Abstract
The coal spontaneous combustion (CSC) in the goaf of a coal mine poses a significant safety hazard. This study investigates the gas production characteristics, apparent activation energy, and limiting parameters of coal samples with varying particle sizes during the low-temperature oxidation stage under both primary and secondary oxidation conditions through a temperature-programmed experiment. The results indicate that smaller particle size leads to higher rates of O2 consumption, CO generation, and heat release intensity in coal samples; The O2 consumption rate, CO generation rate, and heat release intensity during the secondary oxidation of coal samples are relatively high in the initial stage of the experiment. However, in the later stage of the experiment, there is a reversal in these parameters; the selection of the most appropriate model from the 22 commonly observed reaction kinetics mechanism functions revealed that the apparent activation energy of the coal sample decreases during secondary oxidation compared to primary oxidation within the temperature range of 80℃ to 130℃. However, a reversal occurs between temperatures of 140℃ and 170℃, indicating that secondary oxidation initially enhances the low-temperature oxidation characteristics but weakens them in later stages; The various particle sizes under both primary and secondary oxidation conditions significantly influence the limit parameters of CSC, with secondary oxidation being more prone to inducing SC of coal in goaf compared to primary oxidation.
Similar content being viewed by others
Introduction
The phenomenon of coal spontaneous combustion (CSC) involves the exothermic reaction between coal and atmospheric O2, resulting in a gradual increase in temperature until it surpasses the ignition point of coal1,2. Approximately 60% of coal spontaneous combustion (SC) incidents occur in the goaf area of coal mines in China3. The occurrence of SC in the goaf poses a significant threat to personnel safety within the mining vicinity and results in resource wastage and environmental pollution4,5,6,7,8. In order to optimize resource utilization and enhance mining efficiency, it is sometimes necessary to re-mine the residual coal in the working face of the mining area, which leads to a renewed reaction between broken coal fragments and O2, resulting in an elevated temperature to form the secondary oxidation9,10. Secondary oxidation enhances the likelihood of CSC, resulting in more intricate phenomena and escalating the challenges associated with preventing and controlling SC in goaf areas11. Therefore, to prevent the occurrence of hazardous coal mine accidents, it is crucial to investigate the mechanism of secondary oxidation characteristics in coal samples with varying particle sizes.
In recent years, numerous scholars have investigated the primary oxidation characteristics of coal samples with varying particle sizes from perspectives such as gas generation patterns and limiting parameters. Among them, Yu et al.12 examined the impact of particle size on gas generation laws and discovered that smaller particle sizes in coal samples correlate with higher O2 consumption. Zhang et al.13 investigated the impact of coal particle size on CO gas production during the SC process using a custom-designed experimental apparatus. The findings indicated that larger particle sizes were associated with a slower rate of CO generation. Jia et al.14 investigated the oxidation characteristics of six groups of coal samples with varying particle sizes during the heating process and discovered that smaller particle sizes resulted in larger specific surface areas, thereby enhancing the coal-oxygen binding capacity. Wang et al.15 employed a temperature-programmed system to investigate the gas generation characteristics of coals with varying particle sizes during the oxidation process. They identified the indicator gases at different oxidation stages and classified the coal oxidation process into three distinct phases: slow oxidation, rapid oxidation, and accelerated oxidation. Similarly, Li et al.16 utilized the same experimental setup to examine the SC limit parameters of coal samples with different particle sizes. Their findings revealed that the limiting parameters of the coal samples exhibited a quadratic polynomial relationship with particle size.
In the re-mining working face of the mining area, pre-oxidized coal typically undergoes secondary oxidation. Several scholars have investigated the secondary oxidation characteristics of coal samples with varying particle sizes from the perspectives of free radicals and activation energy. For instance, Xu et al.17,18 investigated the secondary oxidation process of coal powder’s active structure based on ERP and FTIP technologies. They analyzed the free radicals before and after primary and secondary oxidations of coal powder using both radical theory and functional group theory. It was found that primary oxidation can enhance the oxidative property of fatty hydrocarbon structures, promoting the secondary oxidation process. Additionally, reducing the particle size of coal powder can shorten the fatty side chains after secondary oxidation, thereby improving the secondary oxidative activity of coal. Yang et al.19 analyzed the concentration of free radicals in coal samples with varying particle sizes during both primary and secondary oxidation processes, and found that the concentration of free radicals in coal samples decreased in the following order: secondary oxidized coal, oxidized coal, and raw coal. Jing et al.20 used TG-DSC experiments to study the characteristics of different particle sizes of secondary oxidized coal and found that as the particle size decreases, the heat release increases and the activation energy during the secondary oxidation SC stage decreases, indicating an increased risk of SC.
During the aforementioned research process, scholars investigated the SC behavior of coal samples with varying particle sizes by separately analyzing primary oxidation and secondary oxidation. Given that coal SC induced by secondary oxidation is relatively complex, this study investigates the low-temperature oxidation characteristics of coal samples with different particle sizes during both primary and secondary oxidation processes through a programmed heating experiment. The gas species analysis of the low-temperature oxidation process is conducted using a gas chromatograph, and based on this, the concentration relationship curves of O2, CO, and CO2 gases are established. Additionally, by comparing the heat release intensity, apparent activation energy, and limiting parameters at different stages of primary and secondary oxidation processes, the primary and secondary SC characteristics of coal samples as well as their differences are summarized based on the collected data. The research findings have certain guiding significance for addressing CSC phenomena occurring in goaf re-mining workfaces and close-range coal seam mining.
Experimental methods and procedures
Preparation of coal samples
The experimental coal samples were obtained from the Zuoyun Donggucheng Coal Mine of the Company of the Coal Import and Export Group in Shanxi, China. Sizeable chunks of fresh brown coal were securely packed into hermetically sealed bags and subsequently transported to the laboratory. The experimental coal samples were subjected to a pressure of 25 MPa using a liquid press device for crushing. Subsequently, the resulting coal samples were categorized into five groups based on particle size: 0.5 mm, 1 mm, 2.36 mm, 5–10 mm, and mixed particle sizes. Conduct weighing of five coal sample groups, each representing a different particle size range, using a precision balance. Ensure that 200 g of coal samples are weighed for each group. The coal sample underwent pre-treatment in a vacuum drying oven at 40℃ for the temperature rise test. The results of the proximate analysis of the experimental coal samples are shown in Table 1, and the particle size range and numbers for the coal samples are presented in Table 2.
