Abstract
Amid global environmental challenges, particularly air pollution caused by toxic and acidic gases like H2S, SO2, CO2, and NO2, public health is increasingly at risk. Metal–organic frameworks (MOFs), distinguished by their crystalline structure, high porosity, tunable pore size, and diverse functionalities, hold great promise for mitigating the capture of these harmful pollutants. In this study, molecular simulation calculations were conducted to investigate the adsorption and diffusion mechanisms of the toxic and acidic gases H2S, SO2, CO2, and NO2 on the novel iron carboxylate (III) Metal–Organic Framework, MIL-100(Fe). The adsorption energy and total energy of the systems were calculated for each gas, with negative values indicating successful adsorption of the gas by MIL-100(Fe). The MIL-100(Fe)/H2S system exhibited the most negative total energy, indicating its superior stability among the studied gas–MOF systems. The highest adsorption energy value was observed for H2S gas at -49.28 kcal/mol, indicating a strong interaction between H₂S molecules and the MIL-100(Fe) framework. Additionally, gas permeability and diffusion coefficient calculations revealed a trend of H2S > CO2 > NO2 > SO2, with H2S exhibiting the highest diffusion coefficient of 7.71 Ų/ps, further supporting its stronger interaction with the MIL-100(Fe)’s adsorption sites. These molecular simulation calculations confirm that MIL-100(Fe) is highly effective at adsorbing toxic gases such as H2S, SO2, CO2, and NO2, highlighting its potential as a promising adsorbent for air purification applications.
Similar content being viewed by others
Introduction
With the economy rapidly expanding and global industrialization on the rise, airborne pollution has evolved into one of the gravest threats to humanity1. Every year, dangerous pollutants are released into the air, which harms the environment and endangers the health of humans and animals2. These toxic pollutants are mainly produced from activities such as burning fossil fuels3, leaking gases4, and industrial vapors5. Among these pollutants are toxic and acidic gases such as hydrogen disulfide (H2S), carbon dioxide (CO2), sulfur dioxide (SO2), and nitrogen dioxide (NO2)6. H2S is a highly toxic and flammable reducing gas that enters the air through oil and gas wells, crude oil refineries, and plants consuming sulfur-containing organic matter, sewage, and polluted ports7. The brain is vulnerable to H2S gas, and neurotransmitter levels are altered in H2S-poisoned patients, causing cognitive decline6. Also, the high concentration of H2S in the air causes poisoning, inflammation of the eyes, skin burns, respiratory diseases, and inactivation of the sense of smell8. A concentration of more than 100 ppm can endanger life and even death6. Another air pollutant is nitrogen dioxide (NO2), which is a highly reactive gas with a yellow or yellowish-brown color9. The main source of NO2 emissions is combustion processes6 from motor vehicles10. NO2 can react with the water in the body tissues to produce nitric acid which leads to severe tissue damage. Reducing lung function and the exacerbation of bronchitis symptoms are known side effects of inhaling this gas. Symptoms of exposure include coughing and a sensation of suffocation. The concentration of the gas and the duration of exposure are key factors influencing its toxicity11.
Sulfur dioxide (SO2), which is known as the most common air pollutant, is an invisible gas with a pungent smell12. The sources of sulfur dioxide production are the burning of fossil fuels, the eruption of active volcanoes, forest fires, and processing of ore containing sulfur. Sulfur dioxide can cause irritation and damage to the eyes, lungs, and throat, and at high concentrations, it may lead to cardiovascular and respiratory complications, or even death. Along with nitrogen dioxide, sulfur dioxide is a major precursor of acid rain, which negatively impacts building materials, wildlife, and vegetation6.
Removing these gaseous pollutants due to both the complexity of pollutant types and the limitations of current materials and technologies used for their removal and providing access to a healthy environment is one of the main challenges of today’s world. While several methods have been documented for the elimination of these pollutants, the physical adsorption method holds particular promise due to its cost-effectiveness and remarkable efficiency. Diverse adsorbents such as Metal-Organic Frameworks (MOFs), Zeolites and Sulfate radical (SO4•–)-based advanced oxidation technologies (AOTs) can be employed for this purpose6,8,13,14.
