Abstract
The stethoscope, crucial in medical diagnosis, links doctors and patients. With the threat of healthcare associated infections (HAIs), understanding stethoscope contamination and medical students’ awareness is imperative. This study conducted from September to December 2022, the research involved 293 stethoscopes from three hospitals in the West Bank. Questionnaires probed students’ practices, and Staphylococcus spp isolations were analyzed. Contamination rates, bacterial species, associations with disinfection practices and other hospital factors were explored. Stethoscope contamination rate was high in the three hospitals (range: 26.5–50.8 CFU/cm2). Staphylococcus spp contaminated 36.9% of stethoscopes. Methicillin Resistant S. aureus (MRSA) and Vancomycin Resistant S. aureus (VRSA) occurred in 16.6% and 1.8% respectively. 36% exhibited resistance to two or more antibiotics. The study revealed significant findings regarding stethoscope contamination. Only 29.4% of stethoscopes used by those who disinfected between patients conformed to permissible contamination levels, in contrast to a markedly greater 60.7% among those who did not disinfect (P < 0.05). Reminders for disinfection in study units resulted in a 32.1% acceptable rate, whereas units without reminders had a significantly higher 67.9% rate (P < 0.001). Stethoscope usage also played a role, with a 34.4% acceptable rate for those examining only patients, compared to a significantly higher 65.8% rate for those examining both patients and peers (P < 0.05). Additionally, significant differences were observed in hospital, rotation, year-wise, disinfection frequency, and the presence of reminders. Improving stethoscope disinfection practices is crucial to improving patient safety and infection control. Recommendations include the implementation of standard protocols, the use of effective disinfectants, the education of health professionals and the integration of routine disinfection into workflows. These measures significantly reduce hospital infections and promote a safety culture, promoting patient confidence and improving health results.
Similar content being viewed by others
Introduction
The stethoscope not only diagnoses but also acts as a doctor-patient channel, allowing engagement and understanding of patients’ backgrounds, lifestyles, and physical characteristics. Auscultation reduces the doctor-patient gap, fostering trust and a stronger doctor-patient relationship1. The stethoscope, derived from Greek words for chest and examination, is a device used to listen to body sounds like heartbeats, bowel movements, and blood flow, aiding doctors in assessing a patient’s health2,3. Healthcare associated infections (HAIs) are a significant concern for healthcare workers and patients, with 3–5% of patients experiencing clinical collapse leading to death or ICU transfer, and those with major infections or septicemia, which contribute to 50% or more hospital mortality4,5.
HAIs have been linked to longer hospital stays and higher resource use, according to research. As per to one estimate, HAIs can cost up to $25,000 per infection6,7. A systematic review and meta-analysis study found that critical care units have a higher prevalence of HAIs than other wards, with adult ICU settings having the highest prevalence (51.3%) in 75 European regions8. Filamentous fungi and bacteria can survive on dry surfaces, causing health issues like HAIs. They can be passed from patient to patient through direct contact with contaminated environments or healthcare personnel’s hands9. Clostridium difficile, Klebsiella pneumoniae, Acinetobacter species, Pseudomonas aeruginosa, and Staphylococcus aureus are the most prevalent bacterial strains causing difficult to-control illnesses in the hospital setting10. Hospitals often use glass, stainless steel, and aluminum surfaces because of their reduced bacterial survival and development rates in comparison to porous materials. Due to their non-porous and inorganic nature, these materials impede the development and survival of microorganisms in contrast to porous and organic materials11. The kinetics of survival of main nosocomial bacteria on different surfaces are investigated in Katzenberger et al.11. surfaces were inoculated with a solution containing type strains of several bacteria, including S. aureus, K. pneumoniae, P. aeruginosa, S. marcescens, E. coli, and E. cloacae. For four weeks, the surfaces were kept in a typical environment. While S. aureus was alive for at least seven days, A. baumannii and E. faecium had the longest survival times. Within two days, most gram-negative bacteria were rendered inactive. Nosocomial transmission of these germs cannot be prevented without consistent daily infection control efforts11.
