Abstract
Ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCO) catalyzes the initial carbon fixation reaction in the Calvin-Benson-Bassham cycle. Among the many forms of RuBisCOs, form-I—a protein complex containing 8 large and 8 small subunits—is the most common, representing over 90% of all known RuBisCOs. Although many form-I RuBisCO structures have been determined, no structure has been reported for a form-IAq RuBisCO. Here, we detail the structure of the heat-stable form-IAq RuBisCO from the thermophilic and anaerobic purple bacterium Thermochromatium (Tch.) tepidum at 1.55 Å resolution. The overall structure of the Tch. tepidum form-IAq RuBisCO resembles both a form-IAc RuBisCO from a chemolithotrophic sulfur bacterium and a synthetic form-I RuBisCO reconstructed from ancestral sequences. However, the Tch. tepidum enzyme shows significantly greater interactions between adjacent small subunits through their extended N-terminal domains that contain a characteristic six-residue insertion unique to form-IAq RuBisCOs. Structural differences of Tch. tepidum RuBisCO from its mesophilic relative Allochromatium vinosum, and key substitutions on the hydrophilic surface of the small subunits suggests the mechanisms of its enhanced thermostability. Our structure represents the first structure of a form-IAq RuBisCO, providing fresh clues for unraveling the evolutionary history of RuBisCO and new details for how this key enzyme remains active at elevated temperatures.
Similar content being viewed by others
Introduction
RuBisCO is the key enzyme of carbon fixation through the Calvin-Benson-Bassham cycle in plants and many microbial autotrophs and converts CO2 to organic form, contributing over 90% of global carbon fixation1,2. RuBisCOs are distributed in all domains of life and are classified into four groups—forms I, II, III and IV—based on their amino acid sequences and structural properties3,4. Form-I RuBisCOs are most common and are present in autotrophic Proteobacteria, cyanobacteria, algae, and higher plants. These enzymes have a hexadecameric (L8S8) structure consisting of eight 55-kDa subunits and eight 15-kDa subunits, referred to as large (RbcL) and small (RbcS) subunits, respectively5,6,7,8,9. Compared with form-I enzymes, forms II–IV RuBisCOs lack RbcS subunits and exist in various large-subunit-only (L2)n complexes that exhibit distinct catalytic activities and activation modes10. Form-IV, also known as “RuBisCO-like proteins”, are most divergent and were discovered in the phototrophic green sulfur bacterium Chlorobaculum tepidum and endospore-forming bacterium Bacillus subtilis where they function in methionine biochemistry rather than CO2 fixation11,12.
As an important enzyme, the evolution of RuBisCO has been debated, and when and how a RbcS subunit was first added to an (L2)n-type RuBisCO to yield a primitive form-I heterocomplex has been the subject of much investigation13,14,15,16,17. Recent analyses of metagenome-assembled genomes (MAGs) have identified a new clade of form-I RuBisCOs termed “form-I Anaero” that encodes RbcS subunits and is hypothesized to be a primitive form of the enzyme14. The RuBisCO-encoding MAGs were obtained from hot spring environments and belong to organisms related to anaerobic, thermophilic Bacteria14; notably, these RuBisCOs branch close to the last common ancestor of all known form-I RuBisCOs14.
Form-I RuBisCOs are encoded by the genes cbbL (large subunit) and cbbS (small subunit) and can be further categorized into four sub-forms: IA (found in Proteobacteria and Cyanobacteria), IB (Cyanobacteria and Prochlorales), IC (Proteobacteria and Chloroflexi), and ID (Proteobacteria and Eukaryotes) based on sequence homology of the proteins18. Biochemical studies of purple bacteria—model phototrophs for the study of photosynthesis—have shown that any of three forms of RuBisCO (I, II, IV) or variants (Aq, Ac, or C) of form-I can be present in these organisms with the genomes of many species encoding two or three forms or form-I variants (Supplementary Table 1). Although many of these enzymes have been isolated and biochemically characterized19only a handful of RuBisCO structures from these phototrophs are available and none from purple sulfur bacteria, the preeminent autotrophs of the purple bacterial group (Supplementary Table 1). For example, the purple bacterium Rhodospirillum (Rsp.) rubrum is well known for its form-II homodimeric (L2) RuBisCO whose unbound and RuBP-bound structures were determined at resolutions of 1.7 Å and 2.6 Å, respectively20,21. The structure of a form-II RuBisCO bound with an activated transition-state analog was reported at 1.85–2.38 Å resolution for the purple nonsulfur bacterium Rhodopseudomonas (Rps.) palustris; this enzyme forms a hexamer composed of three pairs of RbcL homodimers (L2)322. However, despite being the predominant form of RuBisCO in purple bacteria, the only structure of a form-I RuBisCO (form-IC) from these organisms was reported from Cereibacter (C., formerly Rhodobacter) sphaeroides at 3.4 Å resolution23,24.
