Abstract
Water supply and the removal of contaminants are critical challenges for modern societies. Various techniques have been developed to treat industrial wastewater. This study synthesized a Co3O4/CdAl2O4 nanocomposite using a hydrothermal method to remove Direct Blue and Basic Yellow organic dyes from aqueous media. The nanocomposite was characterized through FTIR, XRD, FESEM, EDX, TEM, PL, and UV-DRS spectroscopy to assess its physical, chemical, and optical properties, including morphology, particle size, crystalline structure, chemical composition, and band gap. DRS analysis revealed that the band gaps of Co3O4, CdAl2O4, and the synthesized nanocomposite were 1.5 eV, 3.66 eV, and 2.4 eV respectively. The formation of the heterogeneous Co3O4/CdAl2O4 structure reduced the recombination rate compared to pure Co3O4 and CdAl2O4 samples. The study examined how operational parameters such as pH, contact time, initial dye concentration, and catalyst dosage affected dye removal efficiency. Optimal removal efficiency for Direct Blue was achieved at pH 2 and for Basic Yellow at pH 12 with a dye concentration of 10 ppm in a 20 mL solution using a catalyst dosage of 0.01 g. Maximum removal efficiencies were 91% for Direct Blue and 86% for Basic Yellow. Kinetic studies showed that the dye removal process followed pseudo-first-order kinetics indicative of a surface-controlled reaction. The experimental data fit well with this model, allowing calculation of the reaction rate constant and activation energy determination. The regression coefficients were 0.992 for Direct Blue and 0.991 for Basic Yellow with rate constants of 0.00379 s− 1 and 0.00125 s− 1 respectively. The photocatalytic regeneration results indicate that the synthesized nanocomposite demonstrates excellent reusability for up to four cycles, achieving final degradation rates of 80% and 74% for Direct Blue and Basic Yellow, respectively. Mechanistic studies reveal that superoxide radicals are the primary reactive species, serving as powerful oxidants that break down the chemical bonds of pollutant molecules. This process results in bond cleavage and ultimately mineralizes pollutants into simpler compounds like water and carbon dioxide. Consequently, the cobalt oxide/cadmium alumina nanocomposite, with its superior photocatalytic properties and high stability, shows significant potential for treating industrial wastewater containing organic dyes.
Similar content being viewed by others
Introduction
Water, a crucial yet limited resource on earth, faces serious threats from industrial pollutants, particularly organic dyes. These persistent and toxic substances not only disrupt ecological processes such as photosynthesis but also pose severe health risks to humans, including skin diseases, allergies, and various cancers. Removing these pollutants from aquatic environments, especially drinking water sources, is a top environmental priority. Extensive research is focused on developing efficient and cost-effective methods to detoxify these pollutants1,2. The discharge of dyes in textile wastewater at a rate of 10–15% per dyeing process presents a significant environmental challenge. Traditional treatment methods like coagulation and adsorption have limitations, such as low efficiency in degrading complex dyes and generating hazardous sludge. Additionally, ultraviolet radiation-based methods are costly and can produce harmful by-products3.
Direct Blue and Basic Yellow, as organic colorant pollutants, significantly threaten water resources due to their extensive industrial use. These compounds are toxic to aquatic life, disrupt ecological cycles, pose health risks to humans, and incur economic costs related to water pollution, underscoring the need for effective removal. Among various water treatment methods, advanced oxidation processes (AOPs) have gained attention for efficiently mineralizing complex organic pollutants while producing fewer hazardous by-products and optimizing energy consumption in an environmentally friendly manner. AOPs generate reactive oxygen species like hydroxyl radicals (•OH), breaking strong chemical bonds in dyes and converting them into simple, harmless compounds4.
Photocatalysis, a technique involving nanomaterials under light irradiation to treat aqueous media, has emerged as a recent advancement in nanotechnology5,6. Also, Photocatalysis, an advanced oxidation process (AOPs), has gained attention as an efficient and environmentally friendly method for degrading organic dyes. In this process, photocatalysts play a key role by facilitating the breakdown of dye molecules under visible or UV light irradiation. They produce reactive oxygen species (ROS), such as hydroxyl radicals and superoxide anions, which oxidize and mineralize dyes into harmless byproducts like CO₂ and H₂O7.
Photocatalytic begins with generating electron-hole pairs when a semiconductor photocatalyst is irradiated with photons that have energy exceeding its band gap. Excited electrons in the conduction band can react with electron acceptors such as molecular oxygen to produce highly reactive species like superoxide radicals (•O2−) and hydroxyl radicals (•OH). Meanwhile, holes in the valence band can directly oxidize organic pollutants or interact with electron donors. The strong oxidizing potential of hydroxyl radicals allows for the efficient degradation of a wide range of organic contaminants, including dyes4.
Among the diverse range of semiconductor nanoparticles with photocatalytic properties, CdAl2O4 nanoparticles have garnered considerable interest due to their chemical stability and effective visible light absorption. However, their relatively large bandgap (3.66 eV) results in a high rate of charge carrier recombination, which diminishes photocatalytic efficiency. Various strategies have been proposed to bridge the bandgap from the ultraviolet to the visible spectrum, including creating heterogeneous structures (heterojunctions), doping with metal ions, and other methods for modifying band structures. Notably, heterojunctions have received particular attention for their ability to finely tune optical and electronic characteristics. Cadmium aluminates (CdAl2O4) are important aluminate materials whose application includes the area of catalysts, magnetic, pigments, and refractory materials. The price of the precursor materials applied for the preparation of CdAl2O4 is also inexpensive compared to another catalyst (rare earth)8.
Photocatalysts like cobalt oxide, characterized by a narrower bandgap (1.5 eV), porous structure, high surface area, and biocompatibility, have demonstrated effectiveness as catalysts. Up to date, various synthesis methods have been used for preparing Co3O4 nanoparticles, including hydrothermal/solvothermal, precipitation, sonochemical, sol-gel, solution combustion, and successive ionic layer adsorption and reaction. It is reported that metal doping is an efficient technique to enhance electrical conductivity and catalytic activity9,10. In 2023, Ahmed Helal and his team conducted a study focused on optimizing the synthesis of an efficient g-C3N4/Co3O4 nanocomposite for the catalytic removal of Rhodamine B.