Staphylococcus spp. they are emerging as notable pollutants in the hygiene of the stethoscopes, which raises concerns among health professionals regarding infection control practices, particularly among medical apprentices. Datta et al. referred to the stethoscope as a “friendly enemy”, illustrating the double role it plays in the clinical examination and as a vector for microbial transmission, which requires rigorous hygiene practices12. The importance of adequate stethoscope hygiene becomes evident when the findings of research are considered, which revealed a notable knowledge gap regarding disinfection methods among health personnel in a rural environment. This gap can lead to inconsistent practices, increasing the probability of cross contamination among patients12,13. Alaali et al. (2020) they corroborated even more this documenting suboptimal cleaning practices in community hospitals, highlighting a systemic problem that contributes to high pollution rates14. In addition, Tahir et al. (2022) illustrated the microbiological impacts of disinfection practices among doctors, indicating the need for more compliance and compliance with infections control guidelines. The prevalence of Staphylococcus spp. In medical care environments, the critical responsibility for medical apprentices to implement rigorous infection control protocols, thus safeguarding the health of the patient and the public. Addressing these concerns through standardized training and disinfection practices is essential to improve the general medical care results15.
Stethoscopes are thought to have the potential to spread infectious organisms, which could lead to healthcare-associated infections (HCAI)16. International research shows high bacterial contamination of stethoscopes, with most participants aware of their potential to spread healthcare associated infections. The study by Zehra et al. aimed to assess healthcare professionals’ awareness of stethoscopes as a source of nosocomial infections, their cleaning practices, and the existence of guidelines and accountability criteria. A cross-sectional study of 243 professionals was conducted in tertiary healthcare facilities in Rawalpindi and Islamabad. The majority of participants were aware of stethoscopes as a source of infections and believed in regular cleaning. However, a majority of participants believed their hospitals did not issue protocols for decontamination. The study suggests further research to expand recommendations on stethoscope cleanliness17. HAIs often stem from hygiene neglect, a common cause of medical malpractice claims. To establish legal liability, two key factors must be proven: negligence in preventing stethoscope contamination and a causal link between negligence and patient harm due to pathogen transmission from the stethoscope. Disinfection and sterilization of medical equipment are often used to defend HAI-related misconduct18. During 20 min of typical use, the majority of the devices, including stethoscopes managed to accumulate enough microbes to have microbial loads greater than the permissible limit of 2.5 CFU/cm2 19.
Stethoscope disinfection reduces infection transmission, particularly in controlling Methicillin Resistant S. aureus (MRSA) spread within hospitals, as it is clinically relevant for infection control purposes19. A study reveals forgetfulness, negligence, and lack of disinfectant in the workplace are the top three causes of poor disinfection practice among Ethiopian doctors, requiring strengthening of stethoscope disinfection culture and infection control programs20. The stethoscope, a non-critical medical device, requires proper hygiene due to its role as the doctor’s “third hand.” The Centers for Disease Control and Prevention (CDC) recommend cleaning it with alcohol or bleach-based disinfectant, from patient interactions to weekly21. A study found that 25 out of 62 stethoscopes showed potentially dangerous bacterial growth before alcohol-based disinfectant washing, while only two showed pathogenic isolate development post-cleaning22. Ethanol-Based Hands Sanitizer (EBHS) and Isopropyl alcohol (IPA) pads effectively reduce bacterial load on stethoscope surfaces, but their high cost makes them less widely used in developing nations23. Hands before covering the stethoscope diaphragm with a disposable glove to prevent illness transfer. The CDC recommends using a disposable stethoscope and cleaning it after use on a patient under contact precautions, especially if special disposable devices are not easily accessible24.