Experimental device
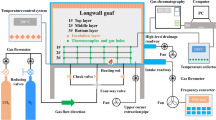
The experimental device for this paper is shown in Fig. 1, which mainly includes gas supply system, temperature control system, coal tank and gas chromatograph. The coal sample tank used in this experiment is a purple copper can with an inner diameter of Ф=39 mm, an outer diameter of Ф=42 mm, and a height of H = 270 mm. The sample is filled to a height of h = 240 mm. The temperature at the center of the coal sample is recorded as the coal temperature using a K-type thermocouple. Heating is conducted through a copper intake pipe with an inner diameter of Ф=3 mm, an outer diameter of Ф=4 mm, and a length of L = 50 m.
Experimental procedure
Connect the coal sample tank to the gas supply system and adjust the airflow rate to 150 ml/min. Then, set the temperature ramp rate of the temperature-programmed experimental device to 0.5℃/min for low-temperature oxidation. The gas produced from the coal sample is collected at intervals of 10℃ during this period and analyzed using a gas chromatograph. When the temperature reaches 170℃, cease heating and switch the air supply in the gas supply system from air to nitrogen for cooling treatment of the coal sample. Subsequently, cool down the coal sample to 30℃ under a nitrogen atmosphere before proceeding with secondary heating. The steps for secondary heating remain identical to those employed during primary heating. Finally, initiate cooling using nitrogen gas at a flow rate of 250 ml/min for a duration of 30 min while simultaneously opening the door of the electric constant temperature drying box to allow for temperature reduction by the temperature-programmed experimental device.
Experimental results and analysis
The variations in O2 consumption during the primary and secondary coal oxidation under different particle sizes
The variations in the dynamics of O2 concentration
The O2 concentration serves as a crucial indicator of the SC process in coal, as it encompasses chemical reactions and consumption of O221. Figure 2 illustrates the variation in O2 concentration within the temperature range of 40℃ to 170℃ during programmed temperature experiments conducted after primary and secondary oxidation of coal samples with different particle sizes. A lower O2 concentration signifies higher O2 consumption during the low-temperature oxidation stage of coal, indicating a more vigorous oxidation reaction.
The figure clearly illustrates that the O2 concentration in the coal sample exhibits a consistent decreasing trend during both oxidation processes. In the initial stage, there is a relatively high level of O2 concentration with a gradual decline. As the temperature increases, the oxidation reaction gradually accelerates, leading to a rapid drop in O2 concentration. Prior to the coal temperature reaching 70℃, there is negligible variation in the O2 volume concentration across different particle sizes during both primary and secondary oxidation processes. The O2 concentration of coal samples P-1# to P-5# remains approximately at 20.5%, while samples S-1# to S-5# maintain a level around 20%. Overall, the degree of O2 consumption in the coal samples is minimal. This is primarily attributed to the relatively low temperature at which the reaction between coal and O2 takes place during the initial stage of oxidation experiment, resulting in a sluggish progression of the coal sample’s oxidation reaction22. The O2 concentration during secondary oxidation in this stage is lower than that of primary oxidation, with sample S-1# exhibiting the lowest concentration. This observation suggests that secondary oxidation enhances the coal-oxygen binding ability of the coal samples in the early stages of the experiment, particularly for the smallest particle size 0.5 mm sample where this strengthening effect is most pronounced.
The later stage of low-temperature oxidation exhibits a noticeable acceleration in the rate of O2 consumption across different particle sizes, resulting in a rapid decline in O2 volume concentration. This phenomenon can be attributed to the escalating temperature and intensifying coal oxidation reaction. Similarly, it is evident that the O2 concentration generally diminishes as the particle size decreases, with mixed particle sizes displaying the lowest concentration. In the secondary oxidation process, the O2 concentration of coal samples 1# to 4# is higher than that of the primary oxidation coal, with the exception of sample S-5#. After reaching a temperature of 150℃, the O2 concentration of sample S-5# becomes lower compared to that of the primary oxidation coal. This observation suggests that secondary oxidation hampers the coal-oxygen binding ability during later stages of low-temperature oxidation for samples 1# to 4#. The coal sample 5# exhibited distinct phenomena during the initial and final stages of the experiment, potentially attributed to the fact that, in comparison with other particle sizes, the mixed particle size coal sample demonstrates a higher degree of particle uniformity and lacks singularity in terms of size within other groups. Some scholars have noted that the variation in O2 concentration is just one of several fundamental parameters necessary for calculating O2 consumption rate23. To accurately compare the strength of coal reaction with O2, it is essential to incorporate an analysis of coal’s O2 consumption rate, as illustrated in Fig. 3.
The variations in the patterns of O2 consumption rate differ
The O2 consumption rate is a crucial parameter in the combustion process, serving as an indicative measure of the intensity of the oxidation reaction between coal and O224. Hence, it plays a significant role in evaluating the probability of CSC. Equation (1) represents the precise formula for calculating the O2 consumption rate:
Where \({V_{{O_2}}}\left( T \right)\) denotes the rate of O2 consumption, mol/(cm3·s); Q denotes the air volume, cm3/s; ν denotes the volume of the coal sample, cm3; \(C_{{{O_2}}}^{{in}}\) denotes the actual air inlet O2 concentration, mol/ cm3; and \(C_{{{O_2}}}^{{out}}\) denotes the actual air outlet O2 concentration, mol/ cm3.