Metal-organic frameworks (MOFs), are a new class of porous crystalline materials15,16,17,18 that have properties such as high and tunable porosity19, high surface area20, and diversity of structural composition18,21. These compounds can be used in various fields such as gas storage/separation22,23, catalysis24, the capture of harmful gases25,26, the adsorption and degradation of chemical warfare agents27,28, and drug delivery29,30. Hence, the ongoing quest is to develop versatile filters capable of eliminating the majority of common toxic gaseous pollutants. Metal-organic frameworks (MOFs) combine metal ions/clusters and organic linkers, leveraging the benefits of both material types31,32 due to their high surface areas33,34, tunable functionalities35,36, and significant thermal stability37,38. Furthermore, numerous techniques have been suggested to enhance their chemical stability, increasing their resilience in extreme conditions.
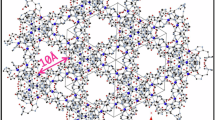
MOFs can be classified into distinct series, including Materials of Lavoisier Institute (MILs) and Zeolitic Imidazolate Frameworks (ZIFs)39,40. Among the different synthesized MOFs based on MILs, mesoporous Fe (III) trimezate (MIL-100(Fe)) stands out as a particularly appealing41,42,43,44,45 member of the MIL family for adsorption applications. MIL-100(Fe) is composed of iron (III) and benzene-1, 3, 5-tricarboxylic acid (H3BTC)46,47,48,49. Its attractiveness is attributed to the presence of unsaturated metal sites50,51, a high surface area, and robust thermal and chemical stability50,51,52, making it the focus of significant research attention. MIL-100 (Fe) has been used to absorb various substances such as colored pollutants52, drugs53, fluoride54, arsenic55.
In this study, we investigated for the first time the adsorption and diffusion mechanisms of four acidic gases—H₂S, SO₂, CO₂, and NO₂— within MIL-100(Fe). This study employed a two-stage simulation process. First, Monte Carlo simulation using the Adsorption Locator module in Materials Studio (physical adsorption) was performed. Subsequently, the output from this stage was refined using molecular dynamics within the Forcite module, run for 100 nanoseconds (ns) to enhance accuracy. Optimize energy, gas diffusion constants, and other parameters (chemical adsorption) were calculated. Molecular simulation analyses provide crucial insights into how MIL-100 (Fe) interacts with and absorbs these toxic and acidic gases. This approach provides molecular-level insight into the interaction strength, mobility, and adsorption capacity of each gas, aiming to support the design and selection of efficient MOF-based materials for air pollutant capture.
Materials and methods
Design and optimization of molecular structures
The MOF crystal structure of MIL-100(Fe), with the molecular formula Fe3O (H2O)2OH(BTC)2, was provide in.cif format56. The molecular structures of H2S, SO2, CO2, and NO2 were retrieved from the PubChem database (PubChem (nih.gov)) in.sdf format. To prepare them for dynamic molecular processes such as adsorption and sorption, the downloaded molecular structures were optimized using Materials Studio 2017 software through the Forcite module Algorithm: smart, Convergence Tolerance: Quality: Ultra-fine, Energy: 2.0e-5 Kcal/mol, Force: 0.001Kcal/mol/Aº, Stress: 0.001 GPa, Displacement: 1.0e-5 Aº, Mix. Iterations: 500, External pressure: 1.0e-4 GPa Forcefield: COMPASS, Charge : Use current, Electrostatic: Ewald and VanderWaals: Atom based (Fig. 1)57.
Molecular dynamics simulation
To compute the gas diffusion coefficient, molecular dynamics calculations were carried out. Four simulation boxes were created, each containing MIL-100(Fe) with dimensions of X = 80 Å, Y = 80 Å, and Z = 80 Å, and with β = 90º and γ = α. These boxes were prepared for the gases H2S, SO2, CO2, and NO2.
