Abstract
Chemotherapy, used to treat hydatid cysts, can cause problems due to various side effects. Consequently, there is growing interest in non-chemical alternatives, such as medicinal plant extracts, as potential new treatments for hydatid cysts. This study investigated the protoscolicidal activity of green tea (Camellia sinensis) and lavender (Lavandula angustifolia) extracts at different concentrations and exposure times under laboratory conditions. Protoscolices were collected aseptically from the liver of sheep infected with hydatid cysts and exposed to three concentrations of C. sinensis and L. angustifolia extracts (10, 25, and 50 mg/mL) for 10, 20, 30, and 60 min. The viability of protoscolices was assessed using 0.1% eosin staining. The results showed that C. sinensis and L. angustifolia extracts at a concentration of 50 mg/mL effectively eliminated all protoscolices after 20 min. The scolicidal effects of C. sinensis and L. angustifolia were significant compared to the control groups. Furthermore, the results showed that these plant extracts have high protoscolicidal activity. However, further studies are needed to evaluate their in vivo effectiveness for treating hydatid cysts in humans and herbivorous animals.
Similar content being viewed by others
Introduction
Cystic echinococcosis (CE), also known as echinococcosis, is a significant zoonotic disease caused by the larval stage of Echinococcus granulosus1. This disease is common in many parts of the world, including Australia, South America, the Middle East, Eastern Europe, South Africa and the Mediterranean2 .
There are three main treatment options for the removal of hydatid cysts: surgery, chemotherapy, and percutaneous aspiration3. In cases that are regarded as not operable, chemotherapy with Albendazole is recommended as the best alternative to surgery4. Despite its effectiveness, chemotherapy can lead to various side effects, such as hepatotoxicity, severe leukopenia, thrombocytopenia, and alopecia5. In addition, resistance to synthetic anthelmintics in treating cystic echinococcosis has led researchers to explore the potential of medicinal herbs as an alternative scolicidal active ingredient that may have fewer side effects3,6.
Green tea, derived from the dried leaves of the C. sinensis plant, contains various physiologically active compounds, including polyphenols, methylxanthines, essential oils, proteins, vitamins, and amino acids7. Earlier studies focused on extracting polyphenols from C. sinensis8,9 and investigating its extracts’ potential antioxidant, antiviral, and antitumor properties10,11,12,13. The most common component in C. sinensis is catechins14which are primarily responsible for the health benefits associated with this plant. These advantages include reducing the plasma lipid levels, reducing inflammation, and exhibiting antibacterial, antiparasitic, anticancer, and antioxidant properties15,16,17,18. Catechins that belong to the Flavan-3-ol family have attracted considerable attention due to their possible therapeutic effects. In particular, their potent antioxidants and antiviral properties can contribute to the prevention of diseases13,19,20.
Lavender, a member of the Labiatae family (Lamiaceae), has been used for therapeutic purposes for centuries21. It is found in various countries and is scientifically classified as Lavandula angustifolia22. This plant is known for its antifungal, antimicrobial, and anti-protozoan properties21,23,24,25,26,27,28. Numerous studies on L. angustifolia have shown its antipsoriatic, antidiabetic, and antidiarrheal effects29,30,31,32. In addition, it has proven antiparasitic activity under both in vivo and in vitro conditions33,34,35,36,37.
No research has investigated the scolicidal effects of green tea (C. sinensis) and lavender (L. angustifolia). Therefore, the present study aims to examine the in vitro effects of C. sinensis and L. angustifolia extracts on eliminating hydatid cysts.
Results
Gas chromatography-mass spectrometry analysis
Based on the gas chromatography-mass spectrometry (GC/MS) analysis of the extracts studied, the most significant chemical components identified in L. angustifolia extract were linalool (26.20%), borneol (22.70%), and alpha-pinene (14.30%) (see Table 1). In C. sinensis extract, the major components were methyl linoleate (24.07%), squalene (11.34%), and N-hexadecanoic acid (9.32%) (Table 1).
In vitro results
The results of our examination of the effectiveness of different concentrations of extracts from C. sinensis and L. angustifolia as protoscolicidal agents are summarized in Tables 2, 3, 4, 5, 6 and 7. Our results show that both the concentration of the extracts and exposure duration significantly influence the viability of protoscolices.
