Abstract
Understanding the intricate relationship between peripheral blood immune profiles and the inflammatory environment within affected tissues is pivotal for uncovering mechanisms driving autoimmune diseases. This study aimed to characterize CD4+ T cell subsets in peripheral blood that mirror the immunological activation of labial salivary glands (LSG) infiltrating T cells in primary Sjögren’s disease (pSjD). Using multicolor flow cytometry and T cell receptor (TCR) sequencing, we identified CXCR3+CXCR5+ T follicular helper 1 (Tfh1) cells as significantly elevated in the circulation of pSjD patients and even more prominently increased in the LSG, with blood PD-1+ICOS+ Tfh1 cells positively correlating with titers of antinuclear, anti-SS-A, and anti-SS-B antibodies. In contrast, CXCR3+CXCR5− Th1 cells were enriched in LSG but reduced in circulation. TCR analysis demonstrated that circulating Tfh1 cells shared a notable clonal similarity with LSG T cells. In the LSG, cytokines such as IL-6, IL-12, IL-21, and TGF-β were upregulated, with TGF-β and TCR recognition promoting Tfh1 differentiation. This microenvironment led to increased production of IL-2, TNF-α, and IL-21, promoting the expansion of CD19+CD38+ B cells. These findings support the notion that circulating activated Tfh1 cells partially mirror glandular T cell activation and highlight TGF-β as a driver of Tfh1 differentiation, presenting a potential therapeutic target.
Similar content being viewed by others
Introduction
Sjögren’s disease (SjD) is an autoimmune disease characterized by lymphocytic infiltration into the lacrimal and salivary glands (SG) leading to chronic inflammation at the exocrine gland1,2. Abnormal response to autoantigen by both T and B cells driven by interactions with local SG epithelial cells, plays a key role in creating the vicious cycle of autoimmune responses in pSjD1,3. In the disease’s earlier phase, lymphocytic infiltration of the SG is dominated by CD4+ αβT cells, whereas B cells accumulate as the disease progresses, suggesting that CD4+ T cells play a pathogenic role in driving B cell hyperactivity4. Thus, understanding how CD4+ T cells expand in the SG environment could lead to the discovery of a promising T cell targeted therapy for use in the early phase of the disease.
Recent studies have identified clonally expanded CD4+ T cells infiltrating the labial SG (LSG) in primary SjD (pSjD)5, with identical autoantigen-reactive clones in both SG and blood6. This suggests that a subset of circulating CD4+ T cells may originate from the SG, reflecting T cell-driven pathogenicity observed in tissue inflammation, where local factors drive distinct CD4+ T cell responses7,8. Technological advances have enabled the analysis of rare cell populations in inflammatory sites, such as tissue-resident T cells, which has given rise to interest in investigating SG T cells. However, the precise relationship between inflammatory organs and peripheral immune environments remains to be elucidated.
The pathogenic phenotypes and functions of CD4+ T cells in both SG and blood have also been investigated with SjD. Of particular interest are T follicular helper cells (Tfh), a T cell subset specialized in helping B cells8,9. As in other B cell-mediated autoimmune diseases, the frequency of Tfh cells is increased in the blood and SG in SjD4,10,11, with the frequency of activated Tfh correlating with disease activity score12,13, which indicates their pathogenic role as a primer for B cell activation. Interestingly, tissue-resident T cells are thought to be related to Tfh cells due to the upregulation of common transcriptional programs14, supporting their key role in initiating immune response at local inflammatory sites. Although the involvement of Tfh subsets, such as Tfh1, Tfh2, Tfh17, and T follicular regulatory cells (Tfr), in SjD has been well documented4,8,12,13,15, the functional distinctions among these subsets and their association with the inflammatory environment remain unclear.
In the current study, we aimed to characterize the immunological profile of CD4+ T cell subsets in peripheral blood that may recapitulate aspects of glandular T cell activation in the LSG of patients with pSjD. We further investigated the role of LSG environmental factors in promoting these CD4+ T cell subsets and their functional characteristics, as well as the immunological responses to glucocorticoid therapy in patients with progressive systemic organ involvement. These insights should deepen our understanding of the pathophysiology of T-cell-driven immune responses in pSjD.
Materials and methods
Study participants
Blood samples were collected from 30 patients with pSjD, and LSG samples were obtained from 18 of them at the time of diagnosis, all meeting the 2016 American College of Rheumatology/ European League Against Rheumatism (ACR/EULAR) classification criteria16. Among these, LSG samples from 7 patients were used for FACS analysis and 11 patients for qPCR analysis. None had prior glucocorticoids or immunosuppressant treatment. For 7 patients who started treatment after diagnosis and had consented to follow up sampling, additional blood samples were collected one month after treatment initiation. For the in vitro analysis, patients were selected from the same cohort of 30 pSjD patients based on the availability of sufficient blood volume at the time of diagnosis. Control samples included peripheral blood from 20 age- and sex-matched healthy controls (HC) and 9 LSG samples. The study followed the Declaration of Helsinki with ethical approval from the University of Tsukuba Hospital (approval number: H24-164). Written informed consent was obtained from all participants. Patient characteristics are detailed in Supplementary Table S1. Anti-SS-A and SS-B antibodies for all pSjD patients were measured using Ouchterlony double-immunodiffusion (DID) assay, with a cutoff ratio of 1.0.
FACS staining of peripheral blood and LSG
Peripheral blood mononuclear cells (PBMCs) were cryopreserved and later analyzed in multiple samples simultaneously. LSG was processed using collagenase D, as previously described17. Staining panels are listed in Supplementary Table S2. Representative flow cytometry data and gating strategy are shown in Supplementary Fig. S1-S3.