Subsequently, the gas molecules were placed within their respective simulation boxes. The initial positions of the gas molecules were automatically generated using the Monte Carlo insertion algorithm implemented in the Sorption module, which places sorbates at energetically favorable sites within the MOF framework while avoiding atomic overlaps. Then, optimization was performed using the same method as previously described. Figure 2 shows the simulation boxes for H2S, SO2, CO2, and NO2 gases that contain MIL-100(Fe). For NVE and NVT calculations, first Anneal calculations with conditions: Annealing cycles: 2, Initial temperature: 298.15 K, temperature: 500 K, Heating ramps per cycle: 5, Dynamics step per ramp: 100 and Forcefield: COMPASS was performed by the Forcite module in Materials Studio 2017 software. Then, NVT calculations with conditions of Initial velocities: Random, Temperature: 298.15 K, Time step: 1 fs, Number of steps: 100,000,000 with Velocity Scale thermostat with Temp. Difference: 10 K, Force field: COMPASS for 100 nanoseconds (ns)were done by the Forcite module. In the next step, NVE calculations with Initial velocities: Random, Temperature: 298 K, Time step: 1 fs, Total simulation time: 100ns, Number of steps: 100,000,000 Force field: COMPASS, by Forcite module was done.
Adsorption calculations
To check the physical adsorption and then the chemical adsorption of H2S, SO2, CO2, and NO2 gases on MIL-100(Fe), adsorption Locator calculations were done with conditions of Number of cycles:3, Step per cycle:10,000, Force field: COMPASS, with Set maximum adsorption distance: 5Å by adsorption locator tools module in Materials Studio 2017 software.
To investigate the adsorption isotherm of H2S, SO2, CO2, and NO2 gases on MIL-100(Fe), sorption calculations were performed with parameters of Fugacity steps: 9, Temperature: 298.15 K with an initial pressure of 101.3 kPa and final pressure of 1013 kPa, Force field: COMPASS, for 100ns by sorption tools module in Materials Studio 2017 software.
Result and discussion
Molecular dynamic simulation analysis
To explore both the physical and chemical adsorption processes of H2S, SO2, CO2, and NO2 gases on MIL-100(Fe), as well as to determine the diffusion coefficients of these gases, molecular dynamics calculations were carried out over a duration of 100 ns timescale.
Figure 2 provides insights into the states of the systems involving H2S/MIL-100(Fe), SO2/MIL-100(Fe), CO2/MIL-100(Fe), and NO2/MIL-100(Fe) both before and after molecular dynamics simulations.
Furthermore, an examination of the system following molecular dynamics that most interaction bond lengths between H2S, SO2, CO2, and NO2 gases and MIL-100(Fe) (Fig. 2) fall within the 2.7–4 angstrom range, indicative of van der Waals interactions.
The results derived from the adsorption Locator and sorption calculations, specifically the total energy and adsorption energy results for H2S, SO2, CO2, and NO2 gases when interacting with MIL-100(Fe) summarized in Table 1. Total energy represents the system’s overall energetic state, encompassing potential and kinetic energy contributions from atomic interactions. The negative values of the total energies (Table 1) indicate that the systems have reached a thermodynamically stable state. The adsorption energy is defined as the energy change when a molecule (adsorbate) is adsorbed onto a surface or within a porous structure (adsorbent), typically calculated as:
Where:
-
EMOF−gas is the total energy of the adsorbent with the adsorbed molecule,
-
EMOF is the energy of the clean adsorbent,
-
Egas is the energy of the isolated gas molecule.
A negative adsorption energy indicates an energetically favorable adsorption process. The highest Adsorption energy value was observed for H2S gas at −49.28 kcal/mol, indicating a strong interaction between H₂S molecules and the MIL-100(Fe) framework. The result of Table 1 unequivocally demonstrate MIL-100(Fe)’s remarkable capability to absorb toxic and acidic gases like H2S, SO2, CO2, and NO2. This implies that MIL-100(Fe) could serve as a highly effective air filtration medium, particularly in industrial facilities afflicted by pollution from these hazardous gases.
