Abstract
This retrospective analysis assesses the effectiveness and safety of pharmacomechanical thrombectomy (PMT) and join CDT (catheter-directed thrombolysis) compared to CDT and with standard anticoagulation therapy for LEDVT (acute lower extremity deep vein thrombosis) with concomitant IVCT (inferior vena cava thrombosis). From January 2014 to January 2020, a total of 114 patients were diagnosed with acute LEDVT and IVCT at Shanxi Bethune Hospital. Patients were separated into three groups: PMT ± CDT (n = 77; 25 in this group also received CDT), CDT ± catheter thrombectomy (CDT ± CT) (n = 21), and conservative management (n = 16). The following outcomes were evaluated using SPSS: thrombus clearance rates, immediate clinical remission, and limb circumference decrease in the patients. The severity of post-thrombotic syndrome (PTS) and venous patency was evaluated at 1-year and 2-year follow-ups. Our findings showed that the PMT ± CDT group had significantly greater rates of immediate clinical remission and thrombus clearance compared with CDT ± CT and conservative treatment. PMT ± CDT achieved a thrombolysis rate of 74.5%, while the CDT ± CT group had a rate of 47.2% (p < 0.001). No method of treatment significantly reduced the overall rate of post-thrombotic syndrome (PTS) (p = 0.301). However, PMT ± CDT had a significantly lower PTS severity than CDT ± CT (p = 0.023). Overall, venous patency scores were significantly better in the PMT ± CDT group at 1-year and 2-year follow-up time points. The results of this study suggest that PMT ± CDT was significantly better than CDT ± CT, as well as conservative management of patients with LEDVT and IVCT concerning both short-term and long-term outcomes. The findings further support a preference towards endovenous management as a treatment approach in clinical practice for patients with such complications.
Similar content being viewed by others
Introduction
Inferior vena cava thrombosis (IVCT) is uncommon. Still, it has serious repercussions as a proximal deep vein thrombosis (DVT), primarily involving the extension of the thrombus from the lower limb veins, as opposed to occurring as an isolated event1,2. The literature refers to an increased incidence of IVCT as a complication of pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT)3,4. Embolic occurrences are debilitating by nature and cause organ damage and dysfunction in the heart and lungs5. The significant morbidity, mortality, and healthcare costs associated with the management of IVCT also make it a global public health issue6,7. Among patients with proximal LEDVT, the incidence of IVCT has been estimated at 2.6–4%8,9,10. The incidence of symptomatic pulmonary embolism in patients with IVCT has been reported to be up to 32.1%, while post-thrombotic syndrome has been reported in 13%11,12,13. Therefore, in patients with LEDVT and IVCT, selecting the proper treatment is important for decreasing mortality and optimizing longer-term quality of life. Recently, with increasing advances in thrombus removal technology and an expanding focus on reducing thrombus burden, percutaneous mechanical thrombectomy and catheter-directed thrombolysis (CDT) have become novel treatment options to standard antithrombotic and inferior vena cava (IVC) filter placement14,15,16. The ATTRACT trial was often quoted as guidance for endovascular approaches to DVT. However, other studies have focused on the utility of pharmacomechanical thrombectomy (PMT) and CDT for IVCT treatment17,18,19. Incorporating these findings is important in the discussion of organizing treatment. However, it should be noted that the study is restricted to a single center in a retrospective design, Shanxi Bethune Hospital, China, and thus may introduce selection bias and generalizability. Nevertheless, the study offers unique findings to IVCT management and highlights the importance of prospective multicenter studies to validate the findings.