TCR sequence of LSG and peripheral blood T cell subsets
Matched LSG and blood samples for TCR sequencing were obtained from three pSjD patients. The clinical characteristics of these patients are provided in Supplementary Table S3. LSG samples were preserved in RNAlater Stabilization Solution (AM7020, Invitrogen, Thermo Fisher Scientific Waltham, USA), and total RNA was extracted using TRIzol (15,596,018, Thermo Fisher Scientific)6. T cell subsets (Th1, Th17, Tfh1, Tfh2, Tfh17, Treg) were sorted using MoFlo XDP cell sorter (Beckman Coulter, Pasadena, USA) based on surface markers (15,18; Supplementary Table S4, Supplementary Fig. S1). Data processing of TCR sequencing was performed as previously described6 and details are provided in Supplementary Data S1. T-cell clones were defined as TCR reads with the same TCR V segment, J segment, and CDR3 nucleotide sequence. Overlapping clones were defined as identical TCR sequences with the same TCR V segment, J segment and CDR3 nucleotide sequence, detected in different samples. To assess repertoire similarity, we calculated the F2 score, which is the clonotype-wise sum of the geometric means of overlapping clonotype frequencies.
Quantitative real-time reverse-transcription polymerase chain reaction (RT-qPCR) of Total LSG
LSG samples were preserved in RNAlater, and RNA was extracted using Isogen (Nippon Gene, Tokyo, Japan). Complementary DNA (cDNA) was synthesized using a Prime Script reverse transcriptase master mix (Takara Bio, Kyoto, Japan). RT-qPCR was performed using TB Green Premix Ex Taq II (Takara Bio). The primers are listed in Supplementary Table S5.
Immunofluorescence staining
LSG tissue sample were fixed in formalin, embedded in paraffin, and sectioned. The sections were deparaffinized and underwent antigen retrieval using a microwave. The sample were then incubated with primary antibodies under the following conditions: anti-CD4 (clone: EPR6855, Abcam) for 2 h at room temperature, anti-CD20 (clone: EP459Y, Abcam) overnight at 4 °C, and anti-TGF-β (clone: EPR21143, Abcam) overnight at 4 °C. After primary antibody incubation, the sections were washed and incubated with secondary antibodies using the Opal Anti-Ms + Rb HRP (AKOYA Biosciences). Fluorescence signals were developed using Opal 3-Plex Kit (AKOYA Biosciences), applying Opal 520, Opal 570, Opal 690 and DAPI. All images of stained sections were obtained using an all-in-one fluorescence microscope (BZ-X800, KEYENCE, Osaka, Japan).
Tfh differentiation in vitro
CD4+ T cells were isolated from PBMCs using microbeads (catalog no. 130-045-101, Miltenyil Biotech, Bergisch Gladbach, Germany) with each sample purity > 95%. Isolated CD4+ T cells were cultured with T-Activator CD3/28 beads (Thermo Fisher Scientific) and cytokines (TGF-β 5 ng/ml, IL-6 25 ng/ml, or IL-12 1 ng/ml) for five days19. After culture, T cell subsets were analyzed by FACS.
Cytokine production of CD4+ T cells
Five days after culturing the CD4+ T cells derived from the peripheral blood of patients with pSjD using Dynabeads Human T-Activator CD3/28 and TGF-β 5 ng/ml, culture supernatants were analyzed for cytokine using the LEGENDplex Human Th cytokine panel (BioLegend). Data were processed using LEGENDplex software.
Co-culture of CD4+ T cells with naïve B cell
CD4+ T cells from 7 pSjD patient samples as well as 4 HC samples were co-cultured with FACS-sorted naïve B cells (CD19+CD27-IgD-) obtained from a healthy donor (Supplementary Fig. S4) under stimulation with TCR-beads and TGF-β 5 ng/ml. After five days, cells were stained with anti-CD19, anti-CD20, anti-CD27, anti-IgD, anti-CD38, and 7AAD for flow cytometry analysis (Supplementary Table S2, Supplementary Fig. S5).
Statistics
Statistical differences between groups were evaluated using the Mann–Whitney U test, Kruskal–Wallis test, Wilcoxon test, or Friedman test, as appropriate. Categorical values were compared using Fisher’s exact test, and correlations were assessed by Spearman r correlation analysis. P values less than 0.05 were considered significant. All statistics were analyzed using Graph Pad Prism version 9.5.0 (GraphPad Software, Boston, USA).
Results
CXCR3+ Tfh1 cells are expanded in both peripheral blood, and LSG, and blood PD-1+ICOS+ cTfh1 positively correlates with anti-SSA and anti-SSB antibodies
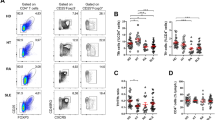
First, to gain insight into the T cell subsets of both peripheral blood and LSG of patients with pSjD, we analyzed blood and LSG T cell subset populations (Supplementary Fig. S1 and S2). Although various T cell subsets have been implicated in the pathogenesis of SjD4, evidence for a T cell population showing common characteristics in both blood and LSG remains inconclusive. When analyzing the peripheral blood T cells of our cohort, consistent with several previous reports4,10,11, we observed a significant increase in circulating effector Tfh (cTfh) population compared to age- and sex-matched controls (Tfh1: p < 0.0001, Tfh2: p < 0.0001, Tfh17: p = 0.0021). Meanwhile, cTh1 was significantly decreased in pSjD (p < 0.0001) (Fig. 1a).
Characteristics of T cell subsets in peripheral blood and LSG of pSjD. (a) Peripheral blood obtained from patients with pSjD (n = 30), and HCs (n = 20) were analyzed using flow cytometry. In peripheral blood, cTh1 was significantly decreased (p < 0.0001) and all the cTfh subsets were significantly increased compared with healthy controls. (Tfh1: p < 0.0001, Tfh2: p < 0.0001, Tfh17: p = 0.0021 by M-W U test). (b) LSG samples with matched peripheral blood from pSjD (n = 7) were analyzed. LSG T cells were composed of nearly all memory phenotypes (naïve T cells: p = 0.01, memory T cells: p = 0.01, by Wilcoxon test). (c) Among the increasing memory CD4+ T cells, both CXCR3+ Th1 cells and Tfh1 cells significantly increased in LSG compared with matched blood samples (Th1: p = 0.03, Tfh1: p = 0.01, by Wilcoxon test). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To deepen our understanding of the relationship between blood and LSG T cells, we analyzed paired blood and LSG samples obtained from the same patient with pSjD. This paired analysis showed that LSG T cells were mainly composed of memory T cells, which were significantly increased in LSG compared to blood (p = 0.01) (Fig. 1b). Among these memory T cell subsets, the populations of CXCR3+ Tfh1 cells and CXCR3+ Th1 cells were significantly expanded in LSG than in blood, highlighting the expansion of CXCR3+ cells in the local inflammatory environment (Tfh1: p = 0.015, Th1: p = 0.03) (Fig. 1c). In contrast, their expansion patterns differed in circulation, with Tfh1 cells increasing and Th1 cells decreasing. These observations prompted us to consider that Tfh1 cells, which exhibit populational expansion in both blood and LSG, may represent the most reflective subset of LSG-infiltrating T cells within the circulatory system.