Indeed, the table above clearly shows the total energy values for the different systems: MIL-100(Fe)/H2S, MIL-100(Fe)/SO2, MIL-100(Fe)/CO2, and MIL-100(Fe)/NO2, which are recorded as −100.78 Kcal/mol, −87.83 Kcal/mol, −57.40 Kcal/mol, and − 67.97 Kcal/mol, respectively. The analysis of the data reveals several significant insights: Among the studied systems, MIL-100(Fe)/H₂S exhibits the highest stability, followed by MIL-100(Fe)/SO₂, which also demonstrates relatively high stability. The MIL-100(Fe)/NO₂ system ranks next in terms of stability, exhibiting a comparatively lower yet notable stability. In contrast, the MIL-100(Fe)/CO₂ system is the least stable among those investigated.
Furthermore, these findings underscore the relative strength of the interactions between MIL-100(Fe) and the respective gases. Specifically, the bond formed between MIL-100(Fe) and H2S is significantly stronger than that between MIL-100(Fe) and SO₂. Additionally, the bond formed between MIL-100(Fe) and NO2 gas demonstrates greater strength than the bond formed between MIL-100(Fe) and CO2 gas. These results provide valuable insights into the energetic characteristics of gas–MOF interactions and support the potential application of MIL-100(Fe) in selective gas adsorption and air purification technologies. The differences in adsorption behavior can also be correlated with the intrinsic physicochemical properties of the gas molecules, including their dipole moments, molecular sizes, and partial charges, which influence the strength and nature of interactions with the MOF surface.
As shown in Table 2, H₂S and SO₂ possess relatively higher dipole moments, making them more interactive with the polar sites of the MIL-100(Fe) framework. In contrast, CO₂, being linear and non-polar, interacts primarily through quadrupole interactions.
Next, molecular dynamics diagrams (Fig. 3) were used to calculate the gas diffusion coefficient. The Einstein relation establishes a connection between the diffusion coefficient and the mean square displacement.
In this context, where Nα is the number of diffusive atoms in the system, d represents the derivative with respect to time, (\(\:\frac{d}{dt}\)) indicating the rate of change of the position of particles over time, ‘t0’ denotes the starting time, and [\(\:\left({r}_{i}\left(t\right)-{r}_{i}({t}_{0}\right)\)] signifies a statistical average across particles/molecules and starting times, as previously clarified.
To determine the gas diffusion coefficient, molecular dynamics simulations were performed, and Mean Square Displacement (MSD) diagrams were generated. The diffusion coefficient for each gas was then obtained by calculating the slope of the corresponding linear equation and applying it to Eq. (2).
In Eq. 2, a is the slope of the line equation for each gas. The diffusion coefficients (D) for the gases H2S, SO2, NO2, and CO2 gasses are determined to be 7.71, 1.90, 4.81, and 4.54 Å2/ps, respectively. As anticipated, among the studied gases, H₂S exhibited both a high diffusion coefficient and a strong adsorption energy within the MIL-100(Fe) framework. This behavior may appear counterintuitive, as strong adsorption is generally expected to hinder molecular mobility due to stronger interactions with the pore surface. However, in this case, the high diffusion rate of H₂S can be attributed to its relatively small molecular size and low molecular weight, which facilitate its movement through the MOF pores despite the strong host–guest interactions. This observation indicates that the diffusion behavior of adsorbed gases is influenced not only by their interaction energies with the framework, but also significantly by their kinetic properties and compatibility with the pore structure.
In accordance with Graham’s law, it is observed that as the molecular mass decreases, the diffusion coefficient tends to increase. The adsorption isotherm diagrams for H2S, SO2, CO2 and NO2 gases on MIL-100(Fe) are shown in (Fig. 4). The observed difference in the adsorption isotherms between H₂S/SO₂ and NO₂/CO₂ is significant and can be explained by a combination of molecular and structural factors. H₂S and SO₂ exhibit stronger interactions with the MIL-100(Fe) framework due to their higher polarity and ability to form specific interactions such as dipole–dipole attractions and hydrogen bonding with the MOF’s active sites. These interactions result in higher adsorption energies and a greater number of adsorbed molecules, as reflected in the steeper and higher isotherm curves. In contrast, CO₂ and NO₂ demonstrate weaker affinities toward the MOF, primarily governed by van der Waals forces and quadrupolar interactions, which are less effective in promoting adsorption under the same conditions. Moreover, the size, shape, and flexibility of H₂S and SO₂ molecules appear to be more compatible with the pore environment of MIL-100(Fe), facilitating their access and confinement within the framework. These combined effects account for the enhanced adsorption performance observed for H₂S and SO₂ compared to NO₂ and CO₂.