By utilizing a gas chromatograph to quantify the O2 volume concentration at the outlet of the coal sample and integrating it with Eq. (1), the O2 consumption rate of coal samples with varying particle sizes during primary and secondary oxidation processes can be deduced as depicted in Fig. 3. The O2 consumption rate of the coal sample exhibits an upward trend as depicted in Fig. 3, with a gradual increase during the initial stages followed by a significant acceleration in the later stages. The low O2 consumption rate in the early stage is attributed to the lower temperature and weaker O2 affinity of coal itself. During this stage, coal primarily undergoes physical adsorption with atmospheric O225. In the late stage of low-temperature oxidation, coal primarily undergoes chemical reactions and chemisorption with O2, resulting in a substantial release of heat. This leads to a significant increase in O2 consumption rate across various particle sizes, exhibiting noticeable disparities. Take primary oxidation as an example. When the coal temperature exceeds 160℃, the O2 consumption rates for each group of coal samples follow the order: P-5#、P-1#、P-2#、P-3# and P-4#. Notably, the mixed particle size coal sample exhibits the highest O2 consumption rate after reaching 100℃, and a similar trend is observed in the subsequent secondary oxidation process. Based on these observations, it can be deduced that overall O2 consumption rates of coal samples decrease with increasing particle size. Moreover, larger particle sizes exhibit a more pronounced inhibitory effect on O2 consumption rate. The reduced coal-oxygen binding ability of larger particle-sized coal samples can be attributed to their decreased internal surface area or specific surface area. Additionally, the thermal desorption of gases adsorbed on these coal samples is impeded due to their larger particle size. Coal samples with a mixture of particle sizes exhibit a distinct behavior, characterized by the highest rate of O2 consumption. This suggests that, in comparison to the smallest particle size (1#), coal samples with equal mass but mixed particle sizes have a reduced surface area than sample 1#, yet due to the variation in particle size among coal samples, they possess a higher effective porosity than sample 1#, resulting in enhanced oxidation characteristics for mixed particle size sample.
In order to clearly differentiate the disparity in O2 consumption rate between the primary and secondary oxidation of coal samples, we define the O2 consumption rates during the primary and secondary oxidations as VP and VS respectively, based on the outcomes derived from two oxidation experiments. The ratio of VP to VS is illustrated in Fig. 3. It can be observed that for coal samples 1# to 4#, the ratio of O2 consumption rates during the preliminary stage prior to reaching a temperature of 110℃ is less than 1. However, beyond this temperature threshold, the ratio surpasses unity for all four groups of coal samples. This signifies that secondary oxidation amplifies the low-temperature oxidative properties of these four groups of coal samples during their initial experimental phase, augmenting their capacity to interact with O2 while diminishing their oxidative characteristics at elevated temperatures. Conversely, there exists no significant discrepancy in terms of mixed particle size ratios, potentially due to variations in internal structure within mixed particle size coal samples and uniformity in particle size distribution.
The variations in carbon-oxygen gas during primary and secondary coal oxidation under varying particle sizes
The variations in the dynamics of carbon-oxygen gas concentration
CO and CO2 gases are generated as byproducts of the coal oxidation process. As the temperature rises, it enhances the oxidation reaction and accelerates gas production. Additionally, CO serves as an indicative gas in the coal oxidation process, its concentration can reveal different stages of coal oxidation26.
The gas chromatograph can be utilized to detect the concentrations of CO and CO2 gases generated at the gas outlet, while Fig. 5a and b illustrate the variations in their concentrations under different experimental conditions. The graph reveals a consistent trend in the concentration changes of CO and CO2 gases for coal samples with identical particle sizes during both primary and secondary oxidation processes. As the temperature increases, both gases exhibit a gradual increase, with the generated CO2 concentration significantly surpassing that of CO. From Fig. 5a, it can be observed that during the initial stage of low-temperature oxidation, the generated CO concentration in both oxidation processes is relatively low and there is no significant difference between different particle sizes. However, once the temperature reaches 90℃, coal sample 5# exhibits an exponential increase in CO concentration with increasing temperature. Although the CO concentrations of the other four groups of coal samples also increase continuously, compared to the primary oxidation, there is a period of gradual transition around 140℃ for secondary oxidation samples. Moreover, during this stage, the CO concentration generated from secondary oxidation is significantly higher than that from primary oxidation and its value is approximately two to three times greater. However, at this point, there is no significant difference among particle sizes indicating that particle size has minimal influence on CO concentration while temperature remains as the primary influencing factor.
The results depicted in Fig. 5b demonstrate that S-5# exhibits a significant CO2 generation rate during the initial stage of low-temperature oxidation, indicating that the mixed particle size sample adsorbed more CO2 at this phase. As the temperature increases, more CO2 is desorbed. Prior to reaching 100℃, there is minimal disparity in the concentration of CO2 generated among coal samples S-1#, S-2#, and S-3#. After surpassing 150℃, the order of CO2 concentration generated by each particle size group becomes as follows: S-2#, S-4#, S-3#, S-5# and finally by S-1#. In contrast to the primary oxidation pattern, lower amounts of CO2 are produced during the early stages of secondary oxidation with concentrations below those observed during primary oxidation. However, as temperatures rise beyond a certain threshold, an exponential increase in CO2 concentration occurs which exceeds that observed during primary oxidation. Notably, there is no gradual transition period around 140℃ and throughout the entire oxidation phase coal sample S-1# consistently exhibits lower levels of CO2 concentration.
The variations in the rate of carbon-oxygen gas production
The rate of CO generation serves as a crucial indicator in determining the extent of coal oxidation. Scholars posit that this rate indirectly reflects the types and quantities of functional groups involved in oxidation and re-reactions, while also characterizing the temperature at which coal undergoes oxidation27. The calculation formulas for the production rates of CO and CO2 under standard O2 concentration are shown in Eqs. (2) and (3):
where \({V_{CO}}(T)\) and \({V_{C{O_2}}}(T)\) denote the rates of CO and CO2 production, mol/(cm3·s); \(C_{{CO}}^{{out}}\) and \(C_{{C{O_2}}}^{{out}}\) denote the air outlet concentrations of CO and CO2, mol/cm3, respectively; \(vn\) is the volume of the reactor, cm3.