Based on these results, we analyzed PD-1 and ICOS expression as activation markers of circulating Tfh subsets in blood and examined their correlation with clinical features. We found that the percentage of PD-1+ICOS+ cells was highest in CXCR3+ Tfh1, compared with Tfh2, and Tfh17 in patients with pSjD (p < 0.0001) (Fig. 2a). Not only was the frequency of PD-1+ICOS+ cells highest in Tfh1 cells, but it also positively correlated with titers of antinuclear antibody (p = 0.016), anti-SS-A (p = 0.003), and anti-SS-B (p = 0.028) antibodies (Fig. 2b, Supplementary Table S6). In addition, a significantly higher frequency of PD-1+ICOS+ cTfh1 cells was observed in the higher focus score group (focus score ≧2) compared to the lower score group (focus score ≦1) (Fig. 2c).
Clinical relevance of PD-1+ICOS+ Tfh1 cells in patients with pSjD. (a) Among the increasing Tfh subsets observed in pSjD (Fig. 1), %PD-1+ICOS+ cells were significantly increased among the CXCR3+ Tfh1 cells. (P < 0.0001) *P < 0.05, by K-W test. (b) %PD-1+ICOS+ Tfh1 cells showed a significant positive correlation with autoantibody titers, such as antinuclear antibody, anti SS-A antibody, anti SS-B antibody (ANA: P < 0.02*, SS-A: p = 0.00*, SS-B: p = 0.03*, by Spearman r test). (c) The percentage of cTfh1 cells among memory CD4+ T cells did not differ between the lower focus score group (focus score: 0–1) and the higher focus score group (focus score: 2–4). The frequency of PD-1+ICOS+ cells in cTfh1 cells was significantly higher in the higher focus score group than in the lower focus score group (%cTfh1 cells: p = 0.59, %PD-1+ICOS+ in cTfh1 cells: p = 0.01, by M-W U test). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
These findings led us to hypothesize that circulating CXCR3+ Tfh1 cells represent a subset reflective of LSG-infiltrating T cell activity. To explore this, we further analyzed changes in blood T cell subsets in seven patients before and one month after initiating glucocorticoid treatment for progressive systemic organ involvement. Following treatment, a significant decrease in the CXCR3+ population, including Tfh1, Th1, and Th17.1 cells, was observed (Supplementary Fig. S6). Although the effect was not exclusive to Tfh1 cells, these results collectively suggest the idea that CXCR3+ Tfh1 cells could serve as one of the indicators of the inflammatory etiology in pSjD.
TCR sequence of peripheral blood CD4+ T cells and LSG T cells indicates cTh1 and cTfh1 subsets as notable overlapping populations between blood and LSG
Next, beyond the paired populational features, to validate whether circulating Tfh1 cells effectively reflect the etiology of T-cell driven inflammation in the tissue, we analyzed the TCR repertoire of sorted peripheral blood circulating T cell subsets (cTh1, cTh17, cTreg, cTfh1, cTfh2, and cTfh17, Supplementary Table S4) together with paired LSG infiltrating T cells obtained from three pSjD patients. Previous findings indicated the presence of LSG-derived autoantigen-reactive T cells in circulation6, prompting us to focus on CD4+ T cell subsets in the blood that shared identical clones with LSG T cells.
Analysis of T cell repertoire clonality revealed variations in its extent among patients. Nonetheless, across all three patients, LSG infiltrating T cells, cTh1, and cTfh1 with cTfh2 cells among cTfh cell subsets appeared as highly expanded T cell clones (Fig. 3a, Supplementary Fig. S7, Supplementary Table S7). We identified T cell clones shared between LSG and at least one of the circulating T cell subsets (hereafter referred to as “overlapping clones”), which were more frequent in cTh1, cTfh1, and cTfh2 cells than in cTh17, cTfh17, or Treg cells (Fig. 3b, Supplementary Fig. S8). Additionally, expanded T cell clones in LSG were more likely to overlap with clones in cTh1 and cTfh1 subsets (Supplementary Fig. S9). The top 5 overlapping clones expanded in LSG were predominantly observed among blood cTh1, cTfh1, and cTfh2 subsets than in cTh17, cTfh17, or Treg cells, indicating preferential expansion within specific circulating T cell subtypes (Supplementary Table S8). Since clone frequency reflects the degree of overlap in a single direction -either from blood to LSG or vice versa- and the frequency varied depending on the direction in certain subsets, we further assessed overall similarity using a bidirectional repertoire similarity score. The heatmap of the repertoire similarity score indicated that the T cell repertoire of LSG was more similar to cTh1 and cTfh1 cells. While patient 1 also showed similarity with cTfh2 cells, all three patients consistently exhibited notable similarity between LSG and both cTh1 and cTfh1 cells (Fig. 3c). Taken together, our analysis indicates that Tfh1 and Th1 cells in the circulation appear to share features with LSG-infiltrating T cells, suggesting their involvement in antigen-driven differentiation.