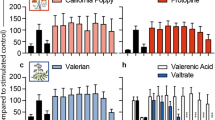
L. angustifolia extract in concentrations of 10, 25, and 50 mg/mL killed 70.75%, 93.09%, and 100% of protoscolices after 60 min, respectively. The control group, treated with a normal saline solution, showed a mortality rate of only 9.72%. Remarkably, all protoscolices were killed after only 20 min of exposure to the 50 mg/mL concentration of L. angustifolia (Table 4; Fig. 1). Similarly, after 60 min of exposure to varying concentrations of C. sinensis extract (10, 25, and 50 mg/mL), the mortality rates were 84.83%, 98.23%, and 100%, respectively, while the control group, treated with normal saline solution, had a mortality rate of 11.34% (Fig. 2).
The protoscolicidal effects of C. sinensis and L. angustifolia extracts in three different concentrations were highly significant compared to the control groups’ overall exposure times (p < 0.05) (Figs. 1 and 2). Our study showed that both C. sinensis and L. angustifolia extracts exhibit protoscolicidal activity in vitro.
Discussion
Surgery is the most preferred method for removing E. granulosus cysts to treat hydatid disease. Various scolicidal agents have been utilized to counteract the contents of hydatid cysts, but a final strategy for exterminating this disease has not yet been determined38. Desired qualities for an effective scolicidal agent include low costs, potency at low concentrations, lack of adverse effects, quick effect, and non-toxicity39.
There has recently become a growing interest in using natural anti-parasite extracts to treat various diseases. Some herbal extracts are emerging as viable alternatives to conventional medications due to their less adverse effects, lower cost, and high availability. Numerous studies have examined the scolicidal effects of various plants, such as Nigella sative40Allium sativum41Quercus infectoria42 Zingiber officinale43Salvadora persica44Ceratonia silique35Taxus baccata45 and Zataria multiflora46in the treatment of hydatid cysts. Additionally, Al Qaisi et al. (2021) demonstrated the in vitro protoscolicidal effects of methanolic extracts from Jordanian medicinal plants, including Ruta graveolens, Peganum harmala, and Citrullus colocynthis47. Furthermore, Al-khlifeh et al. (2021) reported in vitro protoscolicidal effects of Juniperus phoenicea L., Calotropis procera (Aiton) Dryand, and Artemisia judaica L. against Echinococcus granulosus cysts48. Al Qaisi et al. (2023) also explored the preventive effects of some medicinal plant extracts on the development of hydatid cyst infection49. Most of these studies focus more on in vitro evaluations than in vivo applications due to cost efficiency, speed, and a high throughput of in vitro screening methods. These in vitro tests measure the anthelmintic activity directly on the hatching, development, and motility of parasites without influencing the internal physiological functions of the host50. Another advantage of in-vitro studies is that the extracts or compounds, as soon as reliable results are achieved, can be further evaluated in vivo51. However, it is essential to note that compounds or extracts that are effective in vitro may not necessarily have the same level of activity in vivo52.
Several recent studies have documented the antiparasitic effects of C. sinensis and L. angustifolia extracts53,54,55,56,57. This study investigates the scolicidal activity of these extracts in vitro. This is the first research analyzing the effects of C. sinensis and L. angustifolia extracts on hydatid cysts under in vitro conditions.
Our findings demonstrate a dose-dependent and time-dependent scolicidal effect of C. sinensis and L. angustifolia extracts on the protoscolexes of hydatid cysts in vitro. These results align with those of Malekifard et al.35,58who also observed dose and time-dependent sporicidal effects of C. silique and Q. infectoria olivier extracts. In addition, a study by Moazeni et al. (2012) reported similar dose and time-dependent scolicidal effects of Satureja khuzistanica essential oil59.