(RhB) dye11. The objective was to enhance photocatalytic efficiency in the degradation of RhB from aqueous solutions. Their findings indicated that a weight ratio of 2:1 for g-C3N4 to Co3O4 yielded optimal results for RhB removal. Under ideal conditions, this nanocomposite achieved a maximum photocatalytic activity of 87% for RhB removal within 120 min. The success of this approach was attributed to the synergistic interaction between g-C3N4 and Co3O4 nanoparticles12. g-C3N4 nanoparticles feature a high surface area and the capacity to absorb visible light, while Co3O4 nanoparticles exhibit strong oxidation capabilities12. Combining these two types of nanoparticles in the g-C3N4/Co3O4 nanocomposite enhances the active surface area, light absorption capacity, and oxidative power simultaneously13.
The Co3O4 and CdAl2O4 photocatalysts can be used to degrade organic pollutants in water, such as dyes, pharmaceuticals, and other hazardous chemicals. Its ability to absorb visible light makes it effective for environmental remediation. It can also be applied in air purification systems to break down volatile organic compounds (VOCs) and other airborne pollutants. Rajesh et al. fabricated a novel Ni-doped CdAl2O4 nanoparticles to degrade azo dyes under visible light illumination. The existence of azo dyes in textile wastewater is a major problem owing to their possible effect on human health and the environment14. An initial screening of dissimilar Ni-doped CdAl2O4 nanoparticles was determined to classify the most outstanding candidate for BB and BG removal. The Ni (0.075 M) doped CdAl2O4 nanoparticles after 90 min illuminations, 95 and 96% of BB and BG removal were noticed in pH = 5 (acidic) and 5 mg catalyst dosage conditions15. Palanisamy et al. synthesized MnO2 nanorods and Co3O4 nanoparticles combined with eggshell-derived hydroxyapatite to investigate phenol and methylene blue degradation16. Based on photocatalytic degradation, it was found that the HAp-α-MnO2-Co3O4 nanocomposite has the highest photocatalytic activity among all freshly prepared samples. In particular, the MB dye was almost completely degraded (95.5%) by exposure to UV–visible light in 60 min of irradiation, the observed degradation result was relatively higher than that of pristine Hydroxyapatite (Hap), α-MnO2 and Co3O4, respectively. HAp-α-MnO2-Co3O4 nanocomposite efficiently degraded 94.42% colorless phenol, demonstrating its dual ability to break down hazardous dyes and organic compounds under the same conditions16.
In this work, we examined the photodegradation performance under the visible sources of Basic Yellow 28 and Direct Blue 199, which are Azo dyes extensively applied in the food and textile industries. For the first time, a binary CdAl2O4/Co3O4 nanocomposite was synthesized and evaluated as an efficient photocatalyst for the degradation of organic dyes, specifically Basic Yellow and Direct Blue, under visible light irradiation. The individual components of the nanocomposite, Co3O4 and CdAl2O4, were synthesized using sol-gel auto combustion and hydrothermal methods, respectively. The final nanocomposite was prepared through a combination of hydrothermal and sono-chemical techniques. Structural and morphological characteristics of the synthesized nanomaterials were analyzed using photoluminescence (PL), field emission scanning electron microscopy (FESEM), energy-dispersive X-ray spectroscopy (EDX), Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and diffuse reflectance spectroscopy (DRS), Thermogravimetric analysis (TGA), Bruner Emmet Teller (BET), and Raman. The influence of various operational parameters, including catalyst dosage, pH, reaction time, and initial dye concentration, on the efficiency of the photocatalytic process was thoroughly assessed. Additionally, kinetic studies were performed to investigate the dye degradation process.
Experimental
Materials
The materials include Cobalt (II) nitrate hexahydrate (99%), 2-Methylglyoxaline (99%), Ethanol, Methanol, Cadmium nitrate tetrahydrate (99%), Aluminum nitrate nonahydrate (99%), Urea (99%), Sodium hydroxide (99.99%), which were purchased from Merck company.
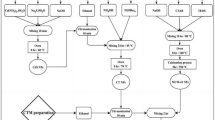
Synthesis of ZIF-67 metal-organic framework
Cobalt nitrate hexahydrate served as a cobalt precursor and was dissolved in methanol, followed by the addition of 2-methylimidazole as a ligand. This mixture initiated a self-assembly reaction that formed the ZIF-67 framework. The resulting precipitate was collected via centrifugation, washed with methanol, and dried at 80 °C to eliminate solvent residues and stabilize the crystalline structure17.
Synthesis of cobalt oxide (Co3O4) from ZIF-67
ZIF-67 was then thermally decomposed in a nitrogen atmosphere to yield cobalt oxide (Co3O4). The calcination process involved heating to 500 °C, which removed organic ligands and created a porous structure. An additional annealing step at 350 °C further stabilized the crystalline phase while preventing excessive particle growth. The inert nitrogen environment effectively inhibited cobalt oxidation, thereby preserving the desired morphology of the resulting Co3O4 nanoparticles17.
Hydrothermal synthesis of CdAl2O4
A mixture of 0.937 g of aluminum nitrate nonahydrate (Al (NO3)3·9H2O) and 2.313 g of cadmium nitrate tetrahydrate (Cd (NO3)2·4H2O) was homogenized using a magnetic stirrer at 300 rpm for 30 min. Following this, 0.9 g of urea and 25 mL of a 1 M sodium hydroxide solution (prepared with deionized water) were added to the mixture while stirring continuously. After one hour, the solution was transferred to a 100 mL hydrothermal reactor and maintained at 160 °C for 18 h. This process yielded a white precipitate, which was thoroughly washed multiple times with deionized water and ethanol, then dried in an oven at 105 °C for 24 h, resulting in the desired CdAl2O4 product18.