Variation in overlap and similarity between blood T cell subsets and LSG T cell clones. (a) LSG and FACS sorted blood T cell subsets were obtained from three patients with pSjD (n = 3) and TCR sequencing was performed. (b) Rank and frequencies of top 100 T cell clones are shown. X-axis represents the rank of clones in log10 scale. Y-axis represents the frequency of clones in log10 scale. The extent of clonal expansion varied among patients, with Patient 1 showing the most clonal expansion and the highest frequency of expanded clones. Across all three patients, LSG infiltrating T cells (black), cTh1(red), and cTfh1(yellow) with cTfh2(blue) cells among cTfh subsets appeared as highly expanded T cell clones, with a sharp decline observed from the higher ranking clone to lower ranking clones. (c) Frequency of the LSG major T cell clones (Top50) overlapping with each blood T cell subset. Their frequencies in the blood subset (top) or in the LSG (bottom) were plotted. cTh1, cTfh1, and cTfh2 cells have more overlapping T cell clones with LSG T cells than other subsets. (gray color: Th1, Th17, black color: Tfh1, Tfh2, Tfh17, white color: Treg). (d) Heatmap of repertoire similarity between LSG and blood T cell subsets. Repertoire similarity was calculated as F2 score: the sum of geometric means of overlapping clone’s frequencies in two TCR repertoires (Reference 46, Supplementary method). The values on the heatmap indicate each F2 score. LSG T cells, cTh1 cells, and cTfh1 cells were clustered in a closer group compared to the other T cell subsets across all three patients.
Collectively, these observations indicate that the increase in activated Tfh1 cells in the blood may reflect local immune activation and could serve as a potential indicator of chronic inflammatory processes within the LSG.
TCR stimulation together with TGF-β, which is highly expressed in LSG in pSjD, induced significant upregulation of CXCR5+ Tfh cells, especially the CXCR3+CXCR5+ Tfh1 population in pSjD
We next sought to elucidate the mechanism of Tfh1 expansion in the SG environment. To assess whether Tfh-associated genes and differentiation-promoting cytokines19 are enriched in pSjD LSG, we conducted qPCR analysis. The expression of CXCR5 was significantly higher in pSjD LSG compared to HC (p = 0.0005) (Fig. 4a). Additionally, cytokines associated with Tfh differentiation, including IL-6, IL-12, IL-21, and TGF-β19,20, were all elevated in pSjD LSG compared to HC (IL-6: p = 0.009, IL-12: p = 0.001, IL-21: p = 0.004, TGF-β: p < 0.0001) (Fig. 4a). Among these cytokines, only TGF-β showed a significant positive correlation with CXCR5 expression in the pSjD LSG (p = 0.0029), as well as with ICOS expression, another critical Tfh marker (p = 0.020) (Fig. 4b). Furthermore, immunofluorescence analysis revealed that TGF-β expression was predominantly localized in areas densely infiltrated by CD4+ T cells. Some cells were double-positive for CD4 and TGF-β, suggesting that a subset of CD4+ T cells may serve as a local source of TGF-β (Fig. 4c).
Increased expression of CXCR5 and Tfh-related cytokines in pSjD LSG, with correlation to TGF-β levels. (a) The mRNA expression levels of CXCR5, IL-6, IL-12, IL-21, and TGF-β were significantly increased in pSjD LSG (n = 11) when compared with HCs (n = 9). (CXCR5: p = 0.0005, IL-12: p = 0.001, IL-21: p = 0.004, TGF-β: P < 0.0001, by M-W U test). (b) Correlation of the expression level of CXCR5 with each cytokine. TGF-β was the only cytokine that showed a significant positive correlation with both CXCR5 and ICOS expression in pSjD LSG (CXCR5: p = 0.0029, ICOS: p = 0.020 by Spearman r test). (c) Immunofluorescence of a representative pSjD LSG sample stained for CD4 (green), CD20 (pink), TGF-β (red), and DAPI. TGF-β expression was predominantly found in areas densely infiltrated by CD4+ T cells, and partial colocalization of CD4 and TGF-β was observed. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To evaluate the effects of these cytokines on Tfh differentiation, we cultured isolated CD4+ T cells from pSjD peripheral blood under CD3/28 stimulation with each cytokine. Of these conditions, TGF-β significantly increased CXCR5 expression (p = 0.0003) (Fig. 5a,b) and preferentially expanded CXCR3+ Tfh1 cells (p = 0.0018) (Fig. 5c). These findings suggest that TGF-β abundant environments, such as the LSG in patients with pSjD, potentially promote Tfh differentiation, particularly skewing toward the Tfh1 phenotype. Given that TGF-β receptor expression is upregulated in response to TGF-β signaling21, we further examined the expression levels of the TGF-β receptor across T cell subsets to assess the extent of TGF-β’s effects in pSjD. Our analysis revealed that TGF-β receptor expression was highest in the Tfh1 subset among all T cell subsets. While Tfh1 cells also exhibited the highest receptor expression in HC, overall TGF-β receptor levels were significantly elevated in most T cell subsets in pSjD compared to controls (Supplementary Fig. S10).
Defining Tfh1 differentiation condition using blood CD4+ T cells from patients with pSjD. (a) Representative FACS plot showing CD4+ T cells isolated from pSjD patient, cultured for 5 days under various conditions: 1) no stimulation, 2) TCR stimulation with CD3/28 beads, 3) TCR and IL-6, 4) TCR and IL-12, and 5) TCR + TGF-β. CXCR5 expression increased after TCR and TGF-β stimulation compared to other cytokines, especially increasing CXCR3 positive Tfh1 population. Gray-colored areas represent isotype control. (b) Statistical analysis of increasing CXCR5+ expression after stimulation of each cytokine showed a significant increase of CXCR5 expression after TCR and TGF-β stimulation. (pSjD: n = 5, non-stimulation vs. TCR + TGF-β stimulation: p = 0.0003, by Friedman test). (c) Among increasing Tfh subsets, CXCR3+ Tfh1 cells were significantly increased after TCR + TGF-β stimulation. (p = 0.0008, by Friedman test). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Taken together, our findings suggest that TGF-β enriched environment in pSjD LSG, with possible autoantigen recognition, promotes Tfh1 differentiation. These circulating Tfh1 cells retain features similar to those of LSG T cells, where ICOS+PD-1+ cTfh1 cells are associated with autoantibody production.