Based on Eqs. (2) and (3), the rates of CO and CO2 generation from coal samples under primary and secondary oxidation processes can be calculated. As depicted in Fig. 6a and b, it is evident that the rates of gas generation from coal samples are generally consistent with their concentration trends. During the initial stages of the experiment at lower temperatures, both gas generation rates remain relatively low without significant differences between particle sizes. However, as temperature continues to rise, both gases’ generation rates increase accordingly. Moreover, during both oxidation processes, the rate of CO2 generation surpasses that of CO28. The change in CO generation rate during the two oxidation processes of coal samples is similar, as observed from Fig. 6a. Throughout the low-temperature oxidation process, there is a gradual increase in CO generation rate for coal samples 1# to 4#, with no significant variation among the different particle sizes. However, coal sample 5# exhibits an exponential growth trend in the later stage of oxidation, where the CO generation rate from secondary oxidation surpasses that from primary oxidation. These findings suggest that particle size does not significantly impact the speed of CO generation rate. Moreover, compared to primary oxidation, secondary oxidation enhances the CO generation rate during the later stages of low-temperature oxidation.
The CO2 generation rate of coal sample S-1# is observed to be lower than the primary oxidation in the temperature range of 40℃ to 170℃, as depicted in Fig. 6b. Subsequently, the CO2 generation rates of the other four groups are initially lower than that of the preoxidized coal sample before reaching temperatures between 120℃ and 140℃. However, beyond this temperature range, they significantly surpass the primary oxidation rate. Notably, during secondary oxidation, there is a distinct order of CO2 generation rates from high to low after reaching temperatures above 160℃ for coal samples: S-2#, S-5#, S-4#, S-3#, and finally S-1#. This contrasts with the pattern observed during primary oxidation where P-1# exhibited the highest CO2 generation rate while P-4# had the lowest. These differences may be attributed to alterations in internal structure resulting from re-oxidation process of pre-oxidized coal.
Variations in the heat release patterns during primary and secondary oxidation CSC are influenced by different particle sizes
Differences in the variation patterns of actual heat release rates
The essence of the SC process of coal lies in the progression of coal oxidation and dissipation of heat. When the heat released from low-temperature coal oxidation surpasses the dissipated heat, the temperature of the coal pile continues to escalate, ultimately resulting in SC. Investigating the intensity of heat release from coal is imperative for comprehending this process25. The formula for calculating the rate of heat release is as follows:
Where \({q_{\hbox{max} }}(T)\), \({q_{\hbox{min} }}(T)\) denote the upper and lower exothermic strengths, kJ/(cm3·s); \(q\left( T \right)\) denotes the actual exothermic strengths, kJ/(cm3·s); \(\Delta {H^{CO}}\),\(\Delta {H^{C{O_2}}}\) denote the average reaction heat of CO and CO2, \(\Delta {H^{CO}}\)= 311.9 kJ/mol, \(\Delta {H^{C{O_2}}}\)= 446.7 kJ/mol; \({q_a}\) denotes the heat of adsorption of the coal chemical reaction, 58.8 kJ/mol.
By utilizing Eqs. (4) and (5), the maximum and minimum heat release rates of coal samples with varying particle sizes under different oxidation conditions are computed. Subsequently, these values are substituted into Eq. (6) to determine the actual heat release intensity of residual coal in goaf, which is graphically represented in Fig. 7. From the figure, it can be visually observed that the actual heat release intensity of coal samples during secondary oxidation exhibits a similar phenomenon to that observed during primary oxidation. In the initial stages of low-temperature oxidation, all five groups of coal samples with varying particle sizes demonstrate relatively low levels of actual heat release intensity. As the temperature continues to rise, an exponential increase trend becomes evident across all five groups of coal samples. In the secondary oxidation process, S-5# coal exhibits the lowest heat release before reaching 70℃ compared to other particle sizes, whereas S-1# demonstrates the highest heat release. Subsequently, after reaching 90℃, S-5# displays significantly higher heat release than the other four groups. In the initial oxidation stage, there is no significant disparity in actual heat release intensity before 70℃ among different particle size coal samples. However, during the later stage of oxidation, smaller particle sizes result in elevated actual heat release intensity. Furthermore, mixed particle size coal samples exhibit the highest actual heat release intensity due to their uniform particle size and abundant internal pore structure. Additionally, based on the figure, it is evident that in the initial phase of the experiment, the actual heat released by the secondary oxidized coal samples generally surpasses that of the primary oxidized coal. In the later stages of the experiment, different phenomena emerge. The heat release intensity during the secondary oxidation process for coal samples 4# and 5# surpasses that of the primary oxidation after reaching temperatures of 160℃ and 130℃ respectively. However, for the remaining three groups of coal samples, their heat release intensities in the later stage of oxidation are all lower than those observed during the primary oxidation phase.
Investigation on the impact of particle sizes on the variation in apparent activation energy during primary and secondary oxidation of coal
The individual aspects of the Chemical function, the Phase boundary reaction, the Diffusion model, the Mampel power rule, and the Avrami-Erofeev equation should be considered separately in order to determine the kinetic models of coal samples with different particle sizes during various reaction stages. Following the principles of the Arrhenius29 equation, heating is conducted at a constant rate of temperature increase, resulting in a transformational expression for the reaction rate:
Where \(\beta\) denotes the constant heating rate, (℃/min); T denotes the sample temperature, K; A denotes the pre-exponential factor, s− 1; E denotes the apparent activation energy, (kJ/mol); R denotes the universal gas constant, 8.314 J/(mol k); \(f(\alpha )\) denotes the derivative of the reaction function; The conversion \(\alpha\) can be expressed as:
Where \(C_{{{O_2}}}^{{{T_i}}}\) denotes the current O2 concentration of \({T_i}\), mol/ cm3.