Synthesis of Co3O4/CdAl2O4 nanocomposite
In the initial step, the prepared CdAl2O4 solution was stirred for 30 min to ensure homogeneity. Next, cobalt oxide nanoparticles, acting as the active phase, were incorporated into the mixture and subjected to ultrasonic irradiation for 30 min to achieve a uniform dispersion within the reaction medium. Urea was subsequently introduced as a reducing agent, and sodium hydroxide was added to adjust the pH of the solution. Finally, the reaction mixture was heated at 160 °C for 18 h in a hydrothermal reactor. Upon completion of the synthesis, the resulting precipitate was washed repeatedly with water and acetone to eliminate impurities, then dried at 105 °C for 24 hours17,18. (Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9).
Photocatalytic test
The photocatalytic degradation experiments were conducted by dispersing a predetermined amount of prepared nanocomposite in 20 mL of Direct Blue (DB) and Basic Yellow (BY) solutions of 10 ppm concentration. The dye solution was stirred in the dark for 30 min to obtain the adsorption-desorption equilibrium for the experiments. To start the visible light irradiation, a Halogen Osram lamp (400 W) was placed at a distance of 5 cm, and the catalyst reaction solution was stirred for 1 h. 3 mL of aqueous solution was collected from the reactor at 15 min intervals. The degradation dye concentration was analyzed using a UV-Vis spectrophotometer at the characteristic wavelengths of Direct Blue and Basic Yellow.
To determine the concentration of DB, a spectrophotometer was used at its maximum absorbance wavelength of 565 nm. The decoloration ratio (DC%) of DB was then calculated using Eq. 119.
The initial and final concentrations of DB can be determined by measuring C₀ (mg/L) and Ct (mg/L), respectively. The same procedure was applied to BY, using its maximum absorbance wavelength (λmax) of 417 nm.
Study of zero point charge (pHpzc)
To determine the point of zero charge (PZC) of the nanocomposite, a 100 mL conical flask containing 50 mL of 0.01 M NaCl solution and 0.5 g of adsorbent was prepared. The pH was adjusted across a range of 2 to 12 using 0.1 M HCl and NaOH solutions. After 24 h of agitation on a shaker, the final pH of each solution was measured. Figure 10 presents the plot of initial pH versus the change in pH (initial pH – final pH), and the PZC of the nanocomposite was identified at the intersection point of the curve.
Result and discussion
FTIR result
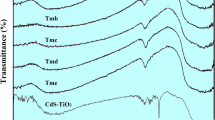
Fourier Transform Infrared (FTIR) spectroscopy is an effective method for identifying functional groups and determining the chemical structure of materials. This study utilized FTIR spectroscopy to examine the structure of CdAl2O4, Co3O4, and Co3O4/CdAl2O4 nanocomposite. The FTIR spectra were recorded in the range of 600 to 4000 cm− 1, with careful interpretation of the observed peaks. According to Fig. 1a, a broad band at approximately 3443 cm− 1 in the FTIR spectrum of Co3O4 nanoparticles is attributed to the stretching vibrations of O-H bonds from water molecules adsorbed on the nanoparticle surface. The peak at 833 cm− 1 is associated with Co3 − 2 anions, confirming the successful formation of Co3O4 nanoparticles through its intensity and position. The peak at 1398 cm− 1 corresponds to NO3 stretching vibrations, indicating nitrate impurities in the sample. Peaks around 659 cm− 1 are linked to O-Co-O bond stretching vibrations. In CdAl2O4 nanoparticles, peaks at 703, 809, and 856 cm− 1 are attributed to Al-O, Cd-O, and Cd-O-Al stretching vibrations, respectively. Weak peaks at 2925 and 2858 cm− 1 correspond to C-H stretching vibrations, likely due to organic impurities or adsorption of organic contaminants on the nanoparticle surface. The broad peak at 3611 cm− 1 is attributed to OH stretching from H2O molecules adsorbed on the catalyst surface. The presence of peaks at 713, 856, 833, and 3443 cm− 1 in the synthesized nanocomposite indicates successful formation of the nanocomposite. Slight shifts in peak positions and intensities compared to pure nanoparticle spectra suggest interactions between Co3O4 and CdAl2O4 within the nanocomposite structure.
XRD result
X-ray diffraction (XRD) is an essential technique for analyzing materials’ crystal structure and phases.
Figure 2 presents a comparison of the X-ray diffraction (XRD) patterns of the synthesized Co3O4, CdAl2O4, and Co3O4/CdAl2O4Co3O4/CdAl2O4 nanocomposite with the standard patterns of Co3O4 (JCPDS card no: 42-1467) and CdAl2O4 (JCPDS card no: 34–0071). The crystallite size of the prepared samples was estimated using the Scherrer equation (Eq. 2)19.
Here, λ represents the X-ray wavelength (0.154178 nm), k is the shape factor (0.94), θ is the diffraction angle of the most intense peak, and βhkl is the full width at half maximum (FWHM) of that peak, measured in radians.
According the Fig. 2a for Co3O4 nanoparticles, peaks were observed at 2θ = 18.73°(111), 31.19°(220), 36.86°(311), 45°(400), 59.35°(511), and 65.23°(440). These align well with standard patterns reported in the literature, indicating large crystallite size and a well-ordered atomic arrangement, confirming the formation of a pure cobalt oxide phase. The absence of additional peaks suggests high purity of the synthesized sample. In contrast, CdAl2O4 nanoparticles (Fig.2b) exhibited peaks at 2θ = 23.56° (220), 30.43° (113), 33.06° (410), 35.54° (232), 43.89° (223), 49.68° (502), 58.29° (324), 65.89° (713), 69.26° (633), and 74.84° (900). These are consistent with literature values. In the Co3O4/CdAl2O4 nanocomposite (Fig. 2c), observed peaks included: 18.73° (111), 23.56° (220), 30.43° (113), 36.86° (311), 43.89° (223), 49.68° (502), 58.29° (324), and others corresponding to both CdAl2O4 and Co3O4 phases11confirming successful nanocomposite formation. Notably, slight shifts and changes in peak intensity compared to pure samples suggest strong interactions between the two phases, leading to a nanocomposite structure with new properties likely due to factors such as lattice strain, small crystallite size, or limited solid solution formation. No other peak signals or impurities are detected in the present products. The outcomes of XRD specify that Co3O4/CdAl2O4 nanocomposite has been prepared successfully. Similar outcomes were obtained by Rajesh et al. Debye Scherrer’s formula computed all obtained products’ mean crystalline size (D). The average crystallite sizes for pure CdAl2O4, Co3O4, and the synthesized nanocomposite are 19.38, 27, and 21.58 nm, respectively.