This study shows that the scolicidal activity of extracts from C. sinensis and L. angustifolia is significant after 20 min of exposure at a concentration of 50 mg/mL, which leads to a 100% mortality rate. The scolicidal effects observed during this concentration and exposure period are comparable to that of other active ingredients such as 95% ethyl alcohol (15 min)600.5-1% cetrimide (10 min)613% H2O2 (15 min)62and 20% hypertonic saline (15 min)63. Norouzi et al. (2020) examined the influence of a hydroalcoholic extract from Taxus baccata L. on the protoscolices of hydatid cysts. Their results showed that an extract concentration of 150 mg/mL killed 66.6% of protoscolices after 60 min45. Sajjadi et al. (2008) examined Allium Sativum extracts and found that the chloroformic extract had the highest protoscolicidal activity in a 200 mg/mL concentration64. Similarly, Moazeni et al. (2014) reported a strong scolicidal effect from the methanolic extract of Zataria multiflora, whereby concentrations of 10 and 25 mg/mL killed 100% of the protoscolices after 3 and 1 min65. In another study, Kavoosi and Purfard (2013) found that all protoscolices were killed within 10 min of exposure to more than 17 µg/mL essential oil concentrations by Zataria multiflora66. In addition, Mahmoudvand et al. (2014) showed that the essential oil from Nigella sativa eliminated 100% of protoscolices in a concentration of 10 mg/mL after 10 min of exposure67. In addition, Rouhani et al. (2013) examined the scolicidal effect of Barberry in different concentrations (0.5, 1, 2, and 4 mg/mL) and different exposure times (5, 15, and 30 min). They found that a 4 mg/mL concentration had a 100% effectiveness ater only 5 min68. The variations in the results in various studies can be attributed to the differences in the types of plants used, concentrations, and exposure times45.
Our research provides evidence of the in vitro protoscolicidal activity of L. angustifolia extract. This study identified linalool as the primary compound, making up 26.20% of the extract. The L. angustifolia extract also contained small amounts of other compounds. Linalool and Linalyl acetate are known to be responsible for most biological activities of lavender69. Supporting our findings, Malekifard et al. (2021) demonstrated that high levels of linalool in L. angustifolia extract are likely the active compounds responsible for its anti-Trichomonas gallinae activity36. Several studies have focused on determining the pharmacological properties of linalool and L. angustifolia. Reports indicate that L. angustifolia extract has therapeutic effects against Toxoplasma gondii, Giardia duodenalis, Trichomonas vaginalis, and Hexamita inflata infections31,34. Furthermore, it is essential to note that L. angustifolia acts by lysing the cells of these parasites to eliminate them34.
In this study, caffeine (24.07%) was identified as the primary component of the C. sinensis extract, and it has been reported to have antiparasitic effects in several studies. Findings from these studies indicated that both C. sinensis extract and caffeine exhibited anti-Acanthamoeba, anti-Trichomonas gallinae, and antileishmanial impact56,70,71. Previous research72 has shown that C. sinensis and its constituents demonstrated antimicrobial activity against multidrug-resistant gram-positive and gram-negative bacteria. In addition, C. sinensis was effective in the inhibition of Leishmania amazonensis73,74reducing exposure to Haemonchus contortus worms75and inhibiting both the promastigote and amastigote forms of Leishmania braziliensis76. Moreover, C. sinensis also inhibited Babesia spp., Eimeria spp., Trichomonas gallinae, and Trypanosoma cruzi18,56,77,78. Research conducted by Fakae et al. demonstrated that beers made from C. sinensis exhibited amoebic activity against Acanthamoeba trophozoites and were effective in preventing the parasite from encysting. Their findings suggested that C. sinensis could serve as a source of inhibitors for A. castellanii growth and encystation79.
Conclusion
In conclusion, our findings demonstrated that both C. sinensis and L. angustifolia could serve as effective scolicidal agents. To fully understand and improve these plant-based compounds’ pharmacological and therapeutic properties, their mechanisms of action should be further investigated80. In addition, further examinations are required to determine possible adverse effects of C. sinensis and L. angustifolia and confirm the scolicidal effectiveness of these herbal medications in vivo.
Methods
Ethical compliance
The study’s ethical considerations were approved by the Animal Ethics Committee at Urmia University, Urmia, Iran (IR-UU-AEC-3/83), and conducted under its regulations.
Collecting protoscolices
Protoscolices were extracted from the livers of infected sheep slaughtered at the Urmia abattoir in northwest Iran. The samples were collected postmortem, following standard abattoir procedures for meat production, with no specific anesthesia or euthanasia methods applied for this study. Under aseptic conditions, the hydatid fluid was transferred to a flask and allowed to stand for 30 min to separate the protoscolices. The supernatant was removed, and the protoscolices were washed three times with PBS solution (pH = 7.2). The viability of the protoscolices was confirmed by motility observations using a standard light microscope after staining with 0.1% Eosin58.