Materials and methods
Data collection
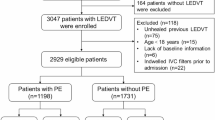
This study retrospectively reviewed the clinical data of patients with acute lower extremity deep vein thrombosis (LEDVT) and inferior vena cava thrombosis (IVCT) at Shanxi Bethune Hospital from January 2014 to January 2020. This study aimed to assess and compare the safety and efficacy of pharmacomechanical thrombectomy (PMT), catheter-directed thrombolysis (CDT), and conservative anticoagulant therapy for IVCT management. Placement of an IVC filter was based on characteristics of thrombus, and some patients underwent filter placement either before or at the time of intervention. It is important to mention that IVC filter placement was not a strict inclusion criterion. Inclusion criteria involved a confirmed LEDVT and IVCT diagnosis by ultrasound imaging and a timeframe of 14 days or less from the onset of symptoms. Patients with severe cardiopulmonary, liver, or kidney dysfunction, a known allergy to contrast agents, or unable to safely tolerate surgery were excluded from this study. Patients were also excluded from this study if there was a contraindication for anticoagulation or thrombolysis, if at a high risk of bleeding, or if there was a psychiatric disease or other related conditions that prevented the patient from adhering to treatment. There were three treatment approaches for patients: PMT ± CDT, CDT ± catheter thrombectomy (CDT ± CT), or conservative management. There were 77 patients in the PMT ± CDT group, of whom 25 underwent adjunctive CDT after undergoing PMT. There were 21 patients in the CDT ± CT group. There were 16 patients in the Conservative Management with anticoagulant therapy group. Participants were treated based on their preferences, physician recommendations, and economic situation, not random assignment or preference. Given the unequal distribution of patient populations between treatment groups, the authors acknowledge possible bias in this comparative analysis, and future studies should implement propensity score matching to help minimize potential confounding effects.
Treatment procedures
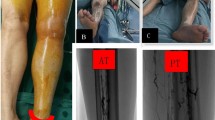
All trial subjects had a sequestered injection of low-molecular-weight heparin (LMWH) for their initial anticoagulation - adjusted for body weight (at a dosage of 100 IU/kg). An IVC filter was not a requisite for being included in this study; however, placement of one was selectively done when clinically expedient. If the thrombus extended above an IVC filter already in place, or there was an IVC thrombus but not a filter in place, placement of an additional IVC filter at the time of PMT or CDT was done. Filters were placed at the higher end of the IVC thrombus or within renal vein segments formed from the iliac vein or IVC to stabilize antegrade flow to non-thrombus areas. DVT above the knee in IVC thrombosis; IVC Filters were removed when the DVT stabilized unless for other specific contraindications. Patients in the PMT ± CDT group underwent percutaneous interventions. In mixed lower-limb DVT, the popliteal was selected for intervention, while in central lower-limb DVT, the femoral vein was selected. An AngioJet connective thrombus removal catheter was introduced, and the jet mode was used to push 250,000–500,000 units of urokinase. After the thrombus was allowed to dwell for 20 min, the catheter was activated to suction mode, and thrombus aspiration was done by moving the catheter at 1 mm/s in the direction of blood flow. If there was > 50% residual stenosis, adjunctive CDT was instituted, using urokinase at a dose of 30,000–50,000 U/24 h at a rate of 100 mL/h, with the routine check of coagulation processes every four hours with an adjustment of urokinase according to fibrinogen levels regarded as high. If residual stenosis remains > 50% after the first CDT treatment, repeated suction or balloon dilation is conducted again. Upon getting a residual thrombus burden that necessitated a larger lumen, the CDT ± CT group agreed upon a method according to a handful of standards. The 8 F large-lumen catheter was inserted with an accompanying 10 F vascular sheath, allowing access to the thrombus once it had crossed underneath the IVC filter. The larger catheter had a 50 ml syringe attached to the Y-valve for negative pressure suction, repeated two to three times until no residual thrombus was detected. IVC iliac patency confirmed with angiography to continue CDT treatment when clinically warranted, with additional reliance on DVT patterns. The Conservative Treatment group was restricted to anticoagulation only for treatment. The anticoagulation choice was at the treating physician’s discretion, and treatment was either rivaroxaban, warfarin, or low molecular weight heparin based on the clinical obstetrical patterns and patient condition.