FE-SEM study
The surface morphology of the synthesized Co₃O₄, CdAl₂O₄, and Co3O4/CdAl2O4 nanocomposite were examined using field emission scanning electron microscopy (FE-SEM). According to the Fig. 3, the Co3O4 nanoparticles were predominantly spherical and exhibited significant agglomeration, whereas CdAl2O4 nanoparticles had a rod-like shape with varying dimensions. It shows the monodisperse crystal formed by the forced nucleation of the Cd (II) and Al (III) under basic condition. High magnification image of CdAl2O4 showed aggregated road like clustered structure. FESEM images of the nanocomposite showed that the original shapes of the nanoparticles were retained after composite formation.
TEM result
Transmission electron microscopy (TEM) provided a detailed examination of morphology, internal structure, and particle size at both nanometer and micrometer scales. TEM images of the Co3O4/CdAl2O4 nanocomposite (Fig. 3d) demonstrated a uniform dispersion of Co3O4 nanoparticles within the CdAl2O4 matrix. This indicates successful nanocomposite formation with proper phase distribution and suggests strong interactions between the particles of both materials. The sample was dispersed in ethanol by ultrasonic vibration and dropped onto carbon-coated copper grids’ surface. It can be seen that nanoparticles have spherical-like, which was covered by CdAl2O4 structure.
EDX result
EDX analysis confirms the presence of the anticipated elements in the structures of Co3O4 and CdAl2O4 nanoparticles, as well as in the corresponding Co3O4/CdAl2O4 nanocomposite, as shown in Figure 4. The morphology and EDX analyses supported the XRD results, confirming that no additional atomic species were present in the nanocomposite20. According to the Fig. 4a, the EDX spectra distinctly reveal the characteristic peaks of O and Co in the Co3O4 sample. Also, the EDX spectra of CdAl2O4 displays peaks for Al, Cd, O, and Co, as illustrated in Fig. (4b). The consistent distribution of these peaks across the entire surface of the synthesized samples indicates a homogeneous dispersion of elements within both the nanoparticle and nanocomposite structures.
TGA result
Thermogravimetric analysis (TGA) is a technique used to study the thermal stability and composition of materials by measuring the change in weight as a function of temperature. When analyzing photocatalysts like Co3O4, CdAl2O4 and the composite, TGA provides insights into their thermal decomposition, phase transitions, and potential interactions between components. The result was shown in Fig. (5) Co3O4 typically shows an initial weight loss at lower temperatures due to the removal of adsorbed water or other volatile impurities. The primary decomposition event for Co3O4 occurs around 900 \(\:℃\). This is attributed to the reduction of Co3O4 to CoO. This reduction results in a weight loss corresponding to the release of oxygen gas. The theoretical weight loss for this transition is approximately 6.6%. CdAl2O4 is generally stable over a wide temperature range due to its spinel structure. Any observed weight loss at lower temperature could be due to surface moisture. The composite exhibited enhanced thermal stability due to synergetic effects between Co and Al sites. The presence of CdAl2O4 influenced the reduction temperature of Co3O4.
BET study
The surface area and porosity of the synthesized nanocomposite textures were evaluated using nitrogen adsorption–desorption measurements at P/P₀ = 0.99 through BET and BJH analyses. Figure 3 shows the N₂ adsorption–desorption isotherms and pore volume curves for Co3O4, CdAl2O4 and the Co3O4/CdAl2O4 nanocomposite. It provides insights into the adsorption and desorption characteristics on solid surfaces, which are crucial for understanding the performance of the synthesized photocatalyst. According to the obtained result in Fig. 6, the CdAl2O4, Co3O4 and the synthesized nanocomposite exhibited type (IV) isotherms. The Co3O4 photocatalyst showed Type (IV) isotherm with pronounced hysteresis loop due to capillary condensation in mesopores. It indicated significant porosity contributing to higher surface area. Besides, the result demonstrated the coexistence between micropores and mesopores of the photocatalyst. Additionally, the surface area of CdAl2O4 was reported 10.55 m2/g, which has a relatively lower surface area compared to Co3O4 (32.73 m2/g). This suggests that its intrinsic photocatalytic activity might be limited by the available active sites for adsorption and reaction. The synthesized nanocomposite surface area was reported 24.68 m2/g, which was intermediate between its individual components, suggesting a synergetic interaction between Co3O4 and CdAl2O421,22.
Raman result
Raman spectroscopy is a powerful tool for characterizing materials, including photocatalysts Co3O4, CdAl2O4, and the synthesized nanocomposite. This technique provides insights into the vibrational modes of the photocatalyst, which can be correlated with structural, electronic, and photocatalytic properties. Co3O4 is a spinel-type oxide with a cubic crystal structure. According to the obtained result in Fig. 7, the Raman spectrum of Co3O4 typically exhibits several characteristic peaks due to its spinel structure. The peak around 690 cm− 1, associated with symmetric stretching vibrations of the oxygen atoms around the octahedral Co3+ ions. The peaks around 480 and 520 cm− 1 are related to various bending and stretching vibrations within the lattice. These peaks provide information about the crystallinity and phase purity of Co3O4. CdAl2O4 is also a spinel-type oxide but with cadmium occupying tetrahedral sites and aluminum in octahedral sites. Peak modes that appear at lower frequencies compared to those in Co3O4 due to differences in mass and bonding characteristics. The synthesized composite showed both pure photocatalyst peaks and it was used to assess interactions between components, phase integration, and structural modifications. Changes in peak sharpness or position can indicate variations in crystallinity or particle size effects.
