomes in this population.
The multivariate regression analysis further elucidated the complex interplay of factors influencing QoL. Female sex, higher education levels, and absence of comorbid chronic diseases were associated with better functional scales. This may be attributed to better access to resources and health literacy among more educated female patients. This findings align with the findings of Julia et al.51, who also suggested that patients with less education felt they had less control over their cancer disease, leading to poorer health-related QoL. In contrast, advanced disease stages and lower physical activity levels were linked to worse outcomes. A possible explanation for this is that patients with low physical activity levels sit for longer periods of each day, increasing the factors of complications after oncological treatment, thereby reducing QoL. Increasing physical activity in such patients may help to improve QoL, particularly in terms of social functioning52. Notably, higher level of physical activity (B = 16.707, P < 0.001) and longer 6-min walking distance (B = 0.041, P < 0.001) demonstrates better overall health and function. These findings emphasize the multifaceted nature of QoL in patients with lung cancer, highlighting the interplay between physical, socioeconomic, and clinical factors.
Interventions targeting physical activity levels and the management of chemotherapy-related comorbid chronic diseases in lung cancer may significantly improve QoL in this population. Future studies should explore the longitudinal effects of these factors and evaluate tailored supportive care strategies to optimize patient prognosis during chemotherapy.
Significant sex differences exist in symptom perception, functional status, and QoL. Female patients reported a higher symptom burden and lower functional scores, whereas male patients demonstrated higher levels of physical activity. Objective physical fitness indicators, such as muscle mass, grip strength, and 6-min walking distance, were directly associated with health-related QoL in patients with lung cancer and were found to be significantly correlated with global health status, functional scores, and symptom burden. In particular, 6-min walking distance was identified as a significant predictor of function and QoL. Clinical interventions should focus on sex specificity, providing more psychological support and symptom management strategies for female patients, while encouraging male patients to maintain physical activity to improve functional status. Simultaneously, physical fitness assessments should be emphasized and integrated into the routine management of patients with lung cancer. Exercise interventions designed to enhance muscle mass and functional capacity may improve the QoL and treatment outcomes of patients with poor physical fitness.
Strengths and limitations
We investigated the health fitness and physical activity levels of patients with lung cancer who received chemotherapy for the first time, providing a clearer explanation of the relationship between these indicators.
Nevertheless, this research has some limitations. First, as a cross-sectional study, we were unable to evaluate the causal relationship between health fitness, physical activity level and QoL among participants. Longitudinal and prospective controlled studies are needed to validate our findings. Second, the study employed convenience sampling, and data were collected from a single tertiary general hospital, which may limit the generalizability of the findings to the broader population of patients undergoing first-line chemotherapy for lung cancer. Therefore, population-based, multicenter studies with large-scale sample sizes are warranted to confirm these findings.
Conclusion
Patients with lung cancer undergoing initial chemotherapy often exhibit varied levels of health-related physical fitness, particularly in muscle strength and cardiorespiratory endurance, which influence their capacity to engage in physical activities. Higher physical fitness and greater physical activities are associated with improved QoL. Early identification of patients with inadequate physical activity levels and poor physical fitness may help clinicians explore the interplay between fitness levels and activity engagement, providing insights into the underlying mechanisms of fitness deterioration. These findings can guide the development of targeted interventions to enhance physical fitness and QoL in this patient population.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F. & Heist, R. S. Lung cancer. Lancet 398(10299), 535–554. https://doi.org/10.1016/S0140-6736(21)00312-3 (2021).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74(1), 12–49. https://doi.org/10.3322/caac.21820 (2024).
Detterbeck, F. C., Mazzone, P. J., Naidich, D. P. & Bach, P. B. Screening for lung cancer: Diagnosis and management of lung cancer: American college of chest physicians evidence-based clinical practice guidelines. Chest 143(5), e78S-e92S. https://doi.org/10.1378/chest.12-2350 (2013).
Leak Bryant, A. et al. Symptoms, mobility and function, and quality of life in adults with acute leukemia during initial hospitalization. Oncol. Nurs. Forum 45(5), 653–664. https://doi.org/10.1188/18.ONF.653-664 (2018).