Adsorption isotherm analysis reveals the following adsorption preference on MIL-100(Fe): H2S > SO2 > NO2 > CO2.
Figure 5 shows the amount of molecular density resulting from sorption calculations. As can be seen in Fig. 5, the molecular density in the case of H2S gas in the system is higher than the case of SO2 gas and SO2 gas is more than NO2 and NO2 molecules are more than CO2. The density plots obtained from the MD simulations provide valuable insight into the spatial distribution and preferential adsorption sites of the gas molecules within the MIL-100(Fe) framework. For H₂S and SO₂, the density maps show a clear accumulation of gas molecules near the metal nodes and inner surfaces of the pores, indicating strong interactions and a high affinity of these gases for the framework. This is consistent with their higher adsorption energies and stronger polar characteristics. In contrast, the distribution of NO₂ and CO₂ appears more dispersed throughout the pore space, suggesting weaker and less site-specific interactions with the MOF. These differences highlight the role of gas–MOF interaction strength and molecular polarity in determining the adsorption behavior, and they further support the observed trends in adsorption isotherms and diffusion coefficients. Molecular density means that MIL-100(Fe) can absorb H2S, SO2, NO2, and CO2 toxic gases.
Conclusion
The results of our analysis, which encompass calculations derived from molecular dynamics, adsorption, and sorption studies concerning the adsorption of H2S, SO2, CO2, and NO2 gases on MIL-100(Fe), reveal MIL-100(Fe)’s remarkable capacity to effectively absorb these noxious gases. Specifically, the computed total energy of the system for MIL-100(Fe)/H2S, MIL-100(Fe)/SO2, MIL-100(Fe)/CO2, and MIL-100(Fe)/NO2 stands at −100.78 Kcal/mol, −87.83 Kcal/mol, −57.40 Kcal/mol, and − 67.97 Kcal/mol, respectively. This suggests a substantial affinity between MIL-100(Fe) and the target gases, showcasing its potential as an efficient absorbent for H2S, SO2, NO2, and CO2. As such, it is evident that the MIL-100(Fe)/H2S system exhibits the greatest stability among the various systems, as evidenced by its lowest energy of −100.78 Kcal/mol. Furthermore, analysis of the gas diffusion coefficients reveals that H₂S also exhibits the highest penetration capability within the MIL-100(Fe) framework, further supporting its strong interaction and mobility within the MOF structure.
Additionally, the calculated adsorption energies for H2S, SO2, NO2, and CO2 gases, which stand at −49.28, −26.89, −18.40, and − 15.35 Kcal/mol, respectively, offer a compelling perspective on the varying strengths and weaknesses of the bonds formed between MIL-100(Fe) and these gases. This data serves as a valuable lens through which to understand the nature of their respective interactions. Furthermore, the adsorption isotherm diagram calculations for toxic gases H2S, SO2, NO2, and CO2 on MIL-100(Fe) reveal a noteworthy trend. Specifically, the average number of absorbed H2S molecules on MIL-100(Fe) surpasses that of the other gases, indicating a higher affinity and capacity for H2S adsorption on this material. In addition, diffusion coefficients (Di) calculated for the MIL-100(Fe)/gas systems—7.71 Ų/ps for H₂S, 1.90 Ų/ps for SO₂, 4.54 Ų/ps for NO₂, and 4.81 Ų/ps for CO₂—reveal that H₂S exhibits the highest mobility within the MOF structure. This trend (H₂S > CO₂ > NO₂ > SO₂) is consistent with expectations based on both the interaction strength with adsorption sites and Graham’s law, which states that gases with lower molecular masses diffuse more rapidly. Collectively, these results highlight the high potential of MIL-100(Fe) as an efficient adsorbent for toxic and acidic gases, particularly H₂S. Its strong adsorption capacity and favorable diffusion properties make it a promising candidate for practical applications. This promising adsorbent can be used in air purification and gas capture technologies, especially in industrial environments such asgas processing plants, oil refineries, and steel manufacturing facilities. The deployment of MIL-100(Fe) in such settings could significantly improve air quality and operational safety.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors.