Photoluminescence spectroscopy
Photoluminescence (PL) is a phenomenon in which a material absorbs high-energy photons and subsequently emits light at longer wavelengths. Analyzing the emission spectrum can provide valuable insights into the electronic structure, the nature and concentration of defects, as well as the processes of non-radiative and radiative recombination. This technique, rooted in quantum mechanical principles and the interaction between light and matter, allows researchers to gain a deeper understanding of material properties. Figure 7d shows the photoluminescence emission spectra of Co3O4, CdAl2O4, and their nanocomposite at room temperature under 390 nm excitation. According to the Fig. 7d, both pure Co3O4 and CdAl2O4 samples demonstrate a high rate of recombination. However, the formation of a heterogeneous structure and the creation of a binary nanocomposite resulting from increased interfacial areas and a range of grain sizes diminishes the likelihood of recombination events. This enhancement ultimately contributes to improved performance of the nanocomposite sample in photocatalytic applications.
Optical properties of synthesized samples
The UV-visible spectra of Co3O4, CdAl2O4, and their nanocomposite are presented in Fig. 8. The UV/vis diffuse reflectance spectroscopy (DRS) analysis of prepared samples provides a detailed exploration of the analytical results and their relationship to the photocatalytic performance of these materials. The primary aim is to deepen our understanding of the mechanisms that drive photocatalytic processes in these systems and to pinpoint the critical factors that affect catalytic efficiency. So, to determine the energy gap (Eg) necessary for electron excitation from the valence band (VB) to the conduction band (CB) during photocatalysis, UV-Vis absorption spectroscopy was employed (Fig. 8b). As shown in the inset of Fig. 8b, UV-Vis spectra were recorded for Co3O4, CdAl2O4, and their nanocomposite. The energy band gaps were calculated using Tauc’s equation (Eq. 3) based on the obtained spectra19.
The optical band gap (Eg) can be determined by extrapolating the linear portion of the (αhν)² versus hν plot known as the Tauc plot to the photon energy (hν) axis, as shown in Fig. 8b. In this method, α is the absorption coefficient, hν is the photon energy, A is a proportionality constant, and n is an exponent that depends on the nature of the electronic transition (n = 1 for direct allowed transitions). According to Tauc’s equation, Eg corresponds to the intercept of the tangent line with the energy axis.
Through an in-depth examination of DRS spectra, coupled with an investigation of the materials’ structural, morphological, and electronic properties, we seek to optimize photocatalytic processes and create more effective catalysts. According to Fig. 8, Cobalt oxide, with a bandgap of 1.5 eV, demonstrates significant light absorption in the visible region, making it an excellent candidate for photocatalytic applications under visible light irradiation. In contrast, CdAl2O4 has a bandgap of 3.66 eV, which limits its absorption to the ultraviolet region, resulting in lower photocatalytic activity compared to Co3O4 when exposed to visible light. The synthesized nanocomposite exhibits a bandgap of 2.4 eV, indicating its ability to absorb light within the visible spectrum.
Standard tests for measuring dye removal efficiency
In the initial phase of the study, we examined the influence of various factors on the efficiency of dye removal from aqueous solutions, evaluating each parameter individually. Key parameters assessed included pH, initial dye concentration, the quantity of adsorbent utilized, and the duration of sample exposure in both dark and light conditions. For each experiment, a predetermined amount of adsorbent was added to a dye solution at a specific concentration, followed by pH adjustment using standard sodium hydroxide and hydrochloric acid solutions. To eliminate any interference from ambient light, samples containing the dye and nanocomposite were kept in the dark for 30 min. After this period, the samples were exposed to light, and dye absorption was measured at specified time intervals using a UV-Vis spectrophotometer. The results indicated that the dye removal efficiency of the nanocomposite was relatively low, suggesting that the anticipated photocatalytic mechanism was not fully activated. To assess the stability of the dye under the experimental conditions, a sample of the dye solution without the nanocomposite was also kept in the dark for 30 min before being exposed to light for 90 min. The findings revealed no significant change in dye concentration, indicating that the dye remained stable under these conditions and did not undergo spontaneous degradation.
Kinetic study of photocatalytic process
The adsorption process was investigated by adding 0.01 g of adsorbent to a dye solution and stirring it under dark conditions for 2 h. Data analysis revealed that the percentage of dye adsorption on the adsorbent surface was less than 25%. Given this minimal amount, it can be concluded that adsorption did not significantly contribute to the dye removal process; therefore, adsorption-based kinetic models were not applied in subsequent analyses. Following this, we explored the kinetics of photocatalytic dye degradation and found that the experimental data aligned well with the pseudo-first-order kinetic model. This suggests that the rate of dye degradation is directly proportional to its concentration at any given moment.
The pseudo-first-order kinetic model is represented by the blow equation –Ln (C0/Ct) = KT (Eq. 5) which is frequently used to describe the degradation rate of organic pollutants in photocatalytic processes. In this equation, C denotes the concentration of the pollutant at time t, C0 is the initial concentration, and k is the pseudo-first-order rate constant. The percentage removal of the pollutant at any time t can be calculated using the following equation (Eq. 1)23; the result was shown in Fig. 9 and the calculated rate constant was reported in Table 1.
Optimization of pH for maximum dye removal
The photocatalytic activity of Co3O4/ CdAl2O4 nanocomposite was evaluated using Direct Blue (DB) and Basic Yellow (BY) as model pollutants under different pH conditions acidic, neutral, and alkaline (Figs. 10, 11). Figure 12 illustrates the effect of pH on the photocatalytic degradation of DB and BY over time. The pH of the system was adjusted using 0.1 M HCl and NaOH solutions. The highest degradation efficiencies, 91% for DB and 86% for BY, were achieved at pH 2 and 12, respectively.
To assess the effect of pH on the removal of BY and DB dyes using a nanocomposite, 20 mL solutions of each dye at a concentration of 10 ppm were prepared across a pH range of 2 to 1214,24. Subsequently, 0.01 g of the nanocomposite was added to each solution. The mixtures were stirred in the dark for 30 min before being exposed to UV light for 60 min. The acidity or alkalinity of the reaction medium can impact photocatalyst performance through various mechanisms, including modifications to the catalyst’s surface charge, ionization of dye molecules, and the generation of different radical species.
pH of point zero charge (PZC) was also provided. The effect of surface charge on the activity of the catalyst was explained. Based on the results obtained, the point of zero charge (pHpzc) of the synthesized composite was determined to be 2.76. The surface of the nanocomposite carries a positive charge at pH values below the pHpzc (pH < pHpzc) and a negative charge at pH values above it (pH > pHpzc). Therefore, at pH levels higher than the pHpzc, the nanocomposite exhibits a high negative surface charge density, which enhances the adsorption of the cationic dye BY. In contrast, at pH levels below the pHpzc, the surface becomes highly positively charged, leading to reduced adsorption of DB25,26.