The integration of Eq. (7) results in:
Where \(g(\alpha )\) denotes an integral form of the \(f(\alpha )\); The Coats-Redfern integral method can be used for conversion30, yielding the following result:
The Achar-Brindley-Sharp-Wentworth differential method can be derived from the Eq. (7) by Arrhenius through taking logarithms on both sides and rearranging, as stated simultaneously:
The values of \(\ln \frac{{g\left( \alpha \right)}}{{{T^2}}}\) and \(\ln \left( {\frac{{d\alpha }}{{dt\cdot f\left( \alpha \right)}}} \right)\) obtained from the calculations of Eqs. (10) and (11) are subjected to linear regression analysis using the data of 1000/T. The resulting slope is denoted as \(- \frac{E}{R}\), while the intercepts are represented by \(\ln \frac{{AR}}{{\beta E}}\) and \(\ln A\) respectively. The equations for \(f\left( \alpha \right)\) and \(g\left( \alpha \right)\) can be found in Table 3.
Due to the lack of clear regularity in the initial experimental data, fitting is conducted at three temperature ranges starting from 80℃, namely 80℃~170℃, 80℃~130℃, and 140℃~170℃. Taking coal sample P-1# as an example, Fig. 8 illustrates the fitted results for parameters \(f\left( \alpha \right)\) and \(g\left( \alpha \right)\) of its F1 model.
By analyzing the slopes and intercepts of the lines depicted in Fig. 8, we can determine the pre-exponential factors (A1, A2) as well as the activation energies (E1, E2) for variables \(f\left( \alpha \right)\) and \(g\left( \alpha \right)\), respectively. Subsequently, employing Eqs. (12) and (13) enables us to compute the ultimate activation energy E and pre-exponential factor A, which are graphically represented in Fig. 9. The calculation methodology for other coal samples with different particle sizes follows a similar approach; hence, it will not be further expounded upon. Through The analysis of the compensation effect figure between A and E for coal samples with varying oxidation states and particle sizes, it is evident that the linear correlation coefficients between E and lnA in the above 22 reaction mechanism functions are all higher than 0.95. This suggests the existence of a reaction mechanism function model that is most suitable for determining kinetic parameters of coal oxidation in different particle sizes.
The final kinetic function is determined based on the criterion of relative error, using the accuracy percentage of the model. Taking coal sample P-1# as an example, the Diffusion model(D2-J、D3-J), the Mampel power rule(P1/4), and the Avrami-Erofeev equation (A-E1/3) exhibit inadequate simulation effects within the temperature range of 80℃ to 170℃. Curve \(\ln \left( {d\alpha /\left( {dT\cdot \left( {f\alpha } \right)} \right)} \right)\) demonstrates a low degree of linearity overlap with this temperature range, thus rendering it unsuitable for solving the oxidative kinetic parameters of the coal sample. In the Chemical function (F1 ~ F3), the linearity of the curve and its alignment with the temperature gradually increase as the reaction order increases. Similarly, the Phase boundary reaction (R1-R4) and the Mampel power rule (P3/2-P1/4) also exhibit comparable patterns, with the Phase boundary reaction demonstrating superior curve alignment compared to the Mampel power rule. Notably, among these models, R1 exhibits the highest degree of coincidence. And the curve linearity of D1 in the Diffusion model has the best overlap with this temperature range, thus D1 is chosen to simulate coal sample P-1#, which calculated activation energy E is 79.7 KJ/mol and A is 4.51 × 105s− 1. The method for selecting other particle size coal samples remains consistent with that of coal sample P-1# and will not be further elaborated. The final results indicate that within the temperature range of 80℃ to 170℃, the Diffusion model (D1) demonstrates superior performance in simulating coal samples P-1#, P-2#, and S-5#, while D2-V is deemed most suitable for simulating coal sample P-5#. The remaining coal samples are better suited for the Avrami-Erofeev equation (A-E4). Moreover, it should be noted that the different models may apply to coal samples of the same particle size during the temperature intervals of 80℃ to 130℃ and 140℃ to 170℃. This observation suggests variations in collision intensity between coal molecules and their reaction levels at different temperature ranges.
The Fig. 10 depict the disparities in apparent activation energy across various temperature ranges and particle sizes. The apparent activation energy E represents the minimum energy required for coal-oxygen reaction, with a lower E value facilitating easier chemical reactivity between coal and O227. It can be observed from Fig. 10b that the activation energy E of coal samples with the same particle size is lower in the secondary oxidation process within the 80℃ to 130℃ temperature range compared to that in the primary oxidation. However, a reverse phenomenon occurs between 140℃ and 170℃ due to the activation of a large number of more active functional groups during the primary oxidation process, increasing the difficulty of activating the functional groups during the early stages of secondary oxidation. From a macroscopic perspective, higher O2 consumption rate, CO generation rate, and heat release intensity are observed for coal samples during the initial stages of experimentation in the secondary oxidation process. However, in the later stages of the experiment, as the temperature of coal increases to a certain extent, its capacity for oxygenation is further enhanced. Consequently, this leads to an accelerated rate of O2 consumption during the initial oxidation process and a substantial release of thermal energy. From Fig. 10a, it can be observed that the secondary oxidation coal sample has a higher risk of SC.
The variation in limit parameters changes during the primary and secondary oxidation with different particle sizes
The process of CSC is influenced by various factors, and the occurrence of such combustion requires specific external conditions. The extreme values that can trigger CSC are referred to as limit parameters, which primarily include the minimum thickness of floating coal \({h_{\hbox{min} }}\), the lower limit O2 concentration \({C_{\hbox{min} }}\), and the upper limit air leakage intensity \({Q_{\hbox{max} }}\)31. When the goaf possesses an adequate thickness of floating coal, a sufficient concentration of O2, and a specific level of air leakage intensity, it will initiate CSC. The calculation formulas for the three critical parameters of SC are as follows:
Where \({h_{\hbox{min} }}\) denotes the minimum floating coal thickness of the loose coal body, cm; \({\rho _g}\) denotes the air density, kg /m3; \({C_g}\) denotes the specific heat capacity of air, J/( kg⋅k); Q denotes the intensity of wind leakage, cm/s; \({T_y}\) denotes the temperature of the coal body enclosing the rock body, ℃; T denotes the temperature of the wind flow, ℃; \({\lambda _e}\) denotes the equivalent thermal conductivity of the loose coal body, J/ ( cm⋅s⋅k); \(Q(T)\) denotes the exothermic intensity at the temperature of the loose coal body T, J/ ( cm3⋅k); \({C_{\hbox{min} }}\) denotes the lower limit O2 concentration of the loose coal body, %; \(C_{e}^{{{O_2}}}\) denotes the O2 concentration in air, %; h denotes the thickness of the loose coal body, cm; \({Q_{\hbox{max} }}\) denotes the upper limit air leakage intensity of loose coal body, cm/s.