To fill a major gap in our structural understanding of form-I RuBisCOs, here we present the cryo-EM structure of a form-IAq enzyme at high resolution (1.55 Å). The enzyme was isolated from the anaerobic and thermophilic purple sulfur bacterium Thermochromatium (Tch.) tepidum (γ-Proteobacteria), a phototrophic microbe that grows autotrophically in sulfidic hot springs in Yellowstone National Park, USA25. To our knowledge, the structure of the Tch. tepidum enzyme is the first form-IAq RuBisCO to be solved and thus provides the missing piece necessary for completing the evolutionary picture of form-I RuBisCOs. Moreover, because the Tch. tepidum RuBisCO is of a “plant-type” and is heat stable26,27its structure may reveal clues to its stability that could someday benefit plant agriculture. As a model purple sulfur bacterium, many Tch. tepidum proteins have shown high thermal and structural stability and have yielded crystal structures with higher resolutions than their counterparts from mesophilic species. These include the Tch. tepidum reaction center complex (RC, 2.2 Å)28light-harvesting–reaction center complex (LH1–RC, 1.9 Å)29various c-type cytochromes30,31and high-potential iron-sulfur protein (HiPIP, 0.48 Å)32. Structural analyses of the Tch. tepidum RuBisCO complement these milestones by providing a detailed view of a protein that does not participate in this phototroph’s photosynthetic light reactions but is nevertheless key to its photosynthetic success.
Results
Overall structure of the Tch. tepidum form-IAq RuBisCO
The Tch. tepidum genome contains two sets of form-I RuBisCO genes, one set encoding form-IAq and one set form-IAc33 (Supplementary Table 1). From our results here only form-IAq is expressed in cells grown at the optimal temperature (48 °C), and biochemical characterization of this enzyme was accomplished more than three decades ago26,27. In the present study, Tch. tepidum RuBisCO was highly purified by ammonium sulfate fractional precipitation. SDS-PAGE and gel filtration analyses demonstrated that Tch. tepidum RuBisCO was composed of two kinds of subunits, a 52.6-kDa large subunit and a 13.8-kDa small subunit, with a total molecular weight of about 530 kDa (Supplementary Fig. 1). The cryo-EM structure of the Tch. tepidum RuBisCO was determined at a global resolution of 1.55 Å (Supplementary Tables 2 and Supplementary Fig. 2). Based on the high-resolution density map, side chains of most residues were sufficiently resolved to identify them and confirm that the purified RuBisCO was indeed form-IAq; the final Tch. tepidum RuBisCO structure verified this conclusion.
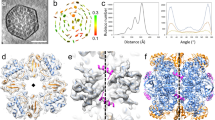
The overall structure of the Tch. tepidum form-IAq RuBisCO consists of eight RbcL subunits and eight RbcS subunits, yielding a hexadecamer with a 422-symmetry like other form-I RuBisCOs (Fig. 1a and b). The four large-subunit dimers assemble into a barreled core forming a solvent accessible channel that runs through the interior (Fig. 1a, Supplementary Fig. 3). Each RbcL subunit contained an N-terminal domain (residues 1–140) and a C-terminal domain (residues 141–472), and two RbcL subunits combined head (N-terminal) to tail (C-terminal) to form a functional dimer (Fig. 1c). The catalytic site of Tch. tepidum RuBisCO is formed by residues Lys167, Lys169, Lys193, Asp195 and Glu196 from the C-terminal domain of one RbcL subunit and three residues (Glu52 and Asn115) from the N-terminal domain of the adjacent RbcL subunit (Fig. 1c); these were expected because residues near the active site of form-I RuBisCOs are highly conserved3 (Fig. 2a). The C-terminal domain from Phe459 to Lys472 in the Tch. tepidum RbcL subunit was not resolved in the map. Moreover, Lys193 appears not to be carbamylated, and no metal ion is present; this structure thus represents the non-activated (and non-liganded) state of the enzyme (Fig. 1c). The four RbcS subunits in the Tch. tepidum form-IAq RuBisCO were positioned in the usual fashion for form-I enzymes but exhibited extensive intersubunit interactions through their extended N-terminal domains (Supplementary Fig. 3c); the latter structural feature of form-IAq RuBisCOs is distinct from that in other form-I RuBisCOs. In addition to RbcS intersubunit interactions, interactions between RbcL and RbcS subunits were also apparent, and all interactions are discussed in detail now.
Cryo-EM structure of Tch. tepidum form-IAq RuBisCO. (a,b) The electrostatic potential density map of Tch. tepidum form-IAq RuBisCO is shown in side (a), and top (b) views. (c) Structure of an RbcL dimer of the Tch. tepidum form-IAq RuBisCO shown in cartoon mode. The active sites are marked with red circles and are enlarged in the right panel.
Comparisons of amino acid sequences and structures of the RbcL subunits between Tch. tepidum form-IAq RuBisCO and the form-I RuBisCOs of Synechococcus elongatus, Cereibacter sphaeroides and Griffithsia monilis. The residue Pro297 and the conserved loop 6 are marked with a cyan pentagram and dashed box, respectively. Residue numbering for sequences and structural annotations (α, α-helix; β, β-strand; η, 310-helix; TT, tight β-turns) are relative to Tch. tepidum Rubisco. Alignments were performed using ClustalW53followed by manual curation of output files. Graphics were generated with ESPript54.