Cytokine production and effects on B cell development by pSjD CD4+ Tcells under Tfh1-polarizing conditions
After identifying CD3/28 and TGF-β stimulation as a Tfh1 polarizing condition in pSjD, we examined the cytokine-producing function of pSjD CD4+ T cells cultured under this condition. Multiplex assay analysis of the culture supernatant revealed a significant increase in the production of IL-2, TNF-α, and IL-21 (IL-2: p = 0.0046, IL-21: p = 0.029, TNF-α: p = 0.0001) (Fig. 6a). Since Tfh cells are specialized CD4+ T cells that interact with B cells8,9,10,11, we further assessed the impact of pSjD CD4+ T cells on B cell development under Tfh1-polarizing conditions. To distinguish B cell intrinsic factor, we co-cultured healthy control blood-derived naïve B cells with pSjD blood-derived CD4+ T cells under CD3/28 and TGF-β stimulation. This resulted in a significant decrease in naïve B cells (p = 0.001), upregulation of CD19 expression on B cells (p = 0.005), and an increase in the CD19+CD38+ B cell population (p = 0.002) (Fig. 6b, Supplementary Fig. S5). In contrast, CD4+ T cells from HC, had less pronounced effects, showing no significant increase in inflammatory cytokine production under TGF-β stimulation. Similarly, the effects on B cell maturation were nonspecific, with similar changes observed under TCR stimulation alone (Supplementary Fig. S11). These findings suggest that the TGF-β-driven effects were more pronounced in pSjD CD4+ T cells, likely due to their higher TGF-β receptor expression, as demonstrated earlier in this study. This reflects the TGF-β enriched environment in the LSG and highlights its potential role in promoting Tfh1 differentiation and affecting B cell responses in pSjD.
Functional analysis of CD4+ T cells cultured under Tfh1 differentiation condition, using blood CD4+ T cells from pSjD patient. (a) After culturing CD4+ T cells under TCR + TGF-β stimulation, culture supernatant was collected, and cytokine production was analyzed using LEGENDplex Human Th cytokine panel 12-plex (BioLegend) (n = 6). Further analysis of IL-21 was performed using ELISA. IL-21, IL-2, and TNF-α production significantly increased when TGF-β was added (IL-21: p = 0.029, IL-2: p = 0.0046, TNF-α: p = 0.0001). (b) Co-culture of CD4+ T cells obtained from patients with pSjD with FACS sorted naïve B cells obtained from a healthy control (n = 7). Under TCR + TGF-β stimulation, we observed a significant decrease in naïve B cells, an upregulation of CD19 MFI, and a significant increase in the percentage of CD19+CD38+ B cells. (Naïve B cells: p = 0.001, CD19 MFI: p = 0.005, CD19+CD38+ B cells: p = 0.002). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by Friedman test.
Discussion
Here, we explored the relationship between the T cell immune environment in peripheral blood and LSG tissue, focusing on the circulating CD4+ T cell subset that most closely mirrors the LSG environment. While the pathogenic role of CD4+ T cells in pSjD has been well established3,4,6,7,8, the precise link between blood and LSG T cells, as well as the underlying mechanisms shaping the immune profile, remains unclear. Our results provide valuable insights into the function and differentiation mechanisms of these CD4+ T cells, suggesting the possibility of monitoring local inflammatory processes through circulation.
In this study, we demonstrated the following three key findings. First, we observed a degree of similarity between Tfh1 cells in the blood and LSG-infiltrating T cells. Second, we identified TGF-β as a factor driving the expansion of Tfh1 cells in vitro, and this cytokine was also enriched in the LSG environment of pSjD patients. Third, we observed CD4+ T cells in the Tfh1 cell-differentiating environment secreting IL-21, IL-2, and TNF-α, which may influence B cell development.
Initially, we analyzed the paired expansion of CD4+ T cell subsets in peripheral blood and LSG, incorporating TCR repertoire analysis to identify specific circulating CD4+ T cells resembling LSG T cells. Our findings revealed that peripheral CXCR3+CXCXR5+ Tfh1 cells shared a notable degree of similarity with LSG T cells, demonstrated by parallel population expansion and higher similarity scores of overlapping clones. Although some heterogeneity was observed in the TCR repertoire analysis, such as Tfh2 cells in patient 1 with a lower focus score, our data indicated repertoire similarities between LSG T cells and both CXCR3+ cTfh1 and cTh1 cells. Alongside cTfh1 cells, CXCR3+CXCR5- cTh1 cells in blood also possessed expanded T cell clones with a similar repertoire to LSG T cells. This observation suggests a potential skewing towards CXCR3+ memory CD4+ T cells at the inflammatory site. Previous studies on Th1 and Tfh cells indicate that these subsets develop in parallel during the early differentiation, and their fate is determined by TCR signal strength and microenvironment22,23,24,25,26. Given that some tissue-resident memory T cells (Trm) are known to express increased CXCR3 in response to the inflammatory environment27, both CXCR3+ non-Tfh and Tfh cells may expand in the inflammatory LSG environment when exposed to LSG-derived autoantigens such as Ro/SSA, La/SSB, and M3 muscarinic acetylcholine receptor1,6,28. In addition, Th1 cells are known to be more susceptible to TCR-activation-induced cell death29, which might explain why Th1 cells are clonally expanded while their population decreases in circulation at the same time. Taken together, although our data are limited in definitively establishing a direct relationship between circulating and tissue-resident T cells, our findings suggest that the LSG environment supports the expansion of antigen-specific Th1 and Tfh1 cells, some of which may be reflected in the circulating Tfh1 cells exhibiting similar features to LSG T cells.