Optimal adsorbent dosage
The influence of photocatalyst dosage on dye removal efficiency was examined within the range of 0.002 to 0.05 g. Findings in Fig. (11a) indicated that the optimal dosage for this system was 0.01 g. Increasing the dosage beyond this level resulted in a decline in dye removal efficiency. This decrease can be attributed to enhanced self-screening effects of the photocatalyst, which hinder light penetration into the solution and reduce the number of photons available to interact with pollutant molecules. Furthermore, higher concentrations of photocatalyst may lead to particle agglomeration, which diminishes the specific surface area and, as a result, reduces catalytic activity. It is also important to note that other factors, such as photocatalyst particle size, specific surface area, type of light used, and light intensity, play significant roles in influencing photocatalytic activity27.
Effect of initial dye concentration
In this study, we explored the effect of initial dye concentration on the photocatalytic removal of Direct Blue (at pH 2) and Basic Yellow (at pH 12) dyes using the Co3O4/CdAl2O4 nanocomposite under visible light irradiation. In these experiments, 0.01 g of the nanocomposite was added to aqueous solutions containing varying initial dye concentrations (ranging from 10 to 50 mg/L). The results in Fig. (11b) revealed that the removal efficiency for both Direct Blue and Basic Yellow dyes significantly decreased as the initial dye concentration increased. This trend can be explained by several factors: (1) Adsorption competition: as dye concentration rises, more dye molecules compete for active sites on the surface of the Co3O4/CdAl2O4 nanocomposite, leading to a reduction in adsorption capacity. (2) Reduced light penetration: higher dye concentrations diminish the penetration of visible light into the solution, resulting in fewer free radicals being generated and a corresponding decrease in dye degradation efficiency. (3) Saturation of active sites: once all active sites on the nanocomposite surface are saturated, the adsorption capacity reaches its maximum limit, rendering further increases in dye concentration ineffective in enhancing removal efficiency.
Effect of contact time on dye removal
A photocatalytic nanocomposite sample underwent a 30-minute dark reaction followed by a 150-minute photocatalytic reaction under optimized conditions. The pollutant’s highest degradation efficiency was observed after 60 min of irradiation in Fig. (11c).
The stabilization of dye degradation efficiency after 60 min can be attributed to several interrelated factors. Firstly, it is possible that the reactive species responsible for dye degradation, such as hydroxyl radicals or other oxidants, reach a saturation point after a certain duration of exposure. This saturation may result from the depletion of available dye molecules or the exhaustion of reactive intermediates necessary for continued degradation. Additionally, the reaction kinetics may shift over time. Initially, the degradation process is rapid as reactive species interact with the dye molecules; however, as the concentration of the dye decreases, the likelihood of effective collisions between reactive species and dye molecules diminishes. This could lead to a plateau in degradation efficiency. Besides, a comparison between this study with other literatures26,28,29 was discussed according to Table 2. It was shown that Co3O4/CdAl2O4 nanocomposite can be an excellent candidate for photocatalytic process in dyes removal.
Photocatalyst reusability
To assess the photocatalyst’s reusability, it was washed with deionized water and dried at 80 °C for 24 h after each cycle before being reused for color removal tests in a fresh dye solution. The experiments involved using 20 mL of Direct Blue dye solution at pH 2 and Basic Yellow dye solution at pH 12, with a dye concentration of 10 ppm and 0.01 g of Co3O4/CdAl2O4 nanocomposite. After treatment, the nanocomposite was washed with deionized water and dried at 80 °C for another 24 h. Results in Fig. 12 indicated that the removal efficiency for Direct Blue and Basic Yellow dyes decreased from 91 to 80% and from 86 to 74%, respectively, after four reuse cycles. This reduction in efficiency is likely due to factors such as fouling of the nanocomposite surface with organic matter, alterations in crystalline structure or particle size, or a decrease in active sites due to repeated use.
XRD analysis in Fig. 13 demonstrated the remarkable stability of the Co3O4/CdAl2O4 nanocomposite after multiple cycles of use and washing. Throughout these processes, no new impurities were adsorbed by the nanocomposite. The slight decrease in XRD peak intensity is mainly attributed to repeated washing and possibly minor changes in crystallite size or specific surface area. Nonetheless, the catalytic performance of the nanocomposite remained largely unaffected. FESEM was used to investigate its structure after multiple cycles. Co3O4 displays a sphere-like structure. The images of the nanocomposite showed that the original shapes of the nanoparticles were retained after multiple cycles30,31.
Photocatalytic degradation mechanism
Benzoquinone (BQ), ammonium oxalate (AO), and isopropanol (IP) were used as scavengers to identify the main reactive species involved in the degradation process in Fig. 14. Benzoquinone, known to effectively scavenge superoxide radicals, BQ was added to our system. The degradation percentage decreased significantly to 62% and 56% for Direct blue and Basic Yellow, respectively, indicating a substantial reduction in activity when superoxide radicals were quenched. This significant drop confirms that superoxide radicals play a crucial role in the degradation process. Ammonium Oxalate, used primarily as a scavenger for hole (h+) radicals, ammonium oxalate was added, resulting in 86% and 81% degradation rate for Direct blue and Basic Yellow, respectively. These values are close to the control (without any scavengers), suggesting that hole radicals are not the primary reactive species. Isopropanol, A known scavenger for •OH quencher, IPA was also tested and resulted in a 83 and 79% degradation for Direct blue and Basic Yellow, respectively, further supporting that hydroxyl radicals have minimal impact on the overall degradation process.
The results from these experiments strongly indicate that superoxide radicals are indeed the main reactive species responsible for degradation in our system. The significant impact of ascorbic acid compared to ammonium oxalate and isopropanol supports this conclusion.