Minimum floating coal thickness
The \({h_{\hbox{min} }}\) is a critical factor that influences the oxidation and heat retention of loose coal32. The occurrence of CSC can only happen when the thickness of the float coal reaches a specific threshold. Taking the air leakage intensity within the range of 0.0004 to 0.0052 m/s as an example, Fig. 11 illustrates its three-dimensional relationship, exemplified by coal sample P-1#. From the figure, it can be observed that the \({h_{\hbox{min} }}\) initially increases and then decreases with an increase in temperature. This phenomenon is attributed to the limited involvement of functional groups during the initial stage of low-temperature oxidation process. Moreover, due to lower temperatures, the efficiency of coal-oxygen combination is compromised, resulting in insufficient heat release to compensate for heat loss. Consequently, there is a progressive thickening of float coal. However, as the temperature surpasses a certain threshold, an intense coal oxidation reaction takes place where the actual heat release intensity exceeds the dissipation caused by temperature difference between coal body itself and its surroundings. As a consequence, there occurs a subsequent reduction in float coal thickness, and the figure illustrates a positive correlation between the \({h_{\hbox{min} }}\) and the intensity of air leakage in the goaf. This implies that as the air leakage intensity increases, so does the \({h_{\hbox{min} }}\). The underlying reason for this phenomenon is that higher air leakage intensities result in greater heat dissipation from residual coal in the goaf, necessitating a thicker loose coal body to accumulate sufficient heat and trigger SC.
The relationship between particle size and the thickness of floating coal during the primary and secondary oxidation processes is analyzed by plotting Fig. 12 based on the maximum thickness data of float coal in each air volume group. In Fig. 12, a and b respectively denote primary oxidation and secondary oxidation. From the figure, it can be observed that when the air leakage intensity remains constant, there is a general trend of increasing \({h_{\hbox{min} }}\) with larger particle sizes. This phenomenon can be attributed to the reduction in specific surface area as particle size increases, resulting in diminished contact area with O2 and decreased capacity for oxygenation. Consequently, less heat is generated, necessitating a larger minimum thickness of floating coal to accumulate sufficient heat for SC conditions. During the secondary oxidation process, Sample 1# exhibits the highest risk to SC, and its \({h_{\hbox{min} }}\) demonstrates a linear increase corresponding to larger particle sizes. The \({h_{\hbox{min} }}\) in sample 5# during one oxidation process is observed to be the lowest, indicating the highest level of potential danger. This phenomenon can possibly be attributed to the mixed particle size coal sample exhibiting a more homogeneous particle distribution and abundant pore structure, while the other four groups display too single particle sizes.
Lower limit O2 concentration
The \({C_{\hbox{min} }}\) of residual coal in the goaf is determined by the thickness of floating coal and the intensity of air leakage, as indicated in Eq. (15). Therefore, it is necessary to analyze each situation separately. In practical terms, the working face in the mine’s goaf should have a lower limit O2 concentration that does not exceed 100%. Taking coal sample P-1# as an example, Figs. 13 and 14 illustrate its three-dimensional relationship. The data presented in Fig. 13 demonstrates that, with a constant thickness of floating coal, the \({C_{\hbox{min} }}\) of the coal sample generally exhibits an initial increase followed by a decrease as temperature rises. This phenomenon can be attributed to the weak ability of coal to react with O2 during the early stages of low-temperature oxidation, resulting in a lower rate of O2 consumption and consequently reduced O2 demand. As temperature continues to rise, the reaction between coal and O2 gradually accelerates. The actual heat released surpasses the heat lost by the coal itself, leading to an increased demand for O2. Ultimately, the decrease in \({C_{\hbox{min} }}\) is due to further enhancement of the reaction between coal and O2, resulting in greater heat release and thus requiring only a lower level of O2 concentration. The phenomenon depicted in Fig. 14 also exhibits a similar trend. With the same air leakage intensity, the \({C_{\hbox{min} }}\) generally demonstrates an initial increase followed by a decrease as temperature rises. Furthermore, it can be observed from the graph that as floating coal thickness increases, the \({C_{\hbox{min} }}\) decreases, while decreasing air leakage intensity leads to an increase in required O2 concentration. This is attributed to thicker floating coal accumulating more heat and higher air leakage intensity introducing more O2, ultimately resulting in a reduced demand for O2.
The relationship between the \({C_{\hbox{min} }}\) and particle sizes under the influence of coal thickness and air leakage intensity during the primary and secondary oxidation processes is illustrated in Fig. 15, as well as Fig. 16. It can be observed that irrespective of the conditions, when both coal thickness and air leakage intensity remain constant, the variation pattern of \({C_{\hbox{min} }}\) follows a similar trend to that of \({h_{\hbox{min} }}\), which generally increases with increasing particle size. Moreover, Fig. 15a and b reveal that sample 5# exhibits the lowest \({C_{\hbox{min} }}\) during the primary oxidation process while sample 1# has the lowest \({C_{\hbox{min} }}\) during the secondary oxidation process indicating a higher risk of SC. The three-dimensional relationship between samples P-5# and S-1# is depicted in Fig. 17.