Second, we elucidated the role of TGF-β, a cytokine enriched in the LSG environment, as a factor promoting the expansion of Tfh1 cells under TCR stimulation in vitro. The increase in Tfh cell populations and transcriptional profiles has been well documented in pSjD, observed in both blood and LSG4,8,9,10,11,12,13,30; however, the mechanisms underlying these phenomena and the specific contributions of each Tfh subset have remained unclear. In this study, our findings highlight TGF-β as a cytokine driving the in vitro expansion of Tfh cells, particularly the Tfh1 cells. Consistent with our findings, previous studies of pSjD SGs have confirmed the increased TGF-β expression in pSjD SGs compared to healthy SGs, possibly in response to chronic inflammation, as seen in other chronic inflammatory diseases31,32. While prior studies have primarily focused on TGF-β as a key driver of fibrosis32,33, it is also recognized as a critical cytokine for Tfh differentiation in humans15,19 and tissue-resident T cells34,35. Although the precise location of Tfh cell priming and maturation remains unresolved, whether it occurs within secondary lymph nodes or local inflammatory site4,8, our findings suggest that local inflammatory cytokines such as TGF-β may contribute to the in-situ expansion of Tfh cells within the salivary glands. Among the TGF-β-induced Tfh cells, we found that CXCR3+ Tfh1 cells were the most upregulated population in pSjD blood samples. Notably, IL-12 has been reported as the key inducer of Tfh1 cells in systemic lupus erythematosus36. Given that Tfh differentiation is regulated by a multifaceted cytokine environment involving the interplay of multiple factors8,9,19, it is unlikely that Tfh1 differentiation can be fully explained by a single cytokine alone, whether it be TGF-β or IL-12. Further studies are needed to determine whether disease-specific differences in Tfh1 differentiation conditions exists, particularly in inflammatory sites.
Third, we focused on the function of T cells under Tfh1 differentiation condition. We observed increased production of IL-21, TNF-α, and IL-2 in the presence of TGF-β and TCR stimulation. In particular, IL-21, a key cytokine produced by Tfh cells, likely reflects enhanced Tfh differentiation and plays a crucial role in B cell maturation8,10. We also identified a potential effect of Tfh1-skewed CD4+ T cells on B cells, as evidenced by higher CD19 MFI and increased CD19+CD38+ B cell population, which may indicate an early activation state of B cells37,38. Although direct comparison with non-pSjD controls is limited due to potential differences in baseline CD4+ T cell composition, the increase in CD19+CD38+ B cells was observed specifically under combined TCR and TGF-β stimulation in pSjD samples, but not with TCR stimulation alone. This suggests that TGF-β provides an additional modulatory effect in the pSjD immune context. In contrast, the induction of class-switch associated markers appeared less dependent on TGF-β, being observed under both TCR stimulation alone and in combination with TGF-β.
Relating to Tfh cytokine production, TNF-α, a proinflammatory cytokine, has recently been shown to be reduced by ICOS blockade in human SjD SG-organ39, suggesting that activated Tfh cells are the primary source. Additionally, IL-2, an intriguing cytokine in the context of Tfh differentiation, inhibits Tfh differentiation via STAT5 mediated skewing of BLIMP1 to BCL-68,25. While IL-2 is essential for the expansion and maintenance of Treg cells8, strong IL-2 signal not only inhibits Tfh cell differentiation but also appears to suppress Tfr cells10,40. However, paradoxically, Tfh themselves are known to produce IL-241, which aligns with our findings. One possible explanation for this paradox is that TGF-β downregulates the IL-2 receptor alpha chain (CD25) expression, thereby modulating IL-2 signaling responsiveness and potentially mitigating the inhibitory effects of IL-2 on Tfh and Tfr differentiation8. Notably, Tfr cells progressively lose CD25 expression as they differentiate toward the germinal center resident phenotype10,40, which could raise the possibility that TGF-β may influence the delicate balance between BCL-6 and IL-2 dependent transcriptional programs that regulate Tfh/Tfr cell fate decision. Despite these observations, further studies are needed to better understand the role of Tfh-derived cytokines in inflammatory sites, particularly in relation to other immune cells and SG epithelial cells.
The present study acknowledges several limitations. First, our analysis focused solely on CD4+ T cell subsets defined by surface markers, which were employed to sort specific populations42. Markers such as CXCR5, while indicative of Tfh cells, are also expressed on B cells4,15 and thus lack cell type specificity. Although ICOS expression was assessed to mitigate this, CXCR5-based analysis in LSG tissue may not precisely distinguish Tfh cells from other CXCR5-expressing populations. Moreover, whether circulating Tfh cells defined by cell surface markers correspond directly to tissue-resident Tfh cells remains controversial, as circulating Tfh cells are considered less mature and may exhibit distinct transcriptional profiles compared to tissue-resident counterparts43. Therefore, although our data provide insights into the immune profile in pSjD, the precise relationship between peripheral and tissue CD4+ T cells remain unresolved. Further investigations, incorporating integrated phenotypic, transcriptomic, and spatial profiling in larger cohorts will be needed to validate and expand these findings.
Second, our study did not account for Tfr cells, which are difficult to reliably distinguish using cell surface markers alone43. As noted, Tfr cells can be either CD25+ or CD25-10,40,43, complicating their identification. Tfr cells, defined as CD4+CXCR5+Foxp3+ T cells, have been reported to increase in pSjD blood12,13 and may phenotypically overlap with Tfh subsets. In general, approximately 4% of Tfr cells (CD4+CXCR5+CD25+Foxp3+) are included in the Tfh gate in blood (CD4+CXCR5+), and up to 19% of Tregs may also be in Tfh subset44. A study defining Tfr cells as CD4+CXCR5+CD25+CD127lo found a predominance of CXCR3+ Th-1 like Tfr cells in blood of pSjD, which were associated with higher IgG levels45. These findings suggest that a portion of the CXCR3+ Tfh1 population in our study may include Tfr cells, potentially aligning with the previous results showing an increased Tfr ratio among Tfh subset in pSjD12,13. However, definitions of Tfr cells remain inconsistent across these studies12,13,45, limiting direct comparison and complicating interpretation of their potential contribution. While our findings are consistent with previous reports linking activated Tfh cells to disease activity12,13, the precise contribution of Tfr cells, particularly within the CXCR3+ Tfh1 cell subset, remains to be fully elucidated and warrants further investigation.