Outcome measures
The collected clinical data included age, sex, comorbidities, IVCT classification, preoperative and postoperative limb circumference differences, immediate clinical remission, thrombus clearance rate, and thrombus clearance grade. Comorbidities and risk factors were noted separately in each treatment group to minimize implications with treatment selection. The IVCT classification was dedicated to the location of the thrombus, with IRCT (infrarenal complete thrombosis) signifying a complete blockage at the level of the renal veins; IRPT (infrarenal partial thrombosis) indicating a partial blockage with the residual flow, and IHCT (intrahepatic complete thrombus) extending beyond the renal veins and into the hepatic segment. Limb circumference measures were determined for each limb 15 cm above the patella’s level to assess the edema reduction. Venous patency was graded as follows: IVC, common iliac vein, external iliac vein, common femoral vein, upper superficial femoral vein, lower superficial femoral vein, and popliteal vein were graded for seven segments in the venous system with the following grading: 0 (complete patency), 1 (partial patency), or 2 (occlusion). The thrombolysis rate was calculated as follows:
Thrombus clearance was categorized as Grade I (< 50% lysis), Grade II (50–94% lysis), and Grade III (≥ 95% lysis). Immediate clinical remission was defined as decreased limb pain and swelling occurring within 24 h after treatment. Follow-up assessments were performed at 1, 3, 6, 12, and 24 months through either office visits or telemedicine. Anticoagulation therapy (rivaroxaban, warfarin, or LMWH) was adjusted as indicated. Venous patency, residual thrombus, and post-thrombotic changes were assessed with coursed ultrasound or computed tomography venography (CTV). Post-thrombotic syndrome (PTS) severity was assessed using the Villalta and VCSS scores and classified individually as mild, moderate, or severe based on symptom severity and venous dysfunction.
Follow-up and patient monitoring
Patients were followed after discharge at one, three, six, twelve, and twenty-four months. A venous ultrasound or venography (CTV) was performed for follow-up assessments to determine the patency of deep veins. The Venous Clinical Severity Score (VCSS) and Villalta Score were documented to assess the severity of post-thrombotic syndrome. Despite close follow-up, in all groups during the two-year follow-up, there were no clinically relevant pulmonary embolism (PE) or recurrent DVT events noted.
Ethics statement
This study was performed in accordance with the Helsinki Declaration principles. Given the study’s retrospective nature, the Shanxi Bethune Hospital Ethics Committee waived ethical approval and informed consent. To protect patient confidentiality, all identifiers were deleted, and data were de-identified prior to analysis. Strict data security measures followed hospital and institutional protocols.
Statistical methods
During the sample size calculation for this study, GPower 3.1.9.7 software was utilized, with the acute symptom response rate designated as the primary outcome indicator. Based on prior clinical experience, the acute symptom response rate for conservative treatment was approximately 40%, while endovascular treatment increased this rate significantly to 80%. Given the frequent use of endovascular treatment in clinical practice at our hospital, the ratio of the two sample groups was set to 3:1 to enhance the representativeness of the sample. A computational analysis conducted with a significance level (α) of 0.05 and statistical power of 0.8 determined that the conservative treatment group required a sample size of 15, while the endovascular treatment group required a sample size of 45.
Statistical analysis was conducted through SPSS 23.0 software. Descriptive statistics were expressed as mean ± standard deviation, and a paired sample t-test was performed to compare differences in venous patency scores and limb circumferences before and after treatment. The classification of post-thrombotic syndrome was evaluated by rank sum test with a significance of p < 0.05.
Results
Data collection and clinical treatment
114 patients met the eligibility criteria for study inclusion, comprising 53 males and 61 females, with a mean age of 56.4 ± 15.0 years. Within the cohort, 16 patients received anticoagulation therapy only, 21 patients underwent CDT ± CT with filter protection, and the remaining patients (n = 77) underwent PMT ± CDT with filter protection. There were no statistically significant differences in baseline characteristics (age, gender, IVCT classification levels, and PE) across groups (Table 1). The two most prevalent comorbidities were hyperhomocysteinemia (n = 24, 21.1%) and filter-related thrombosis (n = 19, 16.7%). Other risk factors included obesity, nephrotic syndrome, pregnancy, malignancy, hematological disorders, autoimmune diseases, congenital IVC anomalies, and Budd-Chiari syndrome (Table 2).
Clinical outcomes and thrombus clearance
The PMT ± CDT group showed significantly greater immediate symptom relief (80.5%, 62 patients) than the CDT ± CT group (61.9%, 13 patients) or the conservative group (43.8%, 7 patients) (p = 0.006, Table 3). Additionally, PMT ± CDT decreased limb circumference significantly more after the procedure (1.78 ± 0.40 cm reduction) than either the CDT ± CT group (1.1 ± 0.73 cm) or the conservative advised treatment (0.2 ± 0.91 cm) (p < 0.001, Table 4). Regarding differences in effectiveness, PMT ± CDT showed greater success with total thrombolysis, 74.5% for PMT ± CDT compared to 47.2% for CDT ± CT (p < 0.001, Table 5; Fig. 1). In terms of thrombus clearance, grading showed much greater success with PMT ± CDT with 16.9% vs. 19.0% for CDT ± CT attaining Grade III (≥ 95% lysis) (Table 6).