Upper limit air leakage intensity
The Fig. 18 illustrates the three-dimensional correlation between the \({Q_{\hbox{max} }}\) of coal sample P-1# within the range of coal thickness from 0.4 to 1.2 m and temperature. From the figure, it can be observed that the \({Q_{\hbox{max} }}\) of coal samples generally exhibits a decreasing trend followed by an increasing trend with an increase in temperature, while maintaining the same thickness of floating coal. This phenomenon can be attributed to the weak reaction ability and insufficient heat release of coal samples at low environmental temperatures, which fails to compensate for the heat lost due to temperature difference during the initial stages of the experiment. Consequently, a lower air leakage intensity is required. As the temperature gradually rises, however, oxidation reactions accelerate and more heat is generated, leading to an increase in corresponding air leakage intensity. Simultaneously, as floating coal thickness increases within a group with equal particle size distribution, greater air leakage intensity becomes necessary for efficient heat removal from coal samples. Therefore, according to this graph’s data set under conditions where particle size remains constant within each group, there exists a positive correlation between floating coal thickness and \({Q_{\hbox{max} }}\).
The relationship between the \({Q_{\hbox{max} }}\) and particle size during the primary and secondary oxidation processes of coal samples is illustrated in Fig. 19a and b. It can be observed that, generally, the \({Q_{\hbox{max} }}\) decreases as the particle size increases. This decrease follows a linear trend during the secondary oxidation process. The reason behind this phenomenon lies in larger particle sizes resulting in a smaller internal surface area of the coal sample, which subsequently reduces both reaction area and heat release rate during oxidation. Consequently, a lower air leakage intensity is required. From the graph, it can be inferred that under specific conditions, both P-5# and S-1# coal samples pose a higher risk of SC.
The three-dimensional relationship of the limit parameters mentioned above is solely exemplified by coal sample P-1#. Upon conducting a comprehensive analysis of the three-dimensional relationships among all other particle size coal samples, it is observed that the maximum values of hmin and Cmin during the secondary oxidation process generally exhibit a decrease compared to those during the primary oxidation, whereas the minimum values of Qmax surpass those observed during the primary oxidation. This suggests that under specific conditions, secondary oxidation escalates the risk of SC in goaf coal.
Conclusions
-
1.
Whether during the primary oxidation or secondary oxidation processes, the mixed particle size coal sample exhibits a more developed pore structure compared to coal samples of other particle sizes. Consequently, the O₂ volume concentration is relatively lower, whereas the O2 consumption rate, the concentration of carbon-oxygen gases, the gas generation rate, and the actual heat release intensity are significantly higher. Therefore, the mixed particle size coal sample is most susceptible to CSC.
-
2.
By comparing the linear regression degrees of 22 reaction mechanism function models, the most appropriate model is selected. It is observed that within the temperature range of 80℃ to 170℃, the Diffusion model (D1) exhibited superior simulation performance for coal samples P-1#, P-2#, and S-5#. The D2-V model demonstrated optimal simulation effectiveness for coal sample P-5#, while the Avrami-Erofeev equation (A-E4) proved more suitable for other coal samples. The activation energy obtained from the optimal model indicates that, in comparison to primary oxidation, secondary oxidation demonstrates enhanced early-stage oxidative capability but weakened late-stage oxidative ability towards coal samples.
-
3.
Under varying conditions of air leakage intensity and thickness of floating coal, the \({h_{\hbox{min} }}\) and \({C_{\hbox{min} }}\)of coal samples increase with larger particle sizes, while the \({Q_{\hbox{max} }}\)decreases with larger particle sizes. Based on the principle of extreme parameters, a preliminary conclusion can be drawn that under specific conditions, sample 1# poses the highest risk of SC during secondary oxidation process, whereas sample 5# presents the highest risk during primary oxidation process. Compared to primary oxidation, secondary oxidation poses a higher risk of inducing CSC.
Data availability
All data generated or analyzed during this study are available from the corresponding author upon request.
References
Zhang, Y. et al. Study on low temperature oxidation heat release characteristics of bituminous coal at different heating rates. Coal Sci. Technol. 50, 104–113. https://doi.org/10.13199/j.cnki.cst.2020-1630 (2022).
Yang, X. et al. Thermal effects and kinetics behaviors of coal spontaneous combustion via synchronous thermal analyzer under different heating rates. Combust. Sci. Technol. 196, 702–715. https://doi.org/10.1080/00102202.2022.2097008 (2024).
Shi, Q. & Qin, B. Experimental research on gel-stabilized foam designed to prevent and control spontaneous combustion of coal. Fuel 254, 115558. https://doi.org/10.1016/j.fuel.2019.05.141 (2019).
Li, M. et al. Thermodynamic variation law and influence mechanism of low-temperature oxidation of lignite samples with different moisture contents. Energy 262, 125605. https://doi.org/10.1016/j.energy.2022.125605 (2023).
Zheng, Y. et al. Study on the evolution characteristics of coal spontaneous combustion and gas coupling disaster region in Goaf. Fuel 349, 128505. https://doi.org/10.1016/j.fuel.2023.128505 (2023).
Qin, C. et al. Prevention and Control of Spontaneous Combustion of Residual Coals in Acid-Soaked Goaf in Gas Drainage Condition. Adv. Civil Eng. https://doi.org/10.1155/2022/1668952 (2022).
Lu, Y. et al. Discharge and ignition characteristics from indentation fracture of coal mine roof. Fuel 291, 120208. https://doi.org/10.1016/j.fuel.2021.120208 (2021).
Xiao, Y. et al. Thermal extraction from a low-temperature stage of coal pile spontaneous combustion by two-phase closed thermosyphon. J. Therm. Anal. Calorim. 144, 587–597. https://doi.org/10.1007/s10973-021-10678-6 (2021).
Bai, Y. Study on secondary oxidation characteristics of coal with different preoxidation degree. Xi’an Univ. Sci. Technol. 106 (2017).
Niu, H., Yang, Y. & Li, S. Effects of pre-oxidized temperature and pre-oxidized oxygen concentration on burning characteristics of pre-oxidized coal. Fuel 332, 125723. https://doi.org/10.1016/j.fuel.2022.125723 (2023).