Preparation of plant extract
C. sinensis and L. angustifolia plants were purchased from a Persian herbal market in June 2023 and verified by the Agriculture Faculty of Urmia University in Urmia, Iran36,56. The extraction method used was based on techniques by Baqer et al. With some modifications81. All dry plant materials, including the leaves of green tea and the dried branches of flowers from lavender, were ground in powder using an electrical mixer (Moulinex, Paris, France). A mixture of 500 mL of 70% ethanol and 100 g of powdered plant material was stirred with a magnetic stirrer for 2 h. The solution was left at room temperature for 24 h and filtered after another mixing round56. The solvent was removed using a rotary evaporator, and the semi-solid residual components were frozen for future use at 4 °C. The extraction process yielded approximately 12.5% (w/w) for L. angustifolia (12.5 g of dried extract from 100 g of dried flower branches) and 15.8% (w/w) for C. sinensis (15.8 g of dried extract from 100 g of dried leaves).
Gas chromatography-mass spectrometry (GC-MS) analysis
The chemical composition of the extracts was analyzed using gas chromatography-mass spectrometry (GC-MS) by Thermo Scientific™ in Paris, France. The helium-based carrier gas was adjusted to a 0.50 mL/min split ratio. The following GS conditions were used: an initial temperature of 40 °C, increasing to 250 °C at 80 °C per minute, and maintaining the temperature for 3 min. The injector and detector temperatures were both set at 250 °C. Individual compounds were identified by comparing their relative retention times in a capillary column with those of authentic samples and by analyzing their peak-to-peak mass spectra against data from reliable sources and published literature56,57.
Scolicidal effects of C. sinensis and L. angustifolia extracts
C. sinensis and L. angustifolia extracts were tested at three different concentrations of 10, 25, and 50 mg/mL, along with various exposure times of 10, 20, 30, and 60 min, to evaluate their effectiveness in eliminating protoscolices of hydatid cysts. For each concentration, 0.5 mL was placed into a test tube, followed by the addition of 0.5 mL of protoscolices solution (containing approximately 450–550 protoscolices suspended in PBS solution). The mixtures were gently mixed and left undisturbed for the specified durations at room temperature. At the end of each time interval, the upper phase of the mixture was carefully collected using a pipette, ensuring that the protoscolices were not disturbed. Staining was performed by adding 2 mL of 0.1% eosin to the settled protoscolices in the tube, which was then gently mixed. The upper portion of the remaining solution was discarded. The remaining protoscolices were then smeared on a glass slide under a light microscope to evaluate their viability. The percentage of dead protoscolices was determined by counting at least 450, often more than 500, protoscolices. Normal saline solution was used as a control group, and all experiments were conducted in triplicate42.
Viability test
This study utilized a 0.1% eosin solution (1 g eosin in 1000 mL distilled water) to analyze the viability of protoscolices. After 15 min of exposure to Eosin, viable protoscolices showed no significant color change, while dead protoscolices became red due to the absorption of eosin red. The mortality rate was calculated by dividing the number of protoscolices killed by the total number of protoscolices42.
Statistical analysis
SPSS (version 26, Chicago) was used for statistical analysis. A Chi-square test was carried out to compare the differences between the test and control groups. A p-value of less than 0.05 (P < 0.05) was considered statistically significant.
Declaration of generative AI in scientific writing
While preparing this work the author(s) used ChatGPT to make the text antive. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the publication’s content.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CE:
-
Cystic echinococcosis
- GC–MS:
-
Gas chromatography–mass spectrometry
References
Eckert, J. & Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 17, 107–135 (2004).
Zulfikaroglu, B., Ozalp, N., Keskek, M. & Koc, M. Primary Echinococcal cyst of the thyroid: report of a case. Surg. Today. 38, 833–835 (2008).
Adas, G. et al. Use of albendazole sulfoxide, albendazole sulfone, and combined solutions as scolicidal agents on hydatid cysts (in vitro study). World J. Gastroenterol. WJG. 15, 112 (2009).
Arif, S. H., Malik, A. A., Khaja, A. R., Dass, T. A. & Naikoo, Z. A. Role of albendazole in the management of hydatid cyst liver. Saudi J. Gastroenterol. 17, 343–347 (2011).
Junghanss, T., da Silva, A. M., Horton, J., Chiodini, P. L. & Brunetti, E. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am. J. Trop. Med. Hyg. 79, 301–311 (2008).