The proposed mechanism in Fig. 15 for photocatalytic dye removal using the Co3O4/CdAl2O4 nanocomposite involves a series of intricate reactions. This process relies on the interaction between light, the catalyst, and dye pollutants, resulting in the breakdown of these pollutants into simpler compounds. When light with appropriate energy strikes the nanocomposite, electrons in the valence band (VB) absorb this energy and become excited to the conduction band (CB)32,33. This excitation generates positive holes (+ h) in the valence band and free electrons (-e) in the conduction band:
The excited electrons in the conduction band can then react with oxygen molecules present in the environment to form superoxide radicals:
These superoxide radicals are potent oxidants that attack and break down the chemical bonds of pollutant molecules, ultimately degrading them into simpler compounds like water and carbon dioxide:
This photocatalytic mechanism for dye removal using Co3O4/CdAl2O4 nanocomposite is a promising approach for purifying water and air. A thorough understanding of this mechanism can lead to process optimization and the development of more efficient catalysts34.
Conclusion
In this study, a novel hybrid nanocomposite, Co3O4/CdAl2O4, was synthesized using hydrothermal and sonochemical methods. The main goal was to create an efficient photocatalyst for treating wastewater contaminated with organic dyes. Detailed characterization through FTIR, EDX, FESEM, TEM, XRD, PL, and UV-vis spectroscopy revealed the nanocomposite’s structural, morphological, and optical properties. The findings showed that the nanocomposite had a porous structure with a high specific surface area and visible light absorption capabilities, making it a promising candidate for water purification applications. Field emission scanning electron microscopy (FESEM) images displayed a uniform distribution of spherical cobalt oxide particles on the rod-like CdAl2O4 substrate. These observations were supported by transmission electron microscopy (TEM), which confirmed the spherical shape of cobalt oxide particles and the rod-like structure of CdAl2O4. By combining cobalt oxide (bandgap of 1.5 eV) with cadmium aluminate (bandgap of 3.66 eV), a nanocomposite with a bandgap of 2.4 eV was achieved. UV-vis spectroscopy results showed enhanced visible and UV light absorption by the nanocomposite compared to its individual components. The reduced bandgap and increased light absorption contributed to its improved photocatalytic performance. Photoluminescence analysis indicated a significant reduction in electron-hole recombination rates in the nanocomposite compared to pristine nanoparticles. This suggested an increase in charge carrier lifetime and consequently enhanced photocatalytic activity. To thoroughly assess the nanocomposite’s performance in dye degradation, various operational parameters such as pH, initial dye concentration, and contact time were examined. Results showed that higher initial dye concentrations significantly decreased the removal efficiency for Direct Blue and Basic Yellow dyes. This effect was likely due to competition among dye molecules for active sites on the nanocomposite surface, reduced light penetration into the solution leading to decreased generation of reactive species for dye degradation, and gradual saturation of active sites as dye concentration increased.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
References
Yousefi, S. R., Alshamsi, H. A., Amiri, O. & Salavati-Niasari, M. Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol. Liq. 337, 116405 (2021).
Heidari-Asil, S. A. et al. Magnetically recyclable ZnCo2O4/Co3O4 nano-photocatalyst: green combustion preparation, characterization and its application for enhanced degradation of contaminated water under sunlight. Int. J. Hydrog. Energy. 47, 16852–16861 (2022).
Hegazy, S., Abdelwahab, N. A., Ramadan, A. M. & Mohamed, S. K. Magnetic Fe3O4-grafted cellulose/graphene oxide nanocomposite for methylene blue removal from aqueous solutions: synthesis and characterization. Next Mater. 3, 100064 (2024).
Ayazi, S., Ghorbani, M. & Abedini, R. Multifunctional composite membranes incorporated by SiO2@ CuFe2O4 nanocomposite for high dye removal, antibacterial and antifouling properties. Chem. Eng. Res. Des. 169, 214–228 (2021).
Falah, S., Ghorbani, M. & Ahmadpour, J. Photocatalytic degradation of anionic and cationic dyes over PPy/CuFe2O4 nanocomposite under visible-light and bactericidal action. J. Taiwan Inst. Chem. Eng. 144, 104767 (2023).
Aazam, E. Environmental remediation of cyanide solutions by photocatalytic oxidation using au/cds nanoparticles. J. Ind. Eng. Chem. 20, 2870–2875 (2014).
Saravanan, A. et al. A detailed review on advanced oxidation process in treatment of wastewater: mechanism, challenges and future outlook. Chemosphere 308, 136524 (2022).
Rajesh, G. et al. Strategies for ameliorating the photodegradation efficiency of Mn-doped CdAl2O4 nanoparticles for the toxic dyes under visible light illumination. Chemosphere 321, 138069 (2023).
Muradov, M. et al. The effect of Cu doping on structural, optical properties and photocatalytic activity of Co3O4 nanoparticles synthesized by sonochemical method. Opt. Mater. 142, 114001 (2023).
Yang, R. et al. Yolk-shell Co3O4@ Fe3O4/C nanocomposites as a heterogeneous Fenton‐like catalyst for organic dye removal. Chem. A Eur. J. 29, e202203097 (2023).
Sanad, M. M., Taha, T. A., Helal, A. & Mahmoud, M. H. Rational optimization of g-C3N4/Co3O4 nanocomposite for enhanced photodegradation of Rhodamine B dye under visible light. Environ. Sci. Pollut. Res. 30, 60225–60239 (2023).
Hamza, A. M. & Alshamsi, H. A. Design of novel Z-scheme g-C3N4/TiO2/CuCo2O4 heterojunctions for efficient visible light-driven photocatalyic degradation of Rhodamine B. Sci. Rep. 14, 23596 (2024).
Alshamsi, H. A. & Jabir, F. A. Green synthesis of Ni0. 5Zn0. 5O/TiO2 for photocatalytic, antibacterial and anticancer activities. J. Mol. Liq. 413, 126037 (2024).