Wang, K. et al. Thermal behavior of the Low-temperature secondary oxidation of coal under different Pre-oxidation temperatures. Combust. Sci. Technol. 194, 1712–1729. https://doi.org/10.1080/00102202.2020.1828378 (2022).
Yu, M., Ma, S. & Chu, T. Influence study of coal particle size on the gases production rule and spontaneous combustion tendency characteristics. J. Henan Polytech. Univ. 35, 1673–9787 (2016).
Zhang, Y., Shi, X., Li, Y. & Liu, Y. Characteristics of carbon monoxide production and oxidation kinetics during the decaying process of coal spontaneous combustion. Can. J. Chem. Eng. 96, 1752–1761. https://doi.org/10.1002/cjce.23119 (2018).
Jia, X. et al. Investigating the effect of coal particle size on spontaneous combustion and oxidation characteristics of coal. Environ. Sci. Pollut. Res. 29, 16113–16122. https://doi.org/10.1007/s11356-021-16929-1 (2022).
Wang, K., Wang, Z., Zhai, X. & Jiang, H. An experimental investigation of early warning index for coal spontaneous combustion with consideration of particle size: a case study. Int. J. Coal Prep. Util. 43, 233–247. https://doi.org/10.1080/19392699.2022.2036730 (2023).
Ma, L. et al. Experiment study on partical size affected to coal spontaneous combustion limit parameters in Goaf. Coal Sci. Technol. 43, 59–64. https://doi.org/10.13199/j.cnki.cst.2015.06.011 (2015).
Xu, Q. et al. Secondary oxidation of crushed coal based on free radicals and active groups. Fuel 290, 120051. https://doi.org/10.1016/j.fuel.2020.120051 (2021).
Xu, Q. et al. Effect of particle size and low-temperature secondary oxidation on the active groups in coal structures. Process. Saf. Environ. 149, 334–344. https://doi.org/10.1016/j.psep.2020.10.056 (2021).
Yang, K., Yang, S. & Xu, Q. Comparative analysis of oxidation characteristics of Raw coal, primary oxidized coal and secondary oxidized coal with different particle sizes. J. Henan Polytech. Univ. 41, 17–22 (2022).
Jing, P. et al. Experimental research on the features of spontaneous combustion during secondary oxidation of coal with various particle sizes. Int. J. Hydrog. Energy. 49, 933–944. https://doi.org/10.1016/j.ijhydene.2023.09.200 (2024).
Guo, C., Jiang, S., Shao, H., Wu, Z. & Bascompta, M. Effect of secondary oxidation of Pre-Oxidized coal on early warning value for spontaneous combustion of coal. Appl. Sci. 13, 3154. https://doi.org/10.3390/app13053154 (2023).
Chao, J. Experimental study on oxidative spontaneous combustion characteristics of loaded crushed coal. Henan Polytech. Univ. 116. (2019).
Dong, Z. et al. Rapid contrastive experimental study on the adiabatic spontaneous combustion period of loose lignite. Acs Omega. 6, 34989–35001. https://doi.org/10.1021/acsomega.1c05667 (2021).
Wang, C. et al. Study on secondary oxidation characteristics of coal gangue at different pyrolysis rank. Fuel 345, 128231. https://doi.org/10.1016/j.fuel.2023.128231 (2023).
Yan, H. et al. Experimental investigation of coal particle size on the kinetic properties of coal oxidation and spontaneous combustion limit parameters. Energy 270, 126890. https://doi.org/10.1016/j.energy.2023.126890 (2023).
Deng, J., Zhao, J., Zhang, Y. & Wang, C. Micro-characteristics of spontaneous combustion of second oxidation with different rank coals. J. China Coal Soc. 41, 1164–1172. https://doi.org/10.13225/j.cnki.jccs.2015.1134 (2016).
Liu, Q., Sun, L., Liu, Z., Wang, G. & Ma, J. Effects of air volume and pre-oxidation on re-ignition characteristics of bituminous coal. Energy 265, 126124. https://doi.org/10.1016/j.energy.2022.126124 (2023).
Zhou, X., Niu, Y. & Bai, G. Experimental study on the influence of air supply on characteristics of lignite spontaneous combustion. Min. Saf. Environ. Prot. 47, 31–35. https://doi.org/10.19835/j.issn.1008-4495.2020.01.007 (2020).
Hu, M. et al. Thermogravimetric kinetics of lignocellulosic biomass slow pyrolysis using distributed activation energy model, Fraser–Suzuki deconvolution, and iso-conversional method. Energ. Convers. Manag. 118, 1–11. https://doi.org/10.1016/j.enconman.2016.03.058 (2016).
Zhai, X., Ge, H., Wang, T., Shu, C. & Li, J. Effect of water immersion on active functional groups and characteristic temperatures of bituminous coal. Energy 205, 118076. https://doi.org/10.1016/j.energy.2020.118076 (2020).
Chen, X., Li, H., Wang, Q. & Zhang, Y. Experimental investigation on the macroscopic characteristic parameters of coal spontaneous combustion under adiabatic oxidation conditions with a mini combustion furnace. Combust. Sci. Technol. 190, 1075–1095. https://doi.org/10.1080/00102202.2018.1428570 (2018).
Zhang, Q., Jia, X. & Wang, D. Experimental evaluation of the effect of sulfur content on the spontaneous combustion characteristics parameters of coal. Thermochim. Acta. 732, 179651. https://doi.org/10.1016/j.tca.2023.179651 (2024).
Author information
Authors and Affiliations
Contributions
P.T.Z. contributed to writing-original draft and formal analysis; Z.Y.Z. worked in methodology and supervision; Z.H.C. helped in formal analysis and supervision; Y.X.Y. contributed to visualization and data curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, P., Zhang, Z., Chai, Z. et al. The variations in the spontaneous combustion characteristics of coal during primary and secondary oxidation under varying particle sizes. Sci Rep 15, 22572 (2025). https://doi.org/10.1038/s41598-025-07091-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07091-6