Finally, TCR repertoire analysis was conducted in only three patients, making the data preliminary and challenging to generalize due to the heterogeneity of pSjD characteristics among individuals. Additionally, two of the three patients analyzed had high focus scores, and these cases were selected because TCR sequencing results are considered more reliable in samples with higher lymphocyte infiltration. This selection approach may have introduced selection bias, particularly in the clonality analysis, where the patient with a lower focus score (Patient 1) exhibited relatively high clonality and a notable degree of similarity between cTfh2 and LSG T cells. Since the lower focus score sample showed fewer clone counts, which may have been influenced by the lower degree of lymphocyte infiltration, both the observed percentage of expanded T cell clones and the similarity scores may have been affected (Supplementary Table S7). Further analysis stratified by the severity of lymphocytic infiltration may help clarify this point. Nonetheless, we identified consistent trends in the TCR repertoire features of blood T cell subsets and the similarity of the repertoire of LSG-infiltrating T cells, despite the heterogeneity between patients.
In conclusion, our study demonstrated that TGF-β enriched environment, such as that seen in sialadenitis of SjD, may play an important role in the expansions of Tfh subsets, especially Tfh1 cells. Potentially, the activated Tfh1 cells in blood reflect the immunological activation of LSG infiltrating T cells, which suggests the usefulness of blood CD4+ T cell analysis for predicting the benefit of T cell-targeted therapy among patients with heterogeneous pSjD.
Data availability
Data supporting this study are included within the article and its supplementary materials. All of the TCR sequence data will be made available through the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) upon publication. For any inquiries regarding the data, please contact the corresponding author, Hiroto Tsuboi, at Hiroto-Tsuboi@md.tsukuba.ac.jp.
Abbreviations
- ACR/EULAR:
-
American College of Rheumatology/ European League against Rheumatism
- ESSDAI:
-
EULAR Sjögren’s Syndrome Disease Activity Index
- LSG:
-
Labial salivary gland
- PBMCs:
-
Peripheral blood mononuclear cells
- pSjD:
-
Primary Sjögren’s disease
- SG:
-
Salivary gland
- SjD:
-
Sjögren’s disease
- TCR:
-
T cell receptors
- Th1:
-
T helper 1 cells
- Th17:
-
T helper 17 cells
- Treg:
-
Regulatory T cells
- Tfh1:
-
T follicular helper 1 cells
- Tfh2:
-
T follicular helper 2 cells
- Tfh17:
-
T follicular helper 17 cells
- Tfh:
-
Follicular T helper cells
- Tfr:
-
T follicular regulatory cells
- Trm:
-
Tissue resident memory T cells
References
Brito-Zerón, P. et al. Sjögren syndrome. Nat. Rev. Dis. Primers 2, 16047 (2016).
Mariette, X. & Criswell, L. A. Primary Sjögren’s syndrome. N. Engl. J. Med. 378(10), 931–939 (2018).
Verstappen, G. M., Pringle, S., Bootsma, H. & Kroese, F. G. M. Epithelial-immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat. Rev. Rheumatol. 17(6), 333–348 (2021).
Verstappen, G. M., Kroese, F. G. M. & Bootsma, H. T cells in primary Sjögren’s syndrome: Targets for early intervention. Rheumatology 60(7), 3088–3098 (2021).
Joachims, M. L. et al. Single-cell analysis of glandular T cell receptors in Sjögren’s syndrome. JCI Insight 1(8), e85609 (2016).
Abe, S. et al. M3 muscarinic acetylcholine receptor-reactive Th17 cells in primary Sjögren’s syndrome. JCI Insight 5(15), e135982 (2020).
Künzli, M. & Masopust, D. CD4+ T cell memory. Nat. Immunol. 24(6), 903–914 (2023).
Walker, L. S. K. The link between circulating follicular helper T cells and autoimmunity. Nat. Rev. Immunol. 22(9), 567–575 (2022).
Crotty, S. T follicular helper cell biology: A decade of discovery and diseases. Immunity 50(5), 1132–1148 (2019).
Deng, J., Wei, Y., Fonseca, V. R., Graca, L. & Yu, D. T follicular helper cells and T follicular regulatory cells in rheumatic diseases. Nat. Rev. Rheumatol. 15(8), 475–490 (2019).
Wei, X. & Niu, X. T follicular helper cells in autoimmune diseases. J. Autoimmun. 134, 102976 (2023).
Verstappen, G. M. et al. Is the T follicular regulatory: Follicular helper T cell ratio in blood a biomarker for ectopic lymphoid structure formation in Sjögren’s syndrome?. Arthritis Rheumatol. 70(8), 1354–1355 (2018).
Fonseca, V. R. et al. The ratio of blood T follicular regulatory cells to T follicular helper cells marks ectopic lymphoid structure formation while activated follicular helper T cells indicate disease activity in primary Sjögren’s syndrome. Arthritis Rheumatol. 70(5), 774–784 (2018).
Peng, C. & Jameson, S. C. The relationship between CD4+ follicular helper T cells and CD8+ resident memory T cells: Sisters or distant cousins?. Int. Immunol. 32(9), 583–587 (2020).
Ueno, H., Banchereau, J. & Vinuesa, C. G. Pathophysiology of T follicular helper cells in humans and mice. Nat. Immunol. 16(2), 142–152 (2015).
Shiboski, C. H. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 76, 9–16 (2017).
Honda, F. et al. Pathogenic roles and therapeutic potential of the CCL8-CCR8 axis in a murine model of IgG4-related sialadenitis. Arthritis Res. Ther. 23(1), 214 (2021).
Maecker, H. T., McCoy, J. P. & Nussenblatt, R. Standardizing immunophenotyping for the human immunology project. Nat. Rev. Immunol. 12(3), 191–200 (2012).
Schmitt, N. et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat. Immunol. 15(9), 856–865 (2014).