Intersubunit interactions in Tch. tepidum RuBisCO
The RbcL subunit of Tch. tepidum RuBisCO interacts extensively with one RbcS subunit mainly through four regions: Gln148–Arg159, Arg186–Gly188, Glu223–Arg227 and Trp403–Gln425. The RbcL subunit also interacts with a second RbcS subunit mainly through Gly171–Tyr182, and several water molecules mediate interactions on the interface between large and small subunits (Fig. 3 and Supplementary Fig. 4). The RbcS subunit of Tch. tepidum RuBisCO interacts with one RbcL subunit through its N-terminal domain (Met3–Tyr39) and C-terminal (Ala103–Glu109) domain and with a second RbcL subunit in the RbcL dimer through its mid-region (Glu55–Lys66) (Supplementary Fig. 4).
Inter-subunit interactions in the Tch. tepidum form-IAq RuBisCO. (a) A structural unit composed of an RbcL dimer and four associated RbcS subunits. (b) Close contacts (< 3.5 Å, hereafter) between RbcL-1 and RbcS-1. (c,d) Close contacts between RbcL-1 and RbcS-2. (e) Close contacts between RbcS-1 and RbcS-2. The conserved residues present in Tch. tepidum form-IAq RuBisCO and all three plant form-I RuBisCOs (Supplementary Fig. 7) are marked with asterisks.
Form-IAq RuBisCOs are characterized by an extended N-terminal domain in the RbcS subunit (Fig. 4 and Supplementary Fig. 4) that contains a six-residue insertion4. Each RbcS subunit of the Tch. tepidum RuBisCO connects two RbcL dimers and further interacts with an adjacent RbcS subunit through this N-terminal insert (Ser10–Asn15). This forms an extended loop (Fig. 3e and Supplementary Fig. 4) that helps stabilize the entire RuBisCO molecule. In addition, the N-terminal domain (Met3–Glu12) of an RbcS subunit interacts with the mid-region (His51–Leu67) of an adjacent RbcS subunit. Among these, Glu12 forms hydrogen bonds with both Glu55 and His56 in the adjacent RbcS subunit (Fig. 3e). Notably, this Glu12 is located in the N-terminal extended loop formed by the unique six-residue insert present in form-IAq RuBisCOs (Fig. 4 and Supplementary Fig. 4).
Sequence alignments of the RbcS subunits between Tch. tepidum form-IAq RuBisCO and other form-I RuBisCOs. Sequence similarities calculated by ClustalW are indicated on the right side of the top rows. The six-residue insertion in the N-terminal domain of form-IAq RuBisCO is boxed in red, and two additional β-strands in the form-IC and ID RuBisCOs are boxed in green. Sequence and structural annotations are the same as in Fig. 2.
Structural comparison of form-I RuBisCOs between Tch. tepidum and other species
Among form-I RuBisCOs with known structures, the Tch. tepidum form-IAq RuBisCO showed the highest structure and amino acid sequence similarities with form-IAc RuBisCO (cloned and expressed in E. coli) from the chemolithotrophic and autotrophic bacterium Halothiobacillus (H.) neapolitanus (γ-Proteobacteria), a bacterium that grows only on an inorganic sulfur compound (such as thiosulfate) as the electron donor and CO2 as carbon source. A search at the Dali server revealed that the structure of the Tch. tepidum form-IAq RuBisCO had the highest Z-scores with the RbcL (PDB: 7SMK) and RbcS (PDB: 6UEW) subunits of the H. neapolitanus form-IAc RuBisCO (Z-scores of 63 and 20, respectively), corresponding to root mean square deviations (RMSDs) of 0.5 Å and 0.6 Å, respectively34,35,36. These high structural similarities reflect high similarities of amino acid sequences as indicated by ClustalW values of 88 and 55 for RbcL and RbcS subunits, respectively, for Tch. tepidum and H. neapolitanus RuBisCOs (Supplementary Fig. 5).
It is noteworthy that in terms of structural similarity to the Tch. tepidum form-IAq RuBisCO indicated by the Dali server, the top group also included a form-I RuBisCO from a sequence reconstruction of the last common RuBisCO ancestor (PDB: 7QSW)14. This sequence showed high Z-scores of 58 (RMSD: 0.8 Å) and 18 (RMSD: 1.0 Å) for RbcL and RbcS subunits, respectively, implying a close relationship between the ancestral sequence and Tch. tepidum RuBisCO. Sequences of the synthetic constructs were designed based on phylogenetic analysis of the form-I Anaero RuBisCO for the ancestral RbcL (AncLS) and RbcS (AncSSU) subunits14. Both AncLS and AncSSU showed high sequence similarities to those of Tch. tepidum RbcL and RbcS subunits (Supplementary Fig. 5). The structure of the Tch. tepidum RbcL subunit is also similar to that of the RbcL subunits of form-IB RuBisCO from Synechococcus (S.) elongatus (PDB: 1RSC, Z-score: 58), form-IC from C. sphaeroides (PDB: 5NV3, Z-score: 51) and form-ID from the red alga Griffithsia (G.) monilis (Eukarya) (PDB: 8BDB, Z-score: 55) with Cα RMSDs from 0.9 to 1.2 Å (Fig. 2b). This is consistent with the high sequence identities between the RbcL subunits of Tch. tepidum, S. elongatus, C. sphaeroides and G. monilis (Fig. 2a).