Pessoa, L. M., Morais, S. M., Bevilaqua, C. M. L. & Luciano, J. H. S. Anthelmintic activity of essential oil of Ocimum gratissimum linn. And Eugenol against Haemonchus contortus. Vet. Parasitol. 109, 59–63 (2002).
Yamamoto, T., Juneja, L. R. & Kim, M. Chemistry and Applications of Green Tea (CRC, 1997).
Perva-Uzunalić, A. et al. Extraction of active ingredients from green tea (Camellia sinensis): extraction efficiency of major catechins and caffeine. Food Chem. 96, 597–605 (2006).
Senanayake, S. P. & J. N. Green tea extract: chemistry, antioxidant properties and food applications–A review. J. Funct. Foods. 5, 1529–1541 (2013).
Adhami, V. M. & Mukhtar, H. Anti-oxidants from green tea and pomegranate for chemoprevention of prostate cancer. Mol. Biotechnol. 37, 52–57 (2007).
Dai, J. & Mumper, R. J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352 (2010).
Feitelson, M. A. et al. Elsevier,. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 35, S25–S54 (2015).
Yang, K. et al. Anti-tumor activity and the mechanism of a green tea (Camellia sinensis) polysaccharide on prostate cancer. Int. J. Biol. Macromol. 122, 95–103 (2019).
Benelli, R., Venè, R., Bisacchi, D., Garbisa, S. & Albini, A. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of Metallo and Serine proteases. (2002).
Bohm, B. Extraction, purification and identification of flavonoids. Introduction Flavonoids 200–204 (1998).
Huang, Y., Zhang, A., Lau, C. & Chen, Z. Y. Vasorelaxant effects of purified green tea epicatechin derivatives in rat mesenteric artery. Life Sci. 63, 275–283 (1998).
Vijaya, K., Ananthan, S. & Nalini, R. Antibacterial effect of theaflavin, polyphenon 60 (Camellia sinensis) and Euphorbia hirta on Shigella spp.—a cell culture study. J. Ethnopharmacol. 49, 115–118 (1995).
Paveto, C. et al. Anti-Trypanosoma Cruzi activity of green tea (Camellia sinensis) catechins. Antimicrob. Agents Chemother. 48, 69–74 (2004).
Sanlier, N., Gokcen, B. B. & Altuğ, M. Tea consumption and disease correlations. Trends Food Sci. Technol. 78, 95–106 (2018).
Katada, S. et al. Effect of tea catechins with caffeine on energy expenditure in middle-aged men and women: a randomized, double-blind, placebo-controlled, crossover trial. Eur. J. Nutr. 59, 1163–1170 (2020).
Cavanagh, H. M. A. & Wilkinson, J. M. Biological activities of lavender essential oil. Phytother. Res. 16, 301–308 (2002).
Woronuk, G., Demissie, Z., Rheault, M. & Mahmoud, S. Biosynthesis and therapeutic properties of Lavandula essential oil constituents. Planta Med. 77, 7–15 (2011).
Perrucci, S. et al. The activity of volatile compounds from Lavandula angustifolia against Psoroptes cuniculi. Phytother. Res. 10, 5–8 (1996).
Ignatowicz, S. Powdered herbs of the mint family (Lamiaceae) as insect repellents for protection of stored wheat grain. (1997).
Plarre, R. et al. Effects of oil of cloves and citronellol, two commercially available repellents, against the webbing clothes moth Tineola Bisselliella Hum.(Lepidoptera: Tineidae). Anz. Schädl. Pflanzenschutz Umweltschutz. 70, 45–50 (1997).
Hori, M. Repellency of Rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol. 24, 1425–1432 (1998).
O’Brien, D. J. Treatment of psoroptic mange with reference to epidemiology and history. Vet. Parasitol. 83, 177–185 (1999).
Ariana, A., Ebadi, R. & Tahmasebi, G. Laboratory evaluation of some plant essences to control Varroa destructor (Acari: Varroidae). Exp. Appl. Acarol. 27, 319–327 (2002).
Mrabti, H. N. et al. Integrative herbal treatments of diabetes in Beni mellal region of Morocco. J. Integr. Med. 17, 93–99 (2019).