Wang, C. et al. Factors associated with quality of life of adult patients with acute leukemia and their family caregivers in China: A cross-sectional study. Health Qual. Life Outcomes 18(1), 8. https://doi.org/10.1186/s12955-020-1269-8 (2020).
Kroenke, K. et al. Prevalence, severity, and co-occurrence of SPPADE symptoms in 31,866 patients with cancer. J. Pain Sympt. Manag. 65(5), 367–377. https://doi.org/10.1016/j.jpainsymman.2023.01.020 (2023).
Albini, A. et al. Physical activity and exercise health benefits: Cancer prevention, interception, and survival. Eur. J. Cancer Prev. 34(1), 24–39. https://doi.org/10.1097/CEJ.0000000000000898 (2025).
Campbell, K. L. et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51(11), 2375–2390. https://doi.org/10.1249/MSS.0000000000002116 (2019).
Rock, C. L. et al. American cancer society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 72(3), 230–262. https://doi.org/10.3322/caac.21719 (2022).
Campbell, K. L. et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51(11), 2375–2390. https://doi.org/10.1249/MSS.0000000000002116 (2019).
Nguyen, T., Tracy, K., Ullah, A. & Karim, N. A. Effect of exercise training on quality of life, symptoms, and functional status in advanced-stage lung cancer patients: A systematic review. Clin. Pract. 13(3), 715–730. https://doi.org/10.3390/clinpract13030065 (2023).
Sloan, J. A. et al. Impact of self-reported physical activity and health promotion behaviors on lung cancer survivorship. Health Qual. Life Outcomes 14, 66. https://doi.org/10.1186/s12955-016-0461-3 (2016).
Avancini, A., Belluomini, L., Quist, M. & Pilotto, S. Lung cancer screening: An opportunity to promote physical activity?. JTO Clin. Res. Rep. https://doi.org/10.1016/j.jtocrr.2024.100651 (2024).
Brodie, D. A. & Stewart, A. D. Body composition measurement: A hierarchy of methods. J. Pediatr. Endocrinol. Metab. 12(6), 801–816. https://doi.org/10.1515/JPEM.1999.12.6.801 (1999).
Zhou, W. et al. Physical activity in surgical lung cancer patients: A systematic review. Support. Care Cancer off. J. Multinatl. Assoc. Support. Care Cancer 30(8), 6473–6482. https://doi.org/10.1007/s00520-022-07018-1 (2022).
Jie, Y. et al. Meta-analysis of the impact of early activity on postoperative rehabilitation and quality of life in patients with lung cancer. Chin. J. Nurs. 57(17), 2095–2102. https://doi.org/10.3761/j.issn.0254-1769.2022.17.008 (2022).
Avancini, A. et al. Physical activity and exercise in lung cancer care: Will promises be fulfilled?. Oncologist 25(3), 555–569. https://doi.org/10.1634/theoncologist.2019-0463 (2020).
Wang, A. et al. Physical activity and sedentary behavior in relation to lung cancer incidence and mortality in older women: The Women’s Health Initiative. Int. J. Cancer. 139(10), 2178–2192. https://doi.org/10.1002/ijc.30281 (2016).
Blair, S. N. et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262(17), 2395–2401. https://doi.org/10.1001/jama.262.17.2395 (1989).
Panel, E. American college of sports medicine roundtable on exercise guide lines for cancer survivors. J. ACSM 42, 1409–1426. https://doi.org/10.1249/MSS.0b013e3181e0c112 (2010).
Ma, Q., Zheng, G., Luo, J., Cao, H. & Hou, L. Exploring factors associated with postoperative physical activity and sedentary behavior in newly diagnosed lung cancer patients: A cross-sectional study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 32(9), 605. https://doi.org/10.1007/s00520-024-08805-8 (2024).
Jinbo, R. et al. Physical function, nutritional status, and quality of life before and after chemotherapy in patients with malignant lymphoma. Medicine 102(6), e32901. https://doi.org/10.1097/MD.0000000000032901 (2023).
Sui, X. et al. Influence of cardiorespiratory fitness on lung cancer mortality. Med. Sci. Sports Exerc. 42(5), 872–878. https://doi.org/10.1249/MSS.0b013e3181c47b65 (2010).