Compared with ligand-bound RuBisCOs, a large conformational change was observed for loop 6 in the Tch. tepidum apo-RuBisCO (Fig. 2b, Supplementary Fig. 6a and 6b), and slightly different conformations were found for the sidechains of the residues around the catalytic site (Supplementary Fig. 6c and 6d). The flexible loop 6 is composed of ten conserved residues (G321TVVGKLEGD330) (Supplementary Fig. 5) located close to the catalytic site, and movement of loop 6 is an important mechanism for catalysis and specificity of the enzyme through transitions between open (unliganded or loosely liganded) and closed (tightly liganded) states37,38. In this regard, the structure of Tch. tepidum form-IAq RuBisCO presented here corresponds to the open state.
Comparisons of the Tch. tepidum, Alc. vinosum, and plant form-I RuBisCOs
Comparative analyses revealed distinct thermostability characteristics between the RuBisCOs of thermophilic Tch. tepidum and mesophilic Alc. vinosum. Despite their high sequence similarity (Fig. 5), which suggests comparable structural folding patterns, the Tch. tepidum RuBisCO exhibits significantly enhanced thermal stability and a higher optimal activity temperature26,27. Detailed sequence alignments demonstrated a striking disparity in substitution rates between subunits in the two species: compared with Alc. vinosum, the RbcS subunits of Tch. tepidum showed 9.3% amino acid substitutions but only 3.6% in RbcL subunits. This differential substitution pattern implies that RbcS modifications may play a predominant role in conferring thermostability on the enzyme. In the Tch. tepidum RuBisCO structure, all 11 substitutions in the RbcS subunit (except for the invisible Asp3) and 13 out of the 17 substitutions (two invisible) in the RbcL subunit are located on the hydrophilic surface of the enzyme exposed to solvents (Fig. 5c). Among the substitutions in the Tch. tepidum RbcS subunit, Gly20, Tyr99 and Thr105 have close contacts (< 3.5 Å) with the RbcL subunit (RbcS-1 in Supplementary Fig. 4), and His56 interacts with both an RbcL subunit and an adjacent RbcS subunit (labeled RbcS-1 and RbcS in Supplementary Fig. 4). These results suggest that these substitutions create subtle structural adjustments in the Tch. tepidum RuBisCO that increase favorable (and/or reduce unfavorable) subunit interactions and optimize interactions between the enzyme and solvent necessary to remain catalytically robust at the temperature of this phototroph’s hot spring habitat.
Comparison of the amino acid sequences of the Tch. tepidum and Alc. vinosum RuBisCO proteins. (a,b) Sequence alignment of the large subunits (a) and small subunits (b) of the Tch. tepidum and Alc. vinosum RuBisCO proteins. The substitutions distributed on the hydrophilic surface of the subunits are marked with hollow triangles (∆). Substitutions buried within the subunits are marked with filled triangles (▲). (c) Distribution of substitutions on the surfaces of the Tch. tepidum RuBisCO subunits in side and top views. Substitutions are colored in red.
When compared to plant form-I RuBisCOs (Arabidopsis thaliana, Spinacia oleracea, Nicotiana tabacum), the Tch. tepidum enzyme shares 72–74% sequence identity in large subunits but only 33–38% in small subunits (Supplementary Fig. 7). This contrast becomes more pronounced when considering substitution quantities: plant enzymes exhibit remarkably higher substitutions, accumulating > 130 variations in large subunits and > 80 variations in small subunits, compared to the limited variations in Alc. vinosum RuBisCO. Intriguingly, 10 large subunit and 8 small subunit substitutions are conserved between Alc. vinosum and plant enzymes, potentially identifying key residues that could be thermostability targets that could inform the design of heat-resistant plant RuBisCOs. Moreover, a critical structural distinction emerges in the RbcS subunit architecture. Plant RuBisCOs uniquely possess a 12-residue insertion containing the conserved “GFVYRE” motif, forming an extended βA-βB loop that protrudes into the central solvent channel (Fig. 6). This structural element, absent in the RuBisCOs of Tch. tepidum and Alc. vinosum, has been previously associated with modulation of holoenzyme stability and catalytic parameters9,38.
Structural comparisons of the two adjacent RbcS subunits (RbcS1 and RbcS2) between Tch. tepidum form-IAq RuBisCO and other representative form-I RuBisCOs. The six-residue insert in the N-terminal domain is shown in red. All other structures are superimposed with that of Tch. tepidum (gray). Form-IAc: H. neapolitanus (cyan, PDB: 7SMK), form-IB: Spinacia oleracea (green, PDB: 1RXO), form-IBc: Synechococcus elongatus PCC6301 (pink, PDB: 1RSC), form-IC: Cereibacter sphaeroides (orange, PDB: 5NV3), form-ID: Griffithsia monilis (slate blue, PDB: 8BDB). βE and βF indicate two additional β-strands in the form-IC and ID RbcS subunits. The extended βA-βB loop in the Spinacia oleracea RbcS subunit is indicated by an arrow.