The critical role of medical interns in the maintenance of stethoscope hygiene practices is essential to mitigate bacterial contamination in clinical environments. As future health service providers, their adherence to hygiene protocols significantly influences the patient’s safety and control measures. Studies indicate that medical interns often exhibit under -ideal hygiene practices due to lack of education and awareness25. In particular, clinical students demonstrate varied attitudes regarding the cleaning of the stethoscope, reflecting the need for directed educational interventions. The training programs were promising in improving the hygiene practices of medical students, emphasizing the importance of hand hygiene alongside the cleaning of the stethoscope. In addition, educational initiatives positively influence the hygiene habits of the stethoscope among medical students, suggesting that systematic training could increase adherence to essential protocols26. Notably, contemporary research highlights an alarming persistence of inadequate cleaning practices among health professionals, including medical interns27. Approaching these gaps through comprehensive training and reinforcing the importance of hygiene can be critical to promoting a culture of patient responsibility and safety during the critical learning phase of medical interns. The integration of rigorous hygiene practices in medical education is crucial to cultivating lifelong compliance and finally reducing the infections acquired at the hospital.
The stethoscope, in addition to its diagnostic role, acts as both a symbolic and practical conduit in the doctor–patient interaction, enhancing communication and trust. It enables professionals to auscultate internal bodily sounds, including heartbeats, bowel movements, and respiratory sounds, so serving a crucial function in physical examination and clinical evaluation1,2. Nonetheless, despite its use in medical practice, the stethoscope also serves as a possible conduit for the spread of healthcare-associated infections (HAIs)3,4.
HAIs pose a significant challenge to global healthcare systems, linked to extended hospitalizations, elevated healthcare expenditures, and increased morbidity and death rates. Their presence in critical care settings is particularly concerning3,4,5. Studies indicate a 51.3% frequency of HAIs in adult intensive care units (ICUs) across 75 European regions8. These infections are frequently attributed to pathogens like Staphylococcus aureus, Clostridium difficile, Klebsiella pneumoniae, and Pseudomonas aeruginosa, many of which have shown the capacity to endure prolonged periods on dry, high-contact surfaces typically present in hospitals, including glass, aluminum, and stainless steel10,11.
The stethoscope is an often used but sometimes neglected instrument in clinical practice. Studies indicate that stethoscopes may contain potentially dangerous bacteria and serve as conduits for cross-transmission among patients12,13. A research performed in Kenyan hospitals revealed that just 9.8% of high-touch surfaces, including medical equipment, conformed to acceptable bacterial contamination guidelines. Stethoscopes are sometimes termed “friendly enemies,” since they are essential diagnostic instruments but may facilitate germ transmission if not adequately sanitized12.
Evidence indicates that inadequate stethoscope hygiene habits among healthcare professionals, especially medical trainees, facilitate the transmission of HAIs17. Medical trainees often engage with patients and participate actively in clinical care; nevertheless, research indicates that they may not consistently comply with infection control guidelines17,27. This is often attributable to a deficiency in awareness, insufficient training, lapses in memory, or the unavailability of disinfectants20,22,23. Inadequate hygiene standards among interns have been recorded in several environments, prompting worries over their potential as vectors for nosocomial diseases18,21.
Although several studies indicate significant knowledge of stethoscope contamination dangers, compliance with cleaning protocols is notably inadequate. For example, just 9.5% of healthcare practitioners in a particular survey said that they disinfected their stethoscopes after each patient encounter25. Alcohol-based disinfectants are efficacious but often underutilized, particularly in resource-limited environments where financial constraints and accessibility may pose challenges18,23. The Centers for Disease Control and Prevention (CDC) advise the routine cleaning of non-critical equipment, such as stethoscopes, preferably after each use or at least weekly24.
The ramifications of insufficient disinfection extend beyond infection control and patient outcomes; they also include legal and ethical significance. Healthcare-associated infections stemming from deficiencies in hygiene protocols may result in malpractice litigation if carelessness and a direct relationship to patient injury are shown. In such instances, insufficient disinfection may serve to illustrate a violation of the duty of care8,16.
In addition to compromising patient outcomes, inadequate stethoscope hygiene carries significant legal and ethical implications. HAIs resulting from lapses in disinfection practices may lead to medical malpractice claims, particularly when negligence in infection control is demonstrably linked to patient harm. A retrospective analysis of civil court judgments in Rome from 2016 to 2020 revealed that healthcare professionals and institutions were frequently held liable for HAIs, highlighting systemic failures in adherence to hygiene protocols and the resulting juridical and economic consequences. Legal experts similarly affirm that breaches in standard infection prevention practices, such as failing to sanitize high-touch instruments like stethoscopes, can serve as grounds for litigation under medical malpractice if a duty of care is proven to have been violated. These findings underscore the importance of rigorous compliance with disinfection guidelines, not only to safeguard patient health but also to mitigate legal exposure for healthcare providers7.