Rtibi, K. et al. Chemical constituents and Pharmacological actions of Carob pods and leaves (Ceratonia siliqua L.) on the Gastrointestinal tract: A review. Biomed. Pharmacother. 93, 522–528 (2017).
Yao, N. et al. In vitro evaluation of lavandula angustifolia essential oil on anti-toxoplasma activity. Front. Cell. Infect. Microbiol. 11, 755715 (2021).
Rai, V. K. et al. Anti-psoriatic effect of Lavandula angustifolia essential oil and its major components Linalool and Linalyl acetate. J. Ethnopharmacol. 261, 113127 (2020).
Boyko, O. & Brygadyrenko, V. Nematicidal activity of essential oils of medicinal plants. Folia Oecol. 48, 42–48 (2021).
Moon, T., Wilkinson, J. M. & Cavanagh, H. M. A. Antiparasitic activity of two Lavandula essential oils against Giardia duodenalis, Trichomonas vaginalis and Hexamita inflata. Parasitol. Res. 99, 722–728 (2006).
Malekifard, F. & Keramati, F. Investigation of the effects of Ceratonia silique extract on protoscolexes of hydratid cyst in vitro. Armaghane Danesh. 23, 69–79 (2018).
Malekifard, F., Tavassoli, M. & Alimoradi, M. In vitro assessment of anti-Trichomonas effects of Zingiber officinale and Lavandula angustifolia alcoholic extracts on Trichomonas gallinae. Vet. Res. Forum 12, 95 (2021).
Karimi, P., Malekifard, F. & Tavassoli, M. Medicinal plant essential oils as promising Anti-Varroa agents: oxidative/nitrosative screens. S. Afr. J. Bot. 148, 344–351 (2022).
Gholami, S. H., Rahimi-Esboei, B., Ebrahimzadeh, M. A. & Pourhajibagher, M. Vitro effect of Sambucus ebulus on scolices of hydatid cysts. Eur. Rev. Med. Pharmacol. Sci. 17, 1760–1765 (2013).
Vuitton, D. A. The WHO informal working group on echinococcosis. Coordinating board of the WHO-IWGE. Parassitologia 39, 349–353 (1997).
Mahmoudvand, H. et al. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int. J. Surg. 12, 399–403 (2014).
Moazeni, M. & Nazer, A. In vitro effectiveness of Garlic (Allium sativum) extract on scolices of hydatid cyst. World J. Surg. 34, 2677–2681 (2010).
Malekifard, F. & Keramati, F. Susceptibility of Protoscoleces of hydatid cyst to various concentrations of oak gall (quercus infectoria olivier) extract at different exposure times in vitro. Zahedan J. Res. Med. Sci. 20 (2018).
Almalki, E., Al-Shaebi, E. M., Al-Quarishy, S., El-Matbouli, M. & Abdel-Baki, A. A. In vitro effectiveness of Curcuma longa and Zingiber officinale extracts on Echinococcus Protoscoleces. Saudi J. Biol. Sci. 24, 90–94 (2017).
Abdel-Baki, A. A. S., Almalki, E., Mansour, L. & Al-Quarishy, S. In vitro scolicidal effects of Salvadora persica root extract against protoscolices of Echinococcus granulosus. Korean J. Parasitol. 54, 61 (2016).
Norouzi, R., Hejazy, M., Azizi, D. & Ataei, A. Effect of Taxus baccata L. Extract on hydatid cyst protoscolices in vitro. Arch. Razi Inst. 75, 473 (2020).
Moazeni, M. & Roozitalab, A. High scolicidal effect of Zataria multiflora on protoccoleces of hydatid cyst: an in vitro study. Comp. Clin. Path. 21, 99–104 (2012).
Al Qaisi, Y. T., Khleifat, K. M. & Oran, S. A. Inhibitory Effects of Some Jordanian Medicinal Plants on In Vitro Viability of Protoscolices of Hydatid Cysts. Trop. J. Nat. Prod. Res. (TJNPR) 5, 707–714 (2021).
Al-khlifeh, E., Saidat, N., Khleifat, K. & Al Qaisi, Y. Phytochemical profile and in vitro Protoscolicidal effects of Juniperus phoenicea L., Calotropis procera (Aiton) dryand, and Artemisia judaica L. against Echinococcus granulosus cysts. J. Pharm. Pharmacogn. Res. 11, 635–650 (2023).