Discussion
Here we detail the first structure of a form-IAq RuBisCO. The enzyme, purified from the thermophilic phototrophic bacterium Tch. tepidum and detailed at high resolution, provides the last missing piece in the structures of known RuBisCOs. The overall structure of Tch. tepidum form-IAq RuBisCO most closely resembles the form-IAc RuBisCO from the chemolithotroph H. neapolitanus and also that of a reconstructed primitive RuBisCO from a hot spring bacterium. The former is unsurprising because both Tch. tepidum and H. neapolitanus are γ-Proteobacteria, whereas the latter may reflect an underlying structural relationship between thermostable RuBisCOs in general and is thus intriguing in terms of both the evolution of form-I RuBisCOs and the structural prerequisites for thermal-stable forms of this enzyme.
The fashioning of RuBisCO from its last common ancestor was accomplished by phylogenetic analysis of a form-I Anaero RuBisCO large (AncLS) subunit reconstructed from a metagenome-assembled genome (MAG). It is hypothesized that this large subunit eventually gained an ancestral small subunit (AncSSU) to yield a primitive form-I (L8S8) heterocomplex14. Extant form-I Anaero RuBisCOs have been detected in anaerobic, thermophilic bacteria of the phylum Calditrichaeota that inhabit iron-rich hot springs39,40. The amino acid sequence of a form-I Anaero RuBisCO (GenBank: RMG64267.1) encoded from the MAG of a species of Calditrichaeota inhabiting a hot spring in Jinata Onsen, Japan revealed relatively high sequence similarities to the Tch. tepidum form-IAq RuBisCO for both RbcL and RbcS subunits (Supplementary Fig. 5), signaling a relationship between form-IAq and form-I Anaero RuBisCOs. Because form-I RuBisCOs are thought to have an anaerobic, thermophilic origin14and no structure of a native form-I Anaero RuBisCO exists, the Tch. tepidum RuBisCO structure detailed herein is currently the best model of a primitive form-I RuBisCO available.
Compared with other form-I RuBisCOs, form-IA RuBisCOs are unique in possessing an N-terminal extension in their RbcS subunits (Supplementary Fig. 5), and form-IAq RuBisCOs contain a unique six-residue insertion in the RbcS N-terminal region absent from form-IAc RuBisCOs4 (Fig. 4). Notably, the structure of the thermostable Tch. tepidum form-IAq RuBisCO shows extensive interactions between the adjacent RbcS subunits, a feature not seen to this extent in other form-I RuBisCOs (Fig. 6). For the most part, these interactions occur through residues in the six-residue insertion that form an extended loop on the top exterior face of the protein (Figs. 3d and 6, Supplementary Fig. 3c). Interactions between this insert and an adjacent RbcS subunit were previously predicted but have now been documented in our work4. Sequence comparisons of the Tch. tepidum and Alc. vinosum RbcS subunits (Fig. 5) bolster the conclusion that substitutions in the Tch. tepidum RbcS evolved to exploit interactions between the extended loop and RbcS subunits and thereby stabilize the enzyme to elevated temperatures.
Although likely essential for heat stability, RbcS subunit interactions may not be the sole mechanism responsible for this characteristic of the Tch. tepidum RuBisCO, as a variety of heat stability mechanisms have been implicated from studies of other Tch. tepidum proteins. For example, although amino acid identity between high-potential iron-sulfur proteins (HiPIPs) of Tch. tepidum and Alc. vinosum is about 90%41, Tch. tepidum HiPIP showed enhanced thermostability attributed to subtle sequence differences that affected the protein’s overall structure41,42,43. Moreover, studies of Tch. tepidum cytochrome c′ and flavocytochrome c revealed several other factors that contributed to the thermostabilities of these soluble proteins30 including differences in (i) the number of hydrogen bonds and charged and polar residues, (ii) the number of residues with shorter sidechains (Gly and Ala), and (iii) the distribution of water molecules on both the surface and interior of the proteins. One or more of these factors may also contribute to thermostability of Tch. tepidum RuBisCO.
Since the Tch. tepidum RuBisCO is a “plant-type” (form-I) enzyme, the RbcS interactions revealed here may provide useful clues for engineering heat stability into plant-type RuBisCOs. Although it is clear that net CO2 assimilation in plants is heat sensitive44, thus far surprisingly little structural information has been published on heat-stable form-I RuBisCOs, as most studies have focused on the dimeric form-III enzymes from hyperthermophilic Archaea that function above 100°C45. However, as Earth’s climate warms, heat-active plant RuBisCOs—especially ones like the Tch. tepidum enzyme that functions optimally at mildly elevated temperatures—will eventually be essential to maintain agricultural productivity at the levels required to feed future human and animal populations. Thus, besides filling the last remaining gap in our understanding of RuBisCO structures, details of the Tch. tepidum enzyme may have applications for creating heat-tolerant plant RuBisCOs.