References
Afifa, Arshad, K., Hussain, N., Ashraf, M. H. & Saleem, M. Z. Air pollution and climate change as grand challenges to sustainability. Sci. Total Environ. 928, 172370 (2024).
Economy, E. C. The great leap Backward-The costs of china’s environmental crisis. Foreign Aff. 86, 38 (2007).
Perera, F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: solutions exist. Int. J. Environ. Res. Public. Health. 15, 16 (2018).
Fu, J., Liu, Y. & Sun, F. Identifying and regulating the environmental risks in the development and utilization of natural gas as a low-carbon energy source. Front. Energy Res. 9, 638105 (2021).
Jaski, A. C. et al. Zein-a plant-based material of growing importance: new perspectives for innovative uses. Ind. Crops Prod. 186, 115250 (2022).
Wen, M. et al. Metal–organic framework-based nanomaterials for adsorption and photocatalytic degradation of gaseous pollutants: recent progress and challenges. Environ. Sci. Nano. 6, 1006–1025 (2019).
Pu, S., Pan, Y., Zhang, L. & Lv, Y. Recent advances in chemiluminescence and Cataluminescence for the detection of volatile sulfur compounds. Appl. Spectrosc. Rev. 58, 401–427 (2023).
Zhang, Y. et al. Electrospun Cu-doped In2O3 Hollow nanofibers with enhanced H2S gas sensing performance. J. Adv. Ceram. 11, 427–442 (2022).
Maurya, A. et al. Microbially-assisted Phytoremediation Toward Air Pollutants: Current Trends and Future Directions (Environ Technol \&, 2023). Innov 103140.
Guaman, M. et al. Traffic Density and Air Pollution: Spatial and Seasonal Variations of Nitrogen Dioxide and Ozone in Jamaica, New York. Atmosphere (Basel) 13:2042 (2022).
Rajakumar, J. P. P. & Choi, J. Helmet-mounted real-time toxic gas monitoring and prevention system for workers in confined places. Sensors 23, 1590 (2023).
Ghayoumi Zadeh, H., Fayazi, A., Rahmani Seryasat, O. & Rabiee, H. A bidirectional long Short-Term neural network model to predict air pollutant concentrations: A case study of tehran, Iran. Trans. Mach. Intell. 5, 63–76 (2022).
Kimura, H. Hydrogen sulfide (H2S) and polysulfide (H2Sn) signaling: the first 25 years. Biomolecules 11, 896 (2021).
Nriagu, J. O. Encyclopedia of Environmental Health (Elsevier, 2019).
Panella, B. & Hirscher, M. Hydrogen physisorption in metal–organic porous crystals. Adv. Mater. 17, 538–541 (2005).
Ranocchiari, M. & van Bokhoven, J. A. Catalysis by metal–organic frameworks: fundamentals and opportunities. Phys. Chem. Chem. Phys. 13, 6388–6396 (2011).
Rowsell, J. L. C. & Yaghi, O. M. Metal–organic frameworks: a new class of porous materials. Microporous Mesoporous Mater. 73, 3–14. https://doi.org/10.1016/j.micromeso.2004.03.034 (2004).
Liu, C., Wang, J., Wan, J. & Yu, C. MOF-on-MOF hybrids: Synthesis and applications. Coord. Chem. Rev. 432, 213743 (2021).
Duan, C. et al. Template synthesis of hierarchical porous metal–organic frameworks with tunable porosity. Rsc Adv. 7, 52245–52251 (2017).
Chae, H. K. et al. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427, 523–527 (2004).
Slater, A. G. & Cooper, A. I. Function-led design of new porous materials. Sci. (80-). 348, aaa8075 (2015).