Al Qaisi, Y. T. et al. The in vivo preventive effect of some medicinal plant extracts on the development of hydatid cyst infection. Asia Pac. J. Sci. Technol. 28 (2023).
Al-Shaibani, I. R. M., Phulan, M. S., Arijo, A. & Qureshi, T. A. Ovicidal and larvicidal properties of Adhatoda vasica (L.) extracts against Gastrointestinal nematodes of sheep in vitro. Pak. Vet. J. 28, 79–83 (2008).
Zips, D., Thames, H. D. & Baumann, M. New anticancer agents: in vitro and in vivo evaluation. Vivo (Brooklyn). 19, 1–7 (2005).
Sangster, N. Pharmacology of anthelmintic resistance. Parasitology 113, S201–S216 (1996).
Panseeta, P. et al. Antiplasmodial and antimycobacterial cyclopeptide alkaloids from the root of Ziziphus mauritiana. Phytochemistry 72, 909–915 (2011).
Dodangeh, S. et al. The amoebicidal activity of Ziziphus vulgaris extract and its fractions on pathogenic Acanthamoeba trophozoites and cysts. Trop. Biomed. 34, 127–136 (2017).
Chung, K. T., Wong, T. Y., Wei, C. I., Huang, Y. W. & Lin, Y. Tannins and human health: a review. Crit. Rev. Food Sci. Nutr. 38, 421–464 (1998).
Rahimi, B., Malekifard, F. & Esmaeilnejad, B. In vitro anti-Trichomonas gallinae effects of Ziziphus vulgaris L. and Camellia sinensis (L.) Kuntze extracts. Vet. Med. Sci. 10, e1432 (2024).
Allahyari, M., Malekifard, F. & Yakhchali, M. Anthelmintic effects of some medicinal plants on different life stages of Fasciola hepatica: evidence on oxidative stress biomarkers, and DNA damage. PLoS Negl. Trop. Dis. 18, e0012251 (2024).
Malekifard, F. & Keramati, F. Susceptibility of Protoscoleces of hydatid cyst to various concentrations of oak gall (quercus infectoria olivier) extract at different exposure times in vitro. Zahedan J. Res. Med. Sci. 20 (2018).
Moazeni, M., Saharkhiz, M. J., Hoseini, A. A. & Alavi, A. M. In vitro scolicidal effect of Satureja khuzistanica (Jamzad) essential oil. Asian Pac. J. Trop. Biomed. 2, 616–620 (2012).
Erzurumlu, K. et al. Effect of albendazole sulfoxide solution on the scolices and the hepatobiliary system. Eur. Surg. Res. 30, 433–438 (1998).
Caglar, R. et al. In vitro effectiveness of different chemical agents on scolices of hydatid cyst. J. Investig. Surg. 21, 71–75 (2008).
Besim, H., Karayalcin, K., Hamamci, O., Güngör, C. & Korkmaz, A. Scolicidal agents in hydatid cyst surgery. HPB Surg. 10, 347–351 (1998).
Kayaalp, C. et al. Hypertonic saline in hydatid disease. World J. Surg. 25, 975–979 (2001).
Sadjjadi, S. M., Zoharizadeh, M. R. & Panjeshahin, M. R. In vitro screening of different Allium sativum extracts on hydatid cysts Protoscoleces. J. Investig. Surg. 21, 318–322 (2008).
Moazeni, M., Larki, S., Oryan, A. & Saharkhiz, M. J. Preventive and therapeutic effects of Zataria multiflora methanolic extract on hydatid cyst: an in vivo study. Vet. Parasitol. 205, 107–112 (2014).
Kavoosi, G. & Purfard, A. M. Scolicidal effectiveness of essential oil from Zataria multiflora and Ferula assafoetida: disparity between phenolic monoterpenes and disulphide compounds. Comp. Clin. Path. 22, 999–1005 (2013).
Mahmoudvand, H. et al. Scolicidal effects of black Cumin seed (Nigella sativa) essential oil on hydatid cysts. Korean J. Parasitol. 52, 653 (2014).
Rouhani, S., Salehi, N., Kamalinejad, M. & Zayeri, F. Efficacy of Berberis vulgaris aqueous extract on viability of Echinococcus granulosus protoscolices. J. Investig. Surg. 26, 347–351 (2013).
de Silva, L. C. M. A. et al. Use of Lavandula angustifolia essential oil as a complementary therapy in adult health care: A scoping review. Heliyon 9 (2023).