Bettariga, F. et al. Effects of resistance training vs high intensity interval training on body composition, muscle strength, cardiorespiratory fitness, and quality of life in survivors of breast cancer: A randomized trial. Breast Cancer Res. Treat. 210(2), 261–270. https://doi.org/10.1007/s10549-024-07559-5 (2025).
Courneya, K. S. et al. Associations between health-related fitness and quality of life in newly diagnosed breast cancer patients. Breast Cancer Res. Treat. 199(3), 533–544. https://doi.org/10.1007/s10549-023-06935-x (2023).
Persoon, S. et al. Effects of exercise in patients treated with stem cell transplantation for a hematologic malignancy: A systematic review and meta-analysis. Cancer Treat Rev. 39(6), 682–690. https://doi.org/10.1016/j.ctrv.2013.01.001 (2013).
Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth 13(Suppl 1), S31–S34. https://doi.org/10.4103/sja.SJA_543_18 (2019).
Ni, P., Chen, J. L. & Liu, N. The sample size estimation in quantitative nursing research. Chin. J. Nurs. 45(4), 378–380. https://doi.org/10.3761/j.issn.0254-1769.2010.04.037 (2010).
Yujia, K. Research on the current situation and influencing factors of physical activity in cancer patients undergoing chemotherapy. North Sichuan Med. Coll. https://doi.org/10.27755/d.cnki.gcbyx.2023.000529 (2023).
Xinxin, C., Guofeng, L., Yang Yingzi, H., Yachen, J. Y. & Yumei, L. Correlation analysis of physical activity levels and symptom clusters in patients with lung cancer undergoing chemotherapy during hospitalization. J. Tubercul. Pulm. Dis. 5(03), 197–206. https://doi.org/10.19983/j.issn.2096-8493.2024048 (2024).
Lijuan, L. Research on the impact of Baduanjin exercise on health fitness and quality of life of patients with cervical cancer. Shanghai Norm. Univ. 2, 22 (2022).
Sweegers, M. G. et al. Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: A meta-analysis of individual patient data. Br. J. Sports Med. 53(13), 812–812. https://doi.org/10.1136/bjsports-2018-099191 (2019).
Imboden, M. T. et al. Cardiorespiratory fitness and mortality in healthy men and women. J. Am. Coll. Cardiol. 72(19), 2283–2292. https://doi.org/10.1016/j.jacc.2018.08.2166 (2018).
Cardiovascular branch of Chinese medical association, cardiopulmonary prevention and rehabilitation professional committee of Chinese association of rehabilitation medicine, editorial committee of Chinese journal of cardiovascular diseases. Clinical use of Chinese experts in six-minute walk-test consensus. Chin. J. Cardiovasc. Dis. 50(5):432–442. https://doi.org/10.3760/cma.J.c.n112148-20211206-01054 (2022).
Wu, M. Reliability and criterion-related validity of four sit and reach tests for measuring hamstring flexibility in male university students. (Master’s dissertation, Jilin University) (2018).
Wang, J. Evaluation of the Credibility and Validity of the International Physical Activity Questionnaire (Refined Version) in the Middle-Aged and Elderly Population ((Master’s Dissertation Shanxi Normal University), 2016).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35(8), 1381–1395. https://doi.org/10.1249/01.MSS.0000078924.61453.FB (2003).
Mengyue, F., Yun, L. & Pingping, H. Calculation of physical activity levels in the international physical activity questionnaire. Chin. J. Epidemiol. 35(8), 961–964. https://doi.org/10.3760/cma.j.issn.0254-6450.2014.08.021 (2014).
Chonghua, W., Cai, L. & Canzhen, Z. Development and evaluation of the Chinese version of the quality of life scale QLQ-LC43 for lung cancer patients. Chin. J. Behav. Med. 8(34), 3–5. https://doi.org/10.3760/cma.j.issn.1674-6554.1999.01.002 (1999).
Zhao, C. Effect of respiratory rehabilitation on quality of life in patients with advanced non-small cell lung cancer. Jining Med. Coll. 2022. https://doi.org/10.27856/dcnki.Gjnyx.2022.000060 (2025).
Zhang, L. et al. Validation of EORTC QLQ-LC43 for Chinese patients with lung cancer. Lung Cancer 85(1), 94–98. https://doi.org/10.1016/j.lungcan.2014.04.006 (2014).