Fang, Q-R., Makal, T. A., Young, M. D. & Zhou, H-C. Recent advances in the study of mesoporous metal-organic frameworks. Comments Inorg. Chem. 31, 165–195 (2010).
Kumar, S. et al. Decoration and utilization of a special class of metal–organic frameworks containing the fluorine moiety. Coord. Chem. Rev. 476, 214876 (2023).
Konnerth, H. et al. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev. 416, 213319 (2020).
Chen, Z. et al. Water-based synthesis of a stable iron-based metal–organic framework for capturing toxic gases. ACS Mater. Lett. 2, 1129–1134 (2020).
Zhang, Y., Cui, X. & Xing, H. Recent advances in the capture and abatement of toxic gases and vapors by metal–organic frameworks. Mater. Chem. Front. 5, 5970–6013 (2021).
Yang, J. et al. Advances in the adsorption and degradation of chemical warfare agents and simulants by Metal-organic frameworks. Coord. Chem. Rev. 493, 215289 (2023).
Ruffley, J. P. et al. Design, synthesis, and characterization of metal–organic frameworks for enhanced sorption of chemical warfare agent simulants. J. Phys. Chem. C. 123, 19748–19758 (2019).
Suresh, K. & Matzger, A. J. Enhanced drug delivery by dissolution of amorphous drug encapsulated in a water unstable metal–organic framework (MOF). Angew Chemie Int. Ed. 58, 16790–16794 (2019).
Cao, J., Li, X. & Tian, H. Metal-organic framework (MOF)-based drug delivery. Curr. Med. Chem. 27, 5949–5969 (2020).
Férey, G. et al. Mixed-valence Li/Fe-based metal–organic frameworks with both reversible redox and sorption properties. Angew Chemie Int. Ed. 46, 3259–3263 (2007).
others Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 43, 5415–5418 (2014).
Al Sharabati, M., Sabouni, R. & Husseini, G. A. Biomedical applications of metal- organic frameworks for disease diagnosis and drug delivery: a review. Nanomaterials 12, 277 (2022).
Zhou, H-C., Long, J. R. & Yaghi, O. M. Introduction to metal–organic frameworks. Chem. Rev. 112, 673–674 (2012).
Wang, W. et al. Two-dimensional metal-organic frameworks: from synthesis to bioapplications. J. Nanobiotechnol. 20, 1–17 (2022).
Bai, Y. et al. Metal-organic frameworks nanocomposites with different dimensionalities for energy conversion and storage. Adv. Energy Mater. 12, 2100346 (2022).
Xie, X. et al. Impressive proton conductivities of two highly stable metal–organic frameworks constructed by substituted Imidazoledicarboxylates. Inorg. Chem. 58, 5173–5182 (2019).
Healy, C. et al. The thermal stability of metal-organic frameworks. Coord. Chem. Rev. 419, 213388 (2020).
Li, Y. et al. Metal-organic frameworks (MOFs) as efficient catalysts for electro-Fenton (EF) reactions: current progress and prospects. Chem. Eng. J. 463, 142287 (2023).
Yusuf, V. F., Malek, N. I. & Kailasa, S. K. Review on Metal–Organic framework classification, synthetic approaches, and influencing factors: applications in energy, drug delivery, and wastewater treatment. ACS Omega. 7, 44507–44531 (2022).
Zhong, G., Liu, D. & Zhang, J. Applications of porous metal–organic framework MIL-100 (M)(M = Cr, fe, sc, al, V). Cryst. Growth \& Des. 18, 7730–7744 (2018).
Fandzloch, M., Maldonado, C. R., Navarro, J. A. R. & Barea, E. Biomimetic 1-aminocyclopropane-1-carboxylic acid oxidase ethylene production by MIL-100 (FE)-based materials. ACS Appl. Mater. \& Interfaces. 11, 34053–34058 (2019).
Karbassiyazdi, E. et al. A juxtaposed review on adsorptive removal of PFAS by metal-organic frameworks (MOFs) with carbon-based materials, ion exchange resins, and polymer adsorbents. Chemosphere 311, 136933 (2023).
Carmo, P. et al. Adsorptive processes for the recovery of VCM emissions using an environmentally friendly MIL-100(Fe) MOF. Microporous Mesoporous Mater. 352, 112510 (2023).