Complications and safety analysis
No serious bleeding events or clinically evident symptomatic pulmonary embolism (PE) were observed in any group. However, there were mild adverse events, including localized hematoma at the puncture site in 6 patients (5.3%), transient hematuria in 4 patients (3.5%), and complications related to the catheter in 3 patients (2.6%). The catheter-related complications included occlusion of the catheter (1 patient), dislodgment of the catheter that required repositioning (1 patient), and a delay in thrombolysis treatment due to the catheter causing mild bleeding at the puncture site (1 patient). Long-term safety aspects of IVC filter placement and retrieval were assessed. Of the 19 patients (16.7%) with pre-existing IVC filters, 12 (63.2%) experienced successful filter retrieval after their stable thrombus. In comparison, 7 patients (36.8%) remained in place due to the ongoing risk of thrombosis. No migration or fracture of the filter nor perforation of the IVC was observed during follow-up.
Long-term follow-up and post-thrombotic syndrome (PTS)
Patients were followed for 1 to 24 months. Venous patency scores were statistically significantly better for patients in the PMT ± CDT group at both 1-year and 2-year follow-up assessments than those treated with CDT ± CT or conservative treatment (Fig. 1). Post-thrombotic syndrome (PTS) incidence was not significantly different between PMT ± CDT and CDT ± CT (p = 0.301, Table 7). The severity of PTS was statistically significantly lower for PMT ± CDT patients; 61.7% of PMT ± CDT patients were classified as mild PTS compared to 46.2% of CDT ± CT patients at the 2-year mark (p = 0.023, Table 8).
Recurrent DVT and study limitations
Throughout the additional 2-year follow-up period, we did not see any recurrent DVT or PE. However, we did not complete longer follow-up beyond 2 years and cannot determine longer-term recurrence rates. Future studies should include longer follow-ups to determine whether the benefits of PMT last and if any late complications arise.
Enhanced data visualization
A Kaplan-Meier survival curve showing cumulative patency over time should be considered to enhance the long-term venous patency data overview. Moreover, a forest plot comparing efficacy and safety outcomes for each treatment group would add to the visualization of outcomes and the ability to see differences in clinical outcomes. These figures will help the reader better understand the study results.
Discussion
IVCT usually originates due to iliofemoral vein thrombosis in the lower limbs and can be classified into primary IVCT and secondary IVCT, which considers pathogenesis mechanisms15,16. Primary IVCT is usually spontaneous and occurs when there is no known cause, such as congenital IVC malformation, that has an incidence in the healthy population of 0.3–0.6%, and VTE risk is at least 60% greater with the occurrence of primary IVCT17,18. Secondary IVCT occurs due to outflow obstruction, as seen in hyperhomocysteinemia, filter-related thrombosis, obesity, antiphospholipid antibody syndrome, Behçet disease, rheumatoid arthritis, and nephrotic syndrome. Secondary IVCT accounts for more than 67% of cases19,20. IVCT has a predominant incidence and mortality, and the clinical symptoms are usually insidious in onset, along with nonspecific clinical signs that are dependent on the severity of the lesions involved and the thrombus burden21,22. IVCT is rare, so often a delayed diagnosis occurs, and no distinct clinical evidence-based guidelines are developed for the condition23.
Compared to anticoagulation therapy alone, PMT ± CDT and CDT ± CT require more healthcare resources and cost more. The higher cost relates to the equipment, procedural cost, increased length of stay, and staffing related to these procedures. PMT ± CDT reported better quality of thrombus clearance and symptom improvement. A formal cost-effectiveness analysis is needed to evaluate if this improved quality/care delivery justifies the higher cost compared to CDT ± CT and/or conservative management of proximal DVT. Future studies should focus on the economic implications of these treatments and determine if better clinical outcomes ultimately save healthcare costs24.