Hajihossein, R., Eslamirad, Z., Rafiei, F., Naderi, G. & Assadi, M. Anti-Acanthamoeba effect of Camellia sinensis extract (black and green tea) in vitro. J. Med. Plants. 19, 163–169 (2020).
Tadesse, A., Hymete, A., Bekhit, A. A. & Mohammed, S. F. Quantification of total polyphenols, catechin, caffeine, L-theanine, determination of antioxidant activity and effect on antileishmanial drugs of Ethiopian tea leaves extracts. Pharmacognosy Res. 7, S7 (2015).
Parvez, M. A. K. et al. Antibacterial activities of green tea crude extracts and synergistic effects of epigallocatechingallate (EGCG) with gentamicin against MDR pathogens. Heliyon 5 (2019).
dos Reis, M. B. G., Manjolin, L. C., Maquiaveli, C. C., Santos-Filho, O. A. & da Silva, E. R. Inhibition of leishmania (Leishmania) amazonensis and rat arginases by green tea EGCG,(+)-catechin and (–)-epicatechin: a comparative structural analysis of enzyme-inhibitor interactions. PLoS One. 8, e78387 (2013).
Inacio, J. D. F., Canto-Cavalheiro, M. M. & Almeida-Amaral, E. E. In vitro and in vivo effects of (–)-epigallocatechin 3-O-gallate on leishmania amazonensis. J. Nat. Prod. 76, 1993–1996 (2013).
Zhong, R. Z., Li, H. Y., Sun, H. X. & Zhou, D. W. Effects of supplementation with dietary green tea polyphenols on parasite resistance and acute phase protein response to Haemonchus contortus infection in lambs. Vet. Parasitol. 205, 199–207 (2014).
Inacio, J. D. F., Gervazoni, L., Canto-Cavalheiro, M. M. & Almeida-Amaral, E. E. The effect of (-)-epigallocatechin 3-O-gallate in vitro and in vivo in Leishmania braziliensis: involvement of reactive oxygen species as a mechanism of action. PLoS Negl. Trop. Dis. 8, e3093 (2014).
Aboulaila, M., Yokoyama, N. & Igarashi, I. Inhibitory effects of (-)-epigallocatechin-3-gallate from green tea on the growth of Babesia parasites. Parasitology 137, 785–791 (2010).
Jang, S. I. et al. Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet. Parasitol. 144, 172–175 (2007).
Fakae, L. B., Stevenson, C. W., Zhu, X. Q. & Elsheikha, H. M. In vitro activity of Camellia sinensis (green tea) against trophozoites and cysts of Acanthamoeba castellanii. Int. J. Parasitol. Drugs Drug Resist. 13, 59–72 (2020).
Ali, R. et al. A systematic review of medicinal plants used against Echinococcus granulosus. PLoS One. 15, e0240456 (2020).
Baqer, N. N., Khuder, M. H. & Amer, N. Antiprotoscolices effects of ethanolic extract of Zingiber officinale against Echinococcus granulosus invitro and invivo. Int. J. 2, 59–68 (2014).
Acknowledgements
This paper has been extracted from Dr Seyed Mohammad Mehdizadeh’s Doctor of Veterinary Medicine (DVM) thesis carried out at Urmia University. The authors would like to sincerely thank the members of the Faculty of Veterinary Medicine and Urmia University Research Council for their approval and support of this research.
Funding
The Research Council of Urmia University has financially supported this research.
Author information
Authors and Affiliations
Contributions
SMM, FM, and BE contributed to the conception, design, data collection, statistical analysis, and drafting of the manuscript. All authors approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All of the protocols were approved by the Faculty of Veterinary Medicine’s Committee on the Ethics of Animal Experiments at Urmia University (IR-UU-AEC-3/83). Every procedure was carried out in accordance with the relevant laws and standards. The study was conducted in compliance with the ARRIVE standards. The owner(s) of the animals gave their informed consent for us to use them in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mehdizadeh, S.M., Malekifard, F. & Esmaeilnejad, B. In vitro evaluation of the susceptibility of Echinococcus granulosus hydatid cysts to lavender and green tea extracts. Sci Rep 15, 21778 (2025). https://doi.org/10.1038/s41598-025-06979-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06979-7