Maher, H. et al. Silica gel-MIL 100 (Fe) composite adsorbents for ultra-low heat-driven atmospheric water harvester. Energy 238, 121741 (2022).
Wu, S.-Y., Lai, M.-T., Hsu, C.-H., Wu, K. C. W. & Gu, Y. Enhancing PEDOT modified electrode for tartrazine sensing through the immobilization of MIL-100(Fe) metal-organic framework. J. Taiwan Ins. Chem. Engin. 155, 105254 (2024).
Barjasteh, M., Vossoughi, M., Bagherzadeh, M. & Bagheri, K. P. Green synthesis of PEG-coated MIL-100 (Fe) for controlled release of Dacarbazine and its anticancer potential against human melanoma cells. Int. J. Pharm. 618, 121647 (2022).
Bhattacharjee, A., Purkait, M. K., Sastri, C. V. & Gumma, S. CeO2 nanoparticles incorporated MIL-100 (Fe) composites for loading of an anticancer drug: effects of HF in composite synthesis and drug loading capacity. Inorganica Chim. Acta. 533, 120784 (2022).
Pangestu, A., Lestari, W. W., Wibowo, F. R. & Larasati, L. Green electro-synthesized MIL-101 (Fe) and its aspirin detoxification performance compared to MOF-808. J. Inorg. Organomet. Polym. Mater. 32, 1828–1839 (2022).
Seo, Y-K. et al. Large scale fluorine-free synthesis of hierarchically porous iron (III) trimesate MIL-100 (Fe) with a zeolite MTN topology. Microporous Mesoporous Mater. 157, 137–145 (2012).
Leclerc, H. et al. Infrared study of the influence of reducible iron (III) metal sites on the adsorption of CO, CO 2, propane, Propene and propyne in the mesoporous metal–organic framework MIL-100. Phys. Chem. Chem. Phys. 13, 11748–11756 (2011).
Huangfu, C. et al. Efficient lithium extraction from aqueous solutions by MIL-100(Fe): A study on adsorption kinetics, thermodynamics and mechanism. Sep. Purif. Technol. 322, 124365. https://doi.org/10.1016/j.seppur.2023.124365 (2023).
Bauza, M., Turnes Palomino, G. & Palomino Cabello, C. MIL-100(Fe)-derived carbon sponge as high-performance material for oil/water separation. Sep. Purif. Technol. 257, 117951. https://doi.org/10.1016/j.seppur.2020.117951 (2021).
Lestari, W. W. et al. Fabrication of composite materials MIL-100(Fe)/Indonesian activated natural zeolite as enhanced CO2 capture material. Chem. Pap. 75, 3253–3263. https://doi.org/10.1007/s11696-021-01558-2 (2021).
Liu, Z. et al. Synthesis of magnetic orderly mesoporous α-Fe2O3 nanocluster derived from MIL-100(Fe) for rapid and efficient arsenic(III,V) removal. J. Hazard. Mater. 343, 304–314. https://doi.org/10.1016/j.jhazmat.2017.09.047 (2018).
Version, D. Metal-Organic framework MIL-100 (Fe) as a novel moisture buffer material for Energy-Efficient indoor humidity control. 100:. (2018). https://doi.org/10.1016/j.buildenv.2018.09.027
Wen, M. et al. Environ. Sci. https://doi.org/10.1039/c8en01167b (2018).
Author information
Authors and Affiliations
Contributions
Rasool Pelalak : Data Analysis, Supervision, Writing and Editing, Data Collection.Thanh T Thi : Writing and Editing. Fereshteh Golestanifar : Writing and Editing, Data Collection. Mohammadreza Aallaei : Writing and Editing, Data Analysis, Data Collection. Zahra Heidari : Supervision, Data Analysis, Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pelalak, R., Thi, T.T., Golestanifar, F. et al. Molecular dynamics insights into the adsorption mechanism of acidic gases over iron based metal organic frameworks. Sci Rep 15, 21924 (2025). https://doi.org/10.1038/s41598-025-07163-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07163-7