Kan, Z. J. Study on quality of life and long-term care needs of elderly patients with chronic diseases in China (Master’s Thesis, Southeast University). https://doi.org/10.27014/dcnki.Gdnau.2019.000352 (2019).
Li, L. Y. & Yuan, P. Quality of life analysis of 53 patients with lung cancer treated with radiotherapy. PLA J. Prev. Med. 36(12), 1592–1593. https://doi.org/10.13704/j.carolcarrollnkijyyx.2018.12.031 (2018).
Zhang, Y. et al. Fear of cancer recurrence in patients with breast cancer and its influencing factors. Chin. J. General Med. 21(20), 2479–2483. https://doi.org/10.3760/cma.j.issn.1671-7368.2018.20.010 (2018).
Yifan, Hu. et al. Quality of life in hospitalized patients after lung cancer surgery and analysis of influencing factors. J. Hainan Med. Coll. 29(12), 922–927. https://doi.org/10.13210/j.carolcarrollnkijhmu.20230320.003 (2023).
Shaoli, Z. et al. Health-related quality of life evaluation and influencing factors in patients with upper digestive tract precancerous lesions. Chin. Oncol. 33(09), 747–755. https://doi.org/10.19401/j.cnki.1007-3639.2024.09.001 (2024).
Luo, Y. et al. Exploring central and bridge symptoms in patients with lung cancer: A network analysis. Semin. Oncol. Nurs. 40(3), 151651. https://doi.org/10.1016/j.soncn.2024.151651 (2024).
Jang, J. Y. et al. Prognostic impact of muscle mass loss in elderly patients with oesophageal cancer receiving neoadjuvant chemoradiation therapy. J. Cachexia Sarcopenia Muscle. 15(3), 1167–1176. https://doi.org/10.1002/jcsm.13462 (2024).
Kok, A. et al. Expectations and experiences of participating in a supervised and home-based physical exercise intervention in patients with head and neck cancer during chemoradiotherapy: A qualitative study. Curr. Oncol. 31(2), 885–899. https://doi.org/10.3390/curroncol31020066 (2024).
Tsukada, K., Seino, Y. & Yamashita, T. The quality of life outcome in patients with head and neck squamous cell cancer treated using chemoradiotherapy. Auris Nasus Larynx 51(4), 674–679. https://doi.org/10.1016/j.anl.2024.04.001 (2024).
Roick, J., Esser, P., Hornemann, B. & Ernst, J. Control beliefs as mediators between education and quality of life in patients with breast, prostate, colorectal, and lung cancer: A large register based study. BMC Psychol. 12(1), 382. https://doi.org/10.1186/s40359-024-01867-7 (2024).
Zainordin, N. H., Karim, N. A., Shahril, M. R. & Abd Talib, R. Physical activity, sitting time, and quality of life among breast and gynaecology cancer survivors. Asian Pac. J. Cancer Prev. 22(8), 2399–2408. https://doi.org/10.31557/APJCP.2021.22.8.2399 (2021).
Funding
Major Project of Nursing Research Project of Wuxi Nursing Society (Z202303); Nursing Research Topic Development Project of Chinese Medical Association Journal 2022–2023 (CMAPH-NRD2022003);Top Wuxi Health Committee Program (M20 2238).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: Jiahui Xu; Hui Lu; Tingting Fang; Acquisition of data: Jiahui Xu; Dongyan Cai; Ping Cai; Yuqing Zhou; Hui Su; Analysis and interpretation of the data: Jianing Hua; Yaoyao Hu; Statistical analysis: Jiahui Xu; Hui Lu; Tingting Fang; Obtaining financing: Hui Lu; Ping Cai; Writing of the manuscript: Jiahui Xu; Hui Lu; Huihong Wang; Tingting Fang; Critical revision of the manuscript for intellectual content: Huihong Wang; Ying Chen; Dongyan Cai; Ping Cai; Yuqing Zhou; Hui Su1. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Affiliated Hospital of Jiangnan University (filenumber: LS2023086).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, J., Lu, H., Fang, T. et al. Health fitness, physical activity, and quality of life in patients undergoing first chemotherapy for lung cancer: a cross-sectional study. Sci Rep 15, 21797 (2025). https://doi.org/10.1038/s41598-025-06834-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06834-9