Livani, M. J. & Ghorbani, M. Fabrication of NiFe2O4 magnetic nanoparticles loaded on activated carbon as novel nanoadsorbent for direct red 31 and direct blue 78 adsorption. Environ. Technol. 39, 2977–2993 (2018).
Rajesh, G. et al. Fabrication of a novel Ni-doped CdAl2O4 nanoparticles and applications in photo-oxidation processes under visible light illumination. Mol. Catal. 535, 112835 (2023).
Palanisamy, G., Bhuvaneswari, K., Lee, J. & Shkir, M. Synergistic photocatalytic performance through Z-Scheme charge transfer in organic dye degradation using α-MnO2 nanorods and Co3O4 nanoparticles combined with eggshell derived hydroxyapatite. Colloids Surf. A. 677, 132300 (2023).
Panahi, M., Ghorbani, M. & Lashkenari, M. S. Construction of CO3O4 derived ZIF/GO electrode for outstanding stability in supercapacitors devices. Int. J. Hydrog. Energy. 47, 9800–9809 (2022).
Kumar, R., Barakat, M., Al-Mur, B. A., Alseroury, F. A. & Eniola, J. O. Photocatalytic degradation of cefoxitin sodium antibiotic using novel BN/CdAl2O4 composite. J. Clean. Prod. 246, 119076 (2020).
Geravi, H. A. & Ghaemy, M. Preparation of molecularly imprinted polymer nanoparticles for selective adsorption of caffeine using dual-functionalized Ag2S quantum Dots. Colloids Surf. A. 680, 132735 (2024).
Karamat, S. et al. High-performance humidity sensors based on reduced graphene oxide sheets decorated with Cobalt and iron doped ZnO nanorods. Mater. Today Commun. 40, 109742 (2024).
Swetha, S. et al. Triple-mechanism driven Fe-doped Nn hetero-architecture of Pr6O11-MoO3 decorated g-C3N4 for Doxycycline degradation and bacterial photoinactivation. Chem. Eng. J. 461, 141806 (2023).
Harikumar, B., Kokilavani, S. & Khan, S. S. Magnetically separable N/S doped Fe3O4 embedded on MoO3 nanorods for photodegradation of cefixime, cr (VI) reduction, and its genotoxicity study. Chem. Eng. J. 446, 137273 (2022).
Mahdi, N. I. Application for fitting of Langmuir and Freundlich equations studies on the experminatl data of adsorption of methylene blue dye by Cobalt oxide surface. Samarra J. Pure Appl. Sci. 5, 66–77 (2023).
Azimifar, M., Ghorbani, M. & Peyravi, M. Fabrication and evaluation of a photocatalytic membrane based on Sb2O3/CBO composite for improvement of dye removal efficiency. J. Mol. Struct. 1270, 133957 (2022).
Steffy, J. et al. Facet engineering in Au nanoparticles buried in Cu2O nanocubes for enhanced catalytic degradation of Rhodamine B and larvicidal application. Sustain. Mater. Technol. 43, e01185 (2025).
Kokilavani, S. et al. Designing Z-scheme AgIO4 Nanorod embedded with Bi2S3 nanoflakes for expeditious visible light photodegradation of congo red and Rhodamine B. Chemosphere 294, 133755 (2022).
Zena, Z. W., Andoshe, D. M., Tufa, L. T., Gemta, A. B. & Dejene, F. B. High performance Co3O4/Sn-ZnO nanocomposite photocatalyst for removal of methylene blue dye. Phys. Scr. 99, 045934 (2024).
Harikumar, B. et al. Robust visible light active CoNiO2–BiFeO3–NiS ternary nanocomposite for photo-fenton degradation of Rhodamine B and Methyl orange: kinetics, degradation pathway and toxicity assessment. J. Environ. Manag. 317, 115321 (2022).
Kokilavani, S. et al. Efficient photocatalytic degradation of Methyl orange and malachite green by Ag3PO4 decorated BiOBr Nanoflower under visible light: performance evaluation, mechanism insights and toxicology of the by-products. J. Alloys Compd. 909, 164703 (2022).
Chinnathambi, A., Nasif, O., Alharbi, S. A. & Khan, S. S. Enhanced optoelectronic properties of multifunctional MnFe2O4 nanorods decorated Co3O4 nanoheterostructure: photocatalytic activity and antibacterial behavior. Mater. Sci. Semiconduct. Process. 134, 105992 (2021).
Janani, B. et al. Designing intimate porous Al2O3 decorated 2D CdO nano-heterojunction as enhanced white light driven photocatalyst and antibacterial agent. J. Alloys Compd. 896, 162807 (2022).
Sayed, N. S., Ahmed, A. S., Abdallah, M. H. & Gouda, G. A. ZnO@ activated carbon derived from wood sawdust as adsorbent for removal of Methyl red and Methyl orange from aqueous solutions. Sci. Rep. 14, 5384 (2024).
Rajesh, G., Kumar, P. S., Alanazi, A. K., Rangasamy, G. & Abo-Dief, H. M. Development of lattice defects and oxygen vacancies in Zn-doped CdAl2O4 nanoparticles for improving the photocatalytic efficiencies of brilliant green and brilliant blue dyes under visible illumination. Catal. Commun. 183, 106762 (2023).
Rajeshwari, M. R., Kokilavani, S. & Khan, S. S. Recent developments in architecturing the g-C3N4 based nanostructured photocatalysts: synthesis, modifications and applications in water treatment. Chemosphere 291, 132735 (2022).
Acknowledgements
The authors are gratefully acknowledged to the Babol Noshirvani University of Technology for the financial supports (BNUT/388001/2021) dedicated to this project.
Author information
Authors and Affiliations
Contributions
Methodology: Z. AraeiInvestigation: Z. Araei and M. GhorbaniFormal analysis: Z. Araei and M. GhorbaniWriting original draft: Z. Araei and M. GhorbaniWriting review and editing: M. GhorbaniSupervision: M. Ghorbani.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
araei, Z., ghorbani, M. Synergistic effect of Co3O4/CdAl2O4 nanocomposite for photocatalytic decontamination of organic dyes under visible light irradiation. Sci Rep 15, 22454 (2025). https://doi.org/10.1038/s41598-025-07095-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07095-2