The improved outcomes of PMT ± CDT compared to CDT ± CT could be attributed to the action of PMT as it permits physical and mechanical fragmentation and aspiration of the thrombus. CDT has the potential to dissolve a clot over an extended time period reliant on fibrinolytic degradation. In contrast, PMT mechanically removes the thrombotic material, permitting quicker and more complete recanalization of venous flow and possibly reduced injury to the endothelium and reduced inflammation, which might predispose to PTS development. CDT’s protracted thrombolytic therapy exposure resulting in clot dissolution, venous obstruction, and further exposure to fibrinolytic agents all might result in increased bleeding complications in the same setting. For PMT, mechanisms, histopathological, and imaging studies are needed to more directly assess the impact on venous endothelial function and long-term patency25.
Our research did not evaluate any recurrent DVT or PE events beyond the two-year follow-up. No recurrence of deep vein thrombosis was observed in this study, potentially attributable to multidimensional interventions. Close follow-up was conducted through face-to-face visits and telemedicine, ensuring timely monitoring of patient recovery status. The effectiveness of anticoagulation therapy administered as rivaroxaban 20 mg daily, with adjustments based on individual patient characteristics, has been verified in a retrospective study by Ilya Schastlivtsev et al., where no recurrence was observed in the rivaroxaban population during a one-year follow-up26. Additionally, the study established continuous communication channels with patients via social platforms such as WeChat, which effectively improved patients’ medication adherence and follow-up compliance. Previous studies have demonstrated that high levels of compliance can significantly reduce the risk of deep vein thrombosis recurrence27. Furthermore, the attention clinicians pay to patients at high risk of recurrence, driven by the domestic medical environment, might also have contributed to the low recurrence rate observed in this study28. Even though no recurrence was observed during the two-year observation period, we cannot conclude whether PMT will provide long-term durability. Longer-duration follow-up studies beyond two years are warranted to assess whether PMT protects long-term recurrence from thrombotic events. Future studies should investigate whether long-term anticoagulation or adjunctive therapy is required to maintain venous patency after PMT29.
PMT is preferable for patients with extensive IVCT, severe symptoms, and a high thrombus burden, as it provides better immediate removal of thrombus and relief of symptoms than CDT. Still, CDT may be reasonable for patients with contraindications to PMT or a lower thrombus burden. Future studies should develop clear, standardized selection criteria for clinicians to determine the most appropriate intervention30. The duration of anticoagulation following PMT or CDT is not well established. Current guidelines suggest three to six months of anticoagulation therapy after DVT; however, the duration of anticoagulation therapy is not clear for patients with IVCT receiving PMT or CDT. Future studies must assess anticoagulation optimization based on clot burden, residual venous obstruction, and patient-specific risk factors31. For high-risk patients, such as those with extensive IVCT and a history of PE, a combination of PMT, prolonged anticoagulation therapy, and imaging surveillance may be warranted for complications. Future clinical trials should determine if encouraging clot removal immediately after PMT and long-term anticoagulation therapy results in better outcomes than optimal medical management32.
Our research is consistent with the most recent guidelines issued by the American College of Chest Physicians (ACCP), European Society of Cardiology (ESC), and the National Institute for Health and Care Excellence (NICE), which recommend anticoagulation as the first-line choice for DVT, and that endovascular interventions be performed in select individuals with acute, severe or extensive iliofemoral DVT. Our study validates the hypothesis that PMT achieves significantly better thrombus clearance and alleviates clinical symptoms, emphasizing its role in treating DVT in appropriately selected patients33. However, unlike the existing guidelines, which fail to make recommendations specifying when PMT is preferable to CDT for ICIVC, our data suggests that PMT ± CDT yields greater short- and mid-term outcomes than CDT ± CT. More studies will be required to validate our findings further and potentially amend treatment recommendations33.
This research was a single-site, retrospective study, which adds to selection bias and restricts generalizability. Multisite prospective studies must validate these results and assess applicability to different clinical settings34. Moreover, the treatment groups were unbalanced (PMT ± CDT = 77, CDT ± CT = 21, conservative = 16), which may have impacted the comparative analyses. In future studies, propensity score matching or statistical adjustment approaches will be appropriate to reduce the impact of selection bias and enhance the findings35. The lack of long-term recurrence data after two years is a limitation of this study. Additionally, treatment allocation was not randomized and was impacted by patient preferences, physician recommendations, and financial constraints, which resulted in confounding by indication, as the patient population who received PMT ± CDT or CDT ± CT may have differed from the conservative treatment group on other or baseline characteristics. Randomized controlled trials (RCTs) are needed to validate that PMT is superior to other treatments and to develop a clearer treatment algorithm for different settings36.