As medical interns are prospective healthcare providers, it is essential to teach appropriate hygiene habits early in their training. Educational programs have shown efficacy in enhancing disinfection practices, particularly with stethoscope hygiene. Organized training programs enhance adherence to infection control practices and foster a culture of safety and responsibility26,28.
This research seeks to examine the hygiene practices and bacterial contamination levels of stethoscopes used by medical trainees, specifically targeting knowledge deficiencies, compliance with disinfection methods, and the effects of instructional interventions.
Materials and methods
Study design
This study involved medical students from various clinical years and rotations at the Faculty of Medicine and Health Sciences at An-Najah National University in the West Bank, Palestine, focusing on the diaphragm and bell of the stethoscope for assessing microbial contamination rates. A statistical study was performed to ascertain the distribution of microbial levels, and subjects were sampled without prior notification.
The research used a cross-sectional study design to gather data from a heterogeneous population sample at a certain time; the study was carried out at a university research laboratory (Central research lab) during five months, from August 2022 to February 2023. We affirm that all techniques were executed in compliance with the relevant standards and regulations as stipulated by our university’s policies.
A descriptive laboratory research was done on medical students at An-Najah National University across three hospitals: Hospital A (32° 13’ 32” N, 35° 14’ 30” E), Hospital B (32° 13’ 22” N, 35° 15’ 45” E), and Hospital C (32° 14’ 23” N, 35° 14’ 45” E). Each participant in the research answered a questionnaire that pertains to their everyday use and practices in hospitals and medical settings. It includes inquiries on their gender, clinical year, and possession of a stethoscope. Additional inquiries evaluated the regularity of disinfection, awareness and comprehension of illness transmission, and sanitation procedures. A designated part inquires about their hospital training, the accessibility of disinfectant solutions, the quality of instruction and reminders on disinfection, and the subjects of their stethoscope examinations (patients or other students). Finally, inquiries on their personal state, including medical issues, hand cleanliness, and nail care. Their responses were assessed to determine knowledge of stethoscope handling, disinfectant use, and compliance with infection control protocols.
The sample size of 293 participants was determined to ensure comprehensive and representative data for our study on the stethoscopes used by medical students. Given the variability among participants—including gender, clinical year, and the specific rotations they were engaged in—a sufficiently large sample was essential to account for these factors and minimize bias.
To determine the appropriate sample size, we used the Raosoft Sample Size Calculator, assuming a prevalence of 50%, a confidence level of 95%, and a 5% margin of error. This yielded a recommended sample size of 293 participants from an estimated total population of approximately 1,235 students. After determining the required sample size, we employed a simple random sampling method. Using a complete list of enrolled students provided by the academic registry, each student was assigned a unique number. We then used a computerized random number generator to select 293 students from the list, ensuring that every individual in the population had an equal chance of being included in the study. This method was chosen to minimize selection bias and enhance the representativeness of the sample. A prevalence of 50% was assumed for the sample size calculation as it provides the most conservative estimate, ensuring a sufficiently large and statistically valid sample in the absence of prior data.
Furthermore, in order to prevent selection bias and guarantee that all pertinent subgroups were fairly represented. We used a nonspecific random sampling technique. In order to compare findings across various phases of medical school, the collected data was classified into three categories that corresponded to the students’ clinical years. This method made it easier to analyse patterns and variations in stethoscope use and associated results. Overall, the chosen sample size provided a robust foundation for reliable statistical analysis while capturing the heterogeneity of the population under study.
Bacterial count and identification of Staphylococci spp
A sterile cotton swab, moistened with sterile normal saline, is used to collect samples from the whole diaphragm of the stethoscope, thereafter placed in a 1 ml sterile normal saline Eppendorf tube. Subsequently, 0.1 ml of each sample was cultured using the spreading technique on Tryptic Soy Agar (TSA) medium in triplicate, followed by incubation of the culture plates at 37 °C for 48 h. Following incubation, colony counting was conducted, and the mean was confirmed18,21,22.