Materials and methods
Purification of Tch. tepidum RuBisCO
Cells of Tch. tepidum were grown anaerobically for 7 days at 49 °C with continuous illumination. Ten liters of cells were collected at 4000 g for 10 min, and the pellet was resuspended in 100 ml Tris buffer (20 mM Tris-HCl, pH 8.0; 1 mM MgCl2; 20 µg/ml DNase I). After pre-cooling on ice for 20 min, cells were broken using an ultrasonic crusher (TOMY, UD-211). The lysate was centrifuged at 4 °C at 17,000 g for 20 min and the pellet discarded. The supernatant was then ultracentrifuged at 150,000 g at 4 °C for 70 min. The resulting supernatant was filtered through a 0.45 μm membrane filter before undergoing anion exchange column chromatography (Q-Sepharose High performance (GE)) using an AKTA-FPLC protein purification system. After loading the sample, the column was washed first with Tris buffer containing 160 mM NaCl and then with a gradient elution with the concentration of NaCl increasing from 160 to 300 mM. The sample eluted with 250–270 mM NaCl was collected and concentrated using 30 kDa ultrafiltration tubes (1000 g, 20 min). Subsequently, the sample was precipitated with 50% and 65% ammonium sulfate successively. The samples precipitated with 65% ammonium sulfate were resuspended with Tris buffer (20 mM Bis-Tris-HCl, pH 6.0) and loaded on a second anion exchange column. The column was eluted with a gradient of 150–300 mM NaCl. The first elution peak was collected, concentrated and further isolated by size exclusion chromatography (Superose 6 Increase 10/300 (GE)) with Tris buffer containing 200 mM NaCl. A high-purity RuBisCO sample was eluted with a sharp peak around 15 ml that was collected and concentrated with 100 kDa ultrafiltration tubes (Millipore) to a suitable final concentration for cryo-EM analysis.
Cryo-EM sample preparation and data collection
To achieve a high-resolution reconstruction, concentrated Tch. tepidum RuBisCO proteins of 50 mg/ml were plunge-frozen in liquid ethane using a Vitrobot (FEI) and the vitrified sample was subsequently imaged on a 300 kV FEI Titan Krios cryo-electron microscope equipped with a Falcon4 detector and a Selectris (TFS) image filter. EasyGlow (Pelco) was used to glow the grids (Quantifoil Cu1.2/1.3 300 mesh) and make the surface hydrophilic to facilitate an even layer on the grid surface. The glow current intensity was set to 20 mA, and the glow time was 2 min. An aliquot of 3 µL purified Tch. tepidum RuBisCO complex sample (50 mg/ml) was applied to a treated grid and then the grids were blotted for 4 s at a humidity of 100% and 20 oC and plunge-frozen in liquid ethane using a Vitrobot (TFS).
Using EPU, cryo-EM images of Tch. tepidum RuBisCO were recorded on an FEI Titan Krios electron microscope operated at 300 kV under a nominal magnification of 270,000. The microscope was carefully aligned before data collection, including the coma-free alignment to minimize the effects of beam tilt. The dose rate of the electron beam was set to ~ 24 e−/Å2/s, and movies were recorded on a Falcon4 detector equipped with Selectris (TFS) for 3 s with a total dose of ~ 72 e−/Å2 on the specimen using EPU (TFS).
Cryo-EM image processing
MotionCor2 was used to correct drift for each image, and using Gctf to measure the contrast transfer function of the corrected images46,47. Relion 4.0 and cryoSPARC3.1 were used to complete subsequent data processing48,49. Through the autopick function in Relion, 2,137,842 particles were selected from 6,571 images and then cryoSPARC was used for the first 3D classification, resulting in 907,092 particles. An additional round of 3D classification was performed on these particles using Relion, resulting in 765,738 particles. After subjecting these particles to 3D refinement, CTF refinement, and polishing in Relion, and imposing the D4 axis of symmetry, a 3D map sharpened at a B-factor of − 25 Å2 was obtained at a resolution of 1.55 Å according to the gold standard FSC (Fourier shell correlation) at 0.143. The specific process is shown in Supplementary Fig. 2.
Modeling and refinement
The initial model of Tch. tepidum RuBisCO was constructed by using the RuBisCO structure from a cyanobacterium (PDB code: 6LRR) as a reference and fitting it into the corresponding density map of Tch. tepidum RuBisCO using UCSF Chimera50. This was followed by manually replacing and editing the model using Coot to obtain the Tch. tepidum atomic model51. Subsequently, PHENIX was used to optimize the atomic model, and manually adjust it using Coot to obtain the final RuBisCO atomic model52. The relevant model building parameters are shown in Supplementary Table 2.
Data availability
The datasets generated and/or analyzed during the current study are available in the wwPDB repository, https://www.rcsb.org/structure/unreleased/9IQO.
References
Field, C. B., Behrenfeld, M. J., Randerson, J. T. & Falkowski, P. Primary production of the biosphere integrating terrestrial and oceanic components. Science 28, 237–240 (1998).
Erb, T. J. & Zarzycki, J. A short history of rubisco: the rise and fall (?) of nature’s predominant CO2 fixing enzyme. Curr. Opin. Biotechnol. 49, 100–101 (2018).
Tabita, F. R., Satagopan, S., Hanson, T. E., Kreel, N. E. & Scott, S. S. Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J. Exp. Bot. 59, 1515–1524 (2007).