Conclusion
This study showed that PMT ± CDT and CDT ± CT are effective treatments for LEDVT using IVCT, resulting in greater thrombus resolution, symptom relief, and venous patency. As PMT ± CDT and CDT ± CT become more widely utilized, their role in improving clinical outcomes and symptoms of PTS is becoming clearer. More studies in this field should focus on defining optimal patient selection, anticoagulation protocols, and long-term outcomes. In general, our findings support the use of PMT ± CDT in clinical practice to enhance the treatment of IVCT.
VCSS and villalta scores at 1-year and 2-year follow-ups. (A–B) Differences in VCSS between each patient group at follow-up visits, year 1 and year 2. (C–D) Villalta scores across groups over the same time points. The lower scores reflect better venous function and reduced severity of post-thrombotic syndrome in patients treated with PMT compared to CDT and conservative management.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Cohen, O. et al. Management strategies and clinical outcomes in patients with inferior vena cava thrombosis: Data from GARFIELD-VTE. J. Thromb. Haemost 20(2), 366–374 (2022).
Dehbi, S. et al. Temporary inferior vena cava filters factors associated with non-removal. Eur. Radiol. 33(4), 2585–2592 (2023).
Liu, H. et al. Inferior vena cava stenosis-induced deep vein thrombosis is influenced by multiple factors in rats. Biomed. Pharmacother. 128, 110270 (2020).
Houghton, D. E. & Carman, T. Caution: Inferior vena cava filters in distal deep vein thrombosis Vasc Med. 1358863X241255968 (2024).
Benedetti, R. et al. Inferior vena cava filters: A clinical review and future perspectives. J. Clin. Med. 13(6) (2024).
Witmer, C. & Raffini, L. Treatment of venous thromboembolism in pediatric patients. Blood 135(5), 335–343 (2020).
Agnelli, G. et al. The MASTER registry on venous thromboembolism: description of the study cohort. Thromb. Res. 121(5), 605–610 (2008).
Stein, P. D., Matta, F. & Yaekoub, A. Y. Incidence of vena cava thrombosis in the United States. Am. J. Cardiol. 102(7), 927–929 (2008).
Jimenez, D. et al. Effect of a pulmonary embolism diagnostic strategy on clinical outcomes in patients hospitalized for COPD exacerbation: A randomized clinical trial. JAMA 326(13), 1277–1285 (2021).
Avgerinos, E. D. et al. Impact of inferior vena cava thrombus extension on thrombolysis for acute iliofemoral thrombosis. J. Vasc Surg. Venous Lymphat Disord. 4(4), 385–391 (2016).
Ye, K. et al. Outcomes of Pharmacomechanical Catheter-directed thrombolysis for acute and subacute inferior vena cava thrombosis: A retrospective evaluation in a single institution. Eur. J. Vasc Endovasc Surg. 54(4), 504–512 (2017).
Xu, D. et al. Triglyceride-rich lipoproteins and cardiovascular diseases. Front. Endocrinol. (Lausanne). 15, 1409653 (2024).
Mouawad, N. J. Percutaneous mechanical thrombectomy to remove post-thrombotic obstructions and manage post-thrombotic syndrome-associated venous leg ulceration. J. Vasc Surg. Venous Lymphat Disord. 11(5), 964–971e1 (2023).
Zhang, R. S. et al. Efficacy and safety of anticoagulation, catheter-directed thrombolysis, or systemic thrombolysis in acute pulmonary embolism. JACC Cardiovasc. Interv. 16(21), 2644–2651 (2023).
Shi, T. et al. Robot-assisted cavectomy versus thrombectomy for level II inferior vena cava thrombus: Decision-making scheme and multi-institutional analysis. Eur. Urol. 78(4), 592–602 (2020).