. Identification was conducted using standard microbiological methods, including assessment of physical and biochemical characteristics. All isolates first underwent Gram staining to confirm Gram-positive cocci in clusters, suggestive of staphylococci. Colonies were then evaluated for morphology (circular, convex, opaque, golden-yellow or white colonies on nutrient or blood agar). Further differentiation was performed using catalase testing (positive for staphylococci), followed by coagulase testing (to distinguish Staphylococcus aureus [coagulase-positive] from coagulase-negative staphylococci [CoNS]). Additional biochemical tests such as mannitol fermentation on mannitol salt agar (MSA) and DNase activity were also used to support identification.
Following 48 h of incubation on TSA, colonies that ferment mannitol (yellow colonies) are presumed to be S. aureus, whereas those that do not ferment mannitol are considered to be CoNS21,22. The examination of Staphylococcal contamination is focused, since we posited that Staphylococcus was mostly accountable for the contamination of skin and surfaces, a hypothesis corroborated by previous studies. To ensure patient safety against cross-infection, since the stethoscope directly contacts the skin, yellow colonies were then cultured using the spreading technique on Mannitol Soy Agar (MSA) for each sample in triplicate, followed by incubation of the culture plates at 37 °C for 48 h. Following incubation, colony counts were recorded, and the average was established22.
For Gram-positive cocci, isolates were subcultured on blood agar and underwent a catalase test; positive results indicate S. aureus and Coagulase-Negative Staphylococci (CoNS). To get positive findings, additional identification is conducted using the coagulase test to confirm Staphylococcus spp. based on the coagulase reaction, we will differentiate between S. aureus and Coagulase-Negative Staphylococci (CoNS). Organisms that exhibit positivity (mannitol fermenter-yellow) are identified as S. aureus, whereas those that are negative are classified as CoNS21,22.
Following the administration of antibiotics (Sect. 2.3), we used API Staph (bio Mérieux, Lyon, France) in accordance with the manufacturer’s instructions to distinguish among various Staphylococcus species. The novobiocin sensitivity disc was used to distinguish S. saprophyticus, with a diameter of less than 16 mm indicating resistance and confirming the presence of S. saprophyticus23.
Oxacillin or cefoxitin are often used as surrogate markers for methicillin in order to identify MRSA resistance. Oxacillin testing was done by disk diffusion method, using a 1-µg disk on Mueller-Hinton agar, where a zone diameter of ≤ 10 mm shows resistance. The selected drug is cefoxitin, which consistently induces the resistance-causing mecA gene; resistance is shown by a zone diameter of ≤ 21 mm. The method used for assessing vancomycin MICs to identify resistance in VRSA is agar screening with six µg/mL vancomycin, where bacterial growth indicates resistance29.
While the emphasis was on Staphylococcus, other bacterial species were not overlooked. MacConkey agar was used for gram-negative bacteria, and any transparent colony produced (lactose nonfermenter) was subjected to the oxidase test for the identification of Pseudomonas spp (oxidase positive). For colonies that tested oxidase negative, a glucose fermentation test was employed; those yielding a negative result were identified as Actinobacter baumannii. However, none of the findings indicated the presence of gram-negative nosocomial bacteria (Data not shown)21,24.
Antibiotics sensitivity test
An antibiotic sensitivity test was conducted on Staphylococcus spp. colonies that proliferated on MSA. The Kirby–Bauer disc diffusion technique was used on Mueller-Hinton (MH) agar as per Weinstein and Lewis29. Utilizing antibiotic classes from Oxoid United Kingdom: Cefoxitin (FOX), Vancomycin (VA), and Oxacillin (OX) (J01CF04) for the assessment of MRSA and VRSA in accordance with Weinstein and Lewis29. Additionally, the sensitivity patterns of Gentamicin (CN) (D06AX07), Erythromycin (E) (J01FA01), Trimethoprim (SXT) (J01EE01), Clindamycin (DA) (J01FF01), and Levofloxacin (LEV) (J01MA12) antibiotics were assessed using a 0.5 McFarland standard as calculated by the densitometer29.