Badger, M. R. & Bek, E. J. Multiple Rubisco forms in proteobacteria: their functional significance in relation to CO2 acquisition by the CBB cycle. J. Exp. Bot. 59, 1525–1541 (2008).
Newman, J. & Gutteridge, S. Structure of an effector-induced inactivated state of ribulose 1,5-bisphosphate carboxylase Oxygenase the binary complex between enzyme and xylulose 1,5-bisphosphate. Structure 2, 495–502 (1994).
Taylor, T. C. & Andersson, I. Structure of a product complex of spinach ribulose-1,5-bisphosphate carboxylase Oxygenase. Biochemistry 36, 4041–4046 (1997).
Valegård, K., Hasse, D., Andersson, I. & Gunn, L. H. Structure of Rubisco from Arabidopsis thaliana complex with 2-carboxyarabinitol-1,5-bisphosphate. Acta Crystallogr. D. 74, 1–9 (2018).
Zhou, Y., Gunn, L. H., Birch, R., Andersson, I. & Whitney, S. M. Grafting Rhodobacter sphaeroides with red algae Rubisco to accelerate catalysis and plant growth. Nat. Plants. 9, 978–986 (2023).
Taylor, T. C., Backlund, A., Bjorhall, K., Spreitzer, R. J. & Andersson, I. First crystal structure of Rubisco from a green alga, Chlamydomonas reinhardtii. J. Bio. Chem. 276, 48159–48164 (2001).
Tabita, F. R. et al. Function, structure, and evolution of the rubisCO-like proteins and their Rubisco homologs. Microbiol. Mol. Biol. Rev. 71, 576–599 (2007).
Hanson, T. E. & Tabita, F. R. A ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco)-like protein from Chlorobium tepidum that is involved with sulfur metabolism and the response to oxidative stress. PNAS 98, 4397–4402 (2001).
Ashida, H. et al. A. A functional link between Rubisco-like protein of Bacillus and photosynthetic Rubisco. Science 302, 286–290 (2003).
Banda, D. M. et al. Novel bacterial clade reveals origin of form I Rubisco. Nat. Plants. 6, 1158–1116 (2020).
Schulz, L. et al. Evolution of increased complexity and specificity at the dawn of form I Rubiscos. Science 378, 155–160 (2022).
Prywes, N., Phillips, N. R., Tuck, O. T., Valentin-Alvarado, L. E. & Savage, D. F. Rubisco function, evolution, and engineering. Annu. Rev. Biochem. 92, 385–410 (2023).
Liu, A. K. et al. Deep-branching evolutionary intermediates reveal structural origins of form I Rubisco. Curr. Biol. 33, 5316–5325 (2023).
Liu, A. K. et al. Structural plasticity enables evolution and innovation of Rubisco assemblies. Sci. Adv. 8, eadc9440 (2022).
Waheeda, K., Kitchel, H., Wang, Q. & Chiu, P. L. Molecular mechanism of Rubisco activase: dynamic assembly and Rubisco remodeling. Front. Mol. Biosci. 10, 1125922 (2023).
Tabita, F. R. Anoxygenic Photosynthetic Bacteria. Advances in Photosynthesis and Respiration Ch 41 (Springer, 1995).
Schneider, G., Lindqvist, Y. & Lundqvist, T. Crystallographic refinement and structure of ribulose-1,5-bisphosphate carboxylase from Rhodospirillum rubrum at 1.7 Å resolution. J. Mol. Biol. 211, 989–1008 (1990).
Lundqvist, T. & Schneider, G. Crystal structure of activated ribulose-1,5-bisphosphate carboxylase complexed with its substrate, ribulose-1,5-bisphosphate. J. Biol. Chem. 266, 12604–12611 (1991).
Satagopan, S., Chan, S., Perry, L. J. & Tabita, F. R. Structure-function studies with the unique hexameric form II ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) from Rhodopseudomonas palustris. J. Biol. Chem. 289, 21433–21450 (2014).
Bhat, J. Y. et al. Mechanism of enzyme repair by the AAA+ chaperone Rubisco activase. Mol. Cell. 67, 744–756 (2017).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Cryst. Sect. D: Struct. Biol. 74, 814–840 (2018).
Madigan, M. T. A novel photosynthetic purple bacterium isolated from a Yellowstone hot spring. Science 225, 313–315 (1984).
Heda, G. D. & Madigan, M. T. Thermal properties and oxygenase activity of ribulose-1,5-bisphosphate carboxylase from the thermophilic purple bacterium, Chromatium tepidum. FEMS Microbiol. Lett. 51, 45–50 (1988).
Heda, G. D. & Madigan, M. T. Purification and characterization of the thermostable ribulose-1,5‐bisphosphate carboxylase/oxygenase from the thermophilic purple bacterium Chromatium tepidum. Eur. J. Biochem. 184, 313–319 (1989).
Nogi, T., Fathir, I., Kobayashi, M., Nozawa, T. & Miki, K. Crystal structures of photosynthetic reaction center and high-potential iron-sulfur protein from Thermochromatium tepidum thermostability and electron transfer. PNAS 97, 13561–13566 (2000).
Yu, L. J., Suga, M., Wang-Otomo, Z. Y. & Shen, J. R. Structure of photosynthetic LH1–RC supercomplex at 1.9 Å resolution. Nature 556, 209–213 (2018).