Offit, K. et al. Regulation of Laboratory-Developed tests in preventive oncology: Emerging needs and opportunities. J. Clin. Oncol. 41 (1), 11–21 (2023).
Chee, Y. L., Culligan, D. J. & Watson, H. G. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br. J. Haematol. 114(4), 878–880 (2001).
Gayer, G. et al. Congenital anomalies of the inferior Vena Cava revealed on CT in patients with deep vein thrombosis. AJR Am. J. Roentgenol. 180(3), 729–732 (2003).
Ortel, T. L. et al. American society of hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 4(19), 4693–4738 (2020).
Veselka, J. et al. Effects of alcohol septal ablation for hypertrophic obstructive cardiomyopathy on doppler Tei index: A midterm follow-up. Echocardiography 22 (2), 105–109 (2005).
Lindsey, P. et al. Thromboembolic risk of endovascular intervention for lower extremity deep venous thrombosis. Ann. Vasc Surg. 49, 247–254 (2018).
Wang, B. et al. Robot-assisted level III-IV inferior vena cava thrombectomy: Initial series with Step-by-step procedures and 1-yr outcomes. Eur. Urol. 78(1), 77–86 (2020).
Bardutz, H. et al. Parkinson’s Disease and the Cardiac Cycle: A Rapid Literature Review and Case Series. Life (Basel) 13(4) (2023).
Decousus, H. et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du risque d’embolie pulmonaire par interruption cave study group. N Engl. J. Med. 338(7), 409–415 (1998).
Liu, X. C. et al. Anatomical distribution of lower-extremity deep venous thrombosis in patients with acute stroke. J. Stroke Cerebrovasc. Dis. 29(7), 104866 (2020).
Schastlivtsev, I. et al. Oral Rivaroxaban versus warfarin after inferior vena cava filter implantation: A retrospective cohort study. Clin. Appl. Thromb. Hemost. 30(1), 10760296241256938 (2024).
Ren, W. et al. Analysis of risk factors for recurrence of deep venous thrombosis in lower extremities. Med. Sci. Monit. 20(1), 199–204 (2014).
Riedl, D. et al. The influence of doctor-patient communication on health outcomes: A systematic review. Z. Psychosom. Med. Psychother. 63(2), 131–150 (2017).
Chen, W. et al. Risk factors and new diagnostic index for deep venous thrombosis of lower extremities in elderly patients with traumatic femoral neck fracture. Front. Surg. 9, 1050347 (2022).
Marston, W. A. Results of the ATTRACT trial do not change the management of acute deep vein thrombosis. J. Vasc Surg. Venous Lymphat Disord. 6(1), 5–6 (2018).
Holper, P. et al. Longterm results after surgical thrombectomy and simultaneous stenting for symptomatic iliofemoral venous thrombosis. Eur. J. Vasc Endovasc Surg. 39(3), 349–355 (2010).
Wang, Y. et al. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior Vena cava/right atrium tumor thrombus: Results of a retrospective cohort study. Ann. Surg. Oncol. 20(3), 914–922 (2013).
Alkhouli, M. et al. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation versus anticoagulation alone in the treatment of inferior vena caval thrombosis. Circ. Cardiovasc. Interv 8(2), e001882 (2015).
Margulis, V. et al. Neoadjuvant SABR for renal cell carcinoma inferior vena cava tumor thrombus-safety lead-in results of a phase 2 trial. Int. J. Radiat. Oncol. Biol. Phys. 110(4), 1135–1142 (2021).
Gong, M. et al. Risk factors and a predictive model for nonfilter-associated inferior Vena Cava thrombosis in patients with lower extremity deep vein thrombosis. Front. Cardiovasc. Med. 9, 1083152 (2022).
Riazi, S. et al. Pre-operative exercise and pyrexia as modifying factors in malignant hyperthermia (MH). Neuromuscul. Disord. 32(8), 628–634 (2022).
Author information
Authors and Affiliations
Contributions
SL & YT. collected the data, SL wrote the main manuscript text, XHM prepared figures, ZWP supervised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, L., Yang, T., Xu, H. et al. Effect of endovascular management and anticoagulation alone on acute deep venous thrombosis in patients with inferior vena cava thrombosis. Sci Rep 15, 21050 (2025). https://doi.org/10.1038/s41598-025-07012-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07012-7