Statistical analysis
Statistical Package for Social Science (SPSS) software version 25 was used for analyzing. Descriptive statistics were used to summarize different variables and presented in the form of texts and tables, Categorical variables were described as frequencies and proportions, and continuous variables tested by Spearman’s correlation test due to the data being not normally distributed. Also, we used Pearson Chi-Square and Fisher’s Exact Test to examine the comparison between categorical variables. We used Kruskal One-Way ANOVA test to compare between variables with more than 3 groups and due to the data not being normally distributed, and being appropriate for comparing means across many groups.
The study formulated the following hypotheses to evaluate the contamination rates and students’ awareness:
-
A.
Null Hypothesis (H0): There is no stethoscope contamination, and high awareness of disinfection among medical students does exist.
-
B.
Alternative Hypothesis (Ha): There is stethoscope contamination, and high awareness of disinfection among medical students does not exist.
The contamination rate of stethoscopes was calculated using the following formula:
Where:
The colony-forming units (CFU) extracted from the cultured samples are expressed as CFU/mL.
R is the radius of the stethoscope’s diaphragm or bell (measured in centimetres).
π is a constant (approximately 3.14).
The contamination density in CFU per square centimetre of surface area is determined using the formula.
The circular surfaces of the stethoscope bell and diaphragm, which were swabbed to measure contamination, are included by this equation. These surfaces were swabbed, the sample was inoculated onto the proper culture medium, and the plates were then incubated as part of the measuring procedure. The colony-forming units that resulted were tallied and expressed as CFU/ml.
The radius of the stethoscope’s diaphragm or bell was measured, and the surface area was calculated using the formula for the area of a circle (π × r²). Finally, the CFU/mL was divided by the calculated surface area to obtain the contamination rate in CFU/cm².
For the purposes of this investigation, a contamination level of 2.5 CFU/cm² was used as the cut-off point to distinguish between acceptable and undesirable bacterial contamination. Values below 2.5 CFU/cm² were considered acceptable, while values equal to or exceeding 2.5 CFU/cm² were classified as indicative of unacceptable contamination. This threshold is based on standards established in previous studies and infection control guidelines, which identify this level as the upper limit for safe microbial presence on medical equipment30,31,32.
This approach ensures that contamination rates are standardized and comparable across different samples and stethoscope surfaces.
The tests were conducted to determine whether there were significant differences in contamination rates among different variables. The tests produced an F-statistic and associated p-value. To determine statistical significance, the resultant p-value from the tests was compared to a pre-set significance level (e.g., α = 0.05). If the p-value is less than the significance level, the null hypothesis is refuted due to strong evidence against it, indicating a significant difference in contamination rates between the variables and vice versa.
Results
Socio-demographic characteristics
In our research, 293 ANNU Medical Students and their stethoscopes were included. The response rate was 100%. The men were 147 (50.2%), while the ladies were 146 (49.8%). The age average was 22.5 ± 0.2. The students were in various years of the clinical program; 98 (33.45%) were in fourth year, the same in sixth year, and 97 (33.1%) were in fifth year. Furthermore, the data was obtained from several Nablus hospitals; 98 samples (33.45%) were taken from A (32° 13’ 32” N, 35° 14’ 30” E), the same from B (32° 13’ 22” N, 35° 15’ 45” E), and 97 samples (33.1%) from C (32° 14’ 23” N, 35° 14’ 45” E). It is worth noting that at the time of data collection, the students were divided into different rotations in the hospital: 64 (21.8%) in internal medicine, 57 (19.4%) in surgery, 58 (19.8%) in pediatrics, 56 (19.1%) in obstetrics and gynecology, and 58 (19.8%) in other departments.
Also, according to our questionnaire, 271 (92.5%) of students participated in online learning throughout the COVID-19 epidemic. 289 of the pupils (98.6%) did not have a chronic condition. The stethoscope 276 (94.2%) was personally owned, while the rest were shared. The Littman brand stethoscopes were used the most (246, or 84%) (Table 1).