Choi, J. & Crotty, S. Bcl6-mediated transcriptional regulation of follicular helper T cells (TFH). Trends Immunol. 42(4), 336–349 (2021).
Duan, D. & Derynck, R. Transforming growth factor-β (TGF-β)-induced up-regulation of TGF-β receptors at the cell surface amplifies the TGF-β response. J. Biol. Chem. 294(21), 8490–8504 (2019).
Nakayamada, S. et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35(6), 919–931 (2011).
Sheikh, A. A. & Groom, J. R. Transcription tipping points for T follicular helper cell and T-helper 1 cell fate commitment. Cell Mol. Immunol. 18(3), 528–538 (2021).
Bartleson, J. M. et al. Strength of tonic T cell receptor signaling instructs T follicular helper cell-fate decisions. Nat. Immunol. 21(11), 1384–1396 (2020).
Olatunde, A. C., Hale, J. S. & Lamb, T. J. Cytokine-skewed Tfh cells: Functional consequences for B cell help. Trends Immunol. 42(6), 536–550 (2021).
Horiuchi, S. et al. Tox2 is required for the maintenance of GC TFH cells and the generation of memory TFH cells. Sci. Adv. 7(41), eabj1249 (2021).
Yenyuwadee, S., Sanchez-Trincado Lopez, J. L., Shah, R., Rosato, P. C. & Boussiotis, V. A. The evolving role of tissue-resident memory T cells in infections and cancer. Sci. Adv. 8(33), eabo5871 (2022).
Sumida, T. et al. The role of M3 muscarinic acetylcholine receptor reactive T cells in Sjögren’s syndrome: A critical review. J. Autoimmun. 51, 44–50 (2014).
Roberts, A. I. et al. The role of activation-induced cell death in the differentiation of T-helper-cell subsets. Immunol. Res. 28(3), 285–293 (2003).
Joachims, M. L. et al. Sjögren’s syndrome minor salivary gland CD4+ Memory T cells associate with glandular disease features and have a germinal center T follicular helper transcriptional profile. J. Clin. Med. 9(7), 2164 (2020).
Mason, G. I., Hamburger, J., Bowman, S. & Matthews, J. B. Salivary gland expression of transforming growth factor beta isoforms in Sjogren’s syndrome and benign lymphoepithelial lesions. Mol. Pathol. 56(1), 52–59 (2003).
Sisto, M. et al. The TGF-β1 Signaling Pathway as an Attractive Target in the Fibrosis Pathogenesis of Sjögren’s Syndrome. Mediators Inflamm. 2018, 1965935 (2018).
Kaieda, S. et al. Mast cells can produce transforming growth factor β1 and promote tissue fibrosis during the development of Sjögren’s syndrome-related sialadenitis. Mod. Rheumatol. 32(4), 761–769 (2022).
Mackay, L. K. et al. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43(6), 1101–1111 (2015).
Fonseca, R. et al. Runx3 drives a CD8+ T cell tissue residency program that is absent in CD4+ T cells. Nat. Immunol. 23(8), 1236–1245 (2022).
Ma, X. et al. Expansion of T follicular helper-T helper 1 like cells through epigenetic regulation by signal transducer and activator of transcription factors. Ann. Rheum. Dis. 77(9), 1354–1361 (2018).
d’Arbonneau, F. et al. BAFF-induced changes in B cell antigen receptor-containing lipid rafts in Sjögren’s syndrome. Arthritis Rheum. 54(1), 115–126 (2006).
Nocturne, G. & Mariette, X. B cells in the pathogenesis of primary Sjögren’s syndrome. Nat. Rev. Rheumatol. 14(3), 133–145 (2018).
Pontarini, E. et al. Unique expansion of IL-21+ Tfh and Tph cells under control of ICOS identifies Sjögren’s syndrome with ectopic germinal centers and MALT lymphoma. Ann. Rheum. Dis. 79(12), 1588–1599 (2020).
Fonseca, V. R., Ribeiro, F. & Graca, L. T follicular regulatory (Tfr) cells: Dissecting the complexity of Tfr-cell compartments. Immunol. Rev. 288(1), 112–127 (2019).
DiToro, D. et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 361(6407), eaao2933 (2018).
Ota, M. et al. Dynamic landscape of immune cell-specific gene regulation in immune-mediated diseases. Cell 184(11), 3006-3021.e17 (2021).
Kumar, S. et al. Developmental bifurcation of human T follicular regulatory cells. Sci. Immunol. 6(59), eabd8411 (2021).
Maceiras, A. R., Fonseca, V. R., Agua-Doce, A. & Graca, L. T follicular regulatory cells in mice and men. Immunology 152(1), 25–35 (2017).
Kim, J. W. et al. Circulating CCR7(lo)PD-1(hi) follicular helper T cells indicate disease activity and glandular inflammation in patients with primary Sjögren’s syndrome. Immune Netw. 19(4), e26 (2019).
Acknowledgements
We thank Thomas Mayers, Medical English Communications Center, University of Tsukuba, for the critical reading of the manuscript. We also would like to thank ImmunoGen Teqs Inc. for the assistance of TCR repertoire analyses, and Y.Yamazaki for excellent technical support for flow cytometric cell sorting.
Funding
This work was supported by the JSPS KAKENHI Grant #JP19K17880, and Outstanding Young Women Researcher Award 2021 by the Japanese Society for Immunology.
Author information
Authors and Affiliations
Contributions
SA, HT, FH, HA, HM and YK recruited patients and collected clinical data. SA and HAo performed all experiments and analyzed the data. HAo analyzed the TCR sequence data. SA wrote the initial draft, Hiroto T and IM edited it. All authors accept equal responsibility for the accuracy of the contents of this paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abe, S., Tsuboi, H., Aoki, H. et al. T follicular helper 1 cells in blood potentially mirror salivary gland-infiltrating T cells in Sjögren’s disease. Sci Rep 15, 23520 (2025). https://doi.org/10.1038/s41598-025-07008-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07008-3