Hirano, Y., Kimura, Y., Suzuki, H., Miki, K. & Wang, Z. Y. Structure analysis and comparative characterization of the cytochrome c′ and Flavocytochrome c from thermophilic purple photosynthetic bacterium Thermochromatium tepidum. Biochemistry 51, 6556–6567 (2012).
Chen, J. H. et al. Properties and structure of a low-potential, penta-heme cytochrome c552 from a thermophilic purple sulfur photosynthetic bacterium Thermochromatium tepidum. Photosynth. Res. 139, 281–293 (2018).
Hirano, Y., Takeda, K. & Miki, K. Charge-density analysis of an iron–sulfur protein at an ultra-high resolution of 0.48 Å. Nature 534, 281–284 (2016).
Sattley, W. M. et al. Complete genome of the thermophilic purple sulfur bacterium Thermochromatium tepidum compared to Allochromatium vinosum and other Chromatiaceae. Photosynth. Res. 151, 125–142 (2022).
Holm, L. Dali server: structural unification of protein families. Nucleic Acids Res. 50, W210–W215 (2022).
Blikstad, C. et al. Identification of a carbonic anhydrase-rubisco complex within the alpha-carboxysome. PNAS 120, e2308600120 (2023).
Oltrogge, L. M. et al. Multivalent interactions between CsoS2 and Rubisco mediate α-carboxysome formation. Nat. Struct. Mol. Biol. 27, 281–228 (2020).
Duff, A. P., Andrews, T. J. & Curmi, P. M. G. The transition between the open and closed States of Rubisco is triggered by the inter-phosphate distance of the bound bisphosphate. J. Mol. Biol. 298, 903–916 (2000).
Andersson, I. & Backlund, A. Structure and function of Rubisco. Plant. Physiol. Biochem. 46, 275–291 (2008).
Fortney, N. W., He, S., Converse, B. J., Boyd, E. S. & Roden, E. E. Investigating the composition and metabolic potential of microbial communities in chocolate pots hot springs. Front. Microbiol. 9, 2075 (2018).
Ward, L. M. et al. Geochemical and metagenomic characterization of Jinata onsen, a proterozoic-analog hot spring, reveals novel microbial diversity including iron-tolerant phototrophs and thermophilic lithotrophs. Microbes Environ. 34, 278–292 (2019).
Moulis, J. M. et al. Primary structure of Chromatium tepidum high–potential iron-sulfur protein in relation to thermal denaturation. Arch. Biochem. Biophys. 305, 186–192 (1993).
Kobayashi, M., Saito, T., Takahashi, K., Wang, Z. Y. & Nozawa, T. Electronic properties and thermal stability of soluble redox proteins from a thermophilic purple sulfur photosynthetic bacterium, Thermochromatium tepidum. Bull. Chem. Soc. Jpn. 78, 2164–2170 (2005).
Liu, L., Nogi, T., Kobayashi, M., Nozawa, T. & Miki, K. Ultrahigh-resolution structure of high-potential iron–sulfur protein from Thermochromatium tepidum. Acta Crystallogr. D. 58, 1085–1091 (2002).
Scafaro, A. P., Posch, B. C., Evans, J. R., Farquhar, G. D. & Atkin, O. K. Rubisco deactivation and chloroplast electron transport rates co–limit photosynthesis above optimal leaf temperature in terrestrial plants. Nat. Commun. 14, 2820 (2023).
Bundela, R., Keown, J., Watkin, S. & Pearce, F. G. Structure of a hyperthermostable dimeric archaeal Rubisco from Hyperthermus butylicus. Acta Crystallogr. D. 75, 536–544 (2019).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 14, 290–296 (2017).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Goddard, T. D. et al. UCSF chimerax: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. 66, 486–501 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D. 68, 352–367 (2012).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new endscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Acknowledgements
We thank the staff from the Center of Cryo-Electron Microscopy (CCEM), Zhejiang University for their technical assistance on cryo-EM data collection, and Dr. Cheng Ma from the Protein facility, School of medicine, Zhejiang University for his assistance on protein purification. This work was supported by a Natural Science Foundation of Zhejiang Province, China (LR22C010001 to J.-H.C.) and National Natural Science Foundation of China (32100202 to J.-H.C). MTM was supported in part by NASA Cooperative Agreement 80NSSC21M0355.
Author information
Authors and Affiliations
Contributions
J.-H.C, X.Z. and Z.‑Y.W.‑O. initialized the research. J.-H.C., W.W. and L.-J.Y. purified the protein sample. S.C. prepared the cryo-sample, collected the data, build the atomic model. J.-H.C., X.Z., Z.‑Y.W.‑O., M.T.M., S.C., W.W. and H.G. analyzed the data, interpreted the structures, prepared the figures and wrote the paper. All authors discussed and commented on the results and the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chang, S., Wang, W., Madigan, M.T. et al. High-resolution structure of the heat-stable form-IAq RuBisCO from the thermophilic purple sulfur bacterium Thermochromatium tepidum. Sci Rep 15, 22941 (2025). https://doi.org/10.1038/s41598-025-07081-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07081-8