The infection, awareness and practice among students’ stethoscope
A total of 293 samples from students’ stethoscopes were tested for bacterial contamination. The majority, 268/293 (91.5%) of pupils, felt that the stethoscope might transmit illness. Approximately 114/293 (39%) felt that both the diaphragm and the bell could convey the microbes, whereas 74/293 (25.3%) believed that only the diaphragm could transmit it. 98/293 (33.45%) of the participants did not disinfect their stethoscope, whereas 79/293, 58/293, and 46/293 (27%, 19.8%, and 15.7%) did so daily, weekly, and monthly, respectively. Approximately 170/293 students (58%) did not sanitize their stethoscopes after each patient assessment. Although 286/293 (97.6%) of them agreed that the stethoscope should be disinfected after each patient. 290/293 (99%) of them said that hand hygiene was necessary to avoid or decrease stethoscope contamination. 223/293 (76.1%) of the students used alcohol to clean their stethoscopes, with about 117/293 (40%) disinfecting the diaphragm, bell, and earpiece in total. 154/293 (52.6%) of the students learned about disinfection throughout their clinical study. It is worth noting that there were no infection control posters in the hospital promoting the significance of cleaning the stethoscope after each use in 185/293 (63.1%) of their study units. However, disinfectants were accessible in 246/293 (84%) of the study units. 183/293 (62.5%) of the students used the same stethoscope to examine both patients and students from the same department. Regarding nails, 213/293 (72.7%) of them did not have long nails (Table S1).
The distribution and frequencies of bacterial isolates among students’ stethoscopes
The overall incidence of stethoscope contamination among students was 229/293 (78.2%), and cultures were produced, revealing a diverse range of bacteria. The distribution was as follows: 2/293 (0.7%) Bacillus spp, 118/293 (40.3%) Yellow Micrococcus (gram-positive cocci), 151/293 (51.5%) Grey wet (gram-negative rods), 11/293 (3.8%) Fungus, and 108/293 (36.9%) distinct species of Staphylococcus. Of them, 70/108 (64.8%) were S. epidermidis, 36/108 (33.3%) were S. aureus, and only one sample included S. saprophyticus 1/108 (0.93%) and S. aureicularis 1/108 (0.93%).
Antimicrobial susceptibility testing for Staphylococcus spp
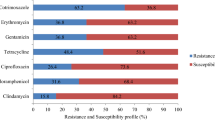
A total of 108 isolated Staphylococcus spp. were evaluated for antimicrobial sensitivity against eight antibiotic-class discs (aminoglycosides, macrolides, sulfonamides, glycopeptides, clindamycins, fluoroquinolones, and B-lactams) used in the treatment of Staphylococcal infection. All S. epidermidis and S. saprophyticus were 108/108 (100%) susceptible to Gentamicin, Cefoxitin, and Vancomycin. Methicillin-resistant S. aureus (MRSA) frequency was 18/108 (16.6%). The majority of S. aureus (98.1%) was sensitive to Gentamicin and Vancomycin, with the exception of two isolates, Vancomycin Resistant S. aureus (VRSA) (1.8%), which were resistant, and 108/108 (100%) of S. aureicularis was susceptible to Gentamicin, Cefoxitin, and Vancomycin (Table 2).
The overall prevalence of resistance to two or more antibiotics was 39/108 (36%). S. aureus 22/108 (20.3%) had the highest prevalence of resistance to two or more antibiotics, followed by S. epidermidis 15/108 (13.9%), S. saprophyticus 1/108 (0.9%), and S. aureicularis 1/108 (0.9%).
Contamination rate and the cleaning practices among the medical students
The students that disinfected their stethoscopes between patients were 86/293 (29.4%) and had an acceptable contamination rate while those who did not disinfect it had 178/293 (60.7%), and the difference was significant with P-value (0.040). The presence of any reminders of equipment disinfection in study unit had 94/293 (32.1%) of the acceptable contamination rate and who didn’t have reminder had 199/293 (67.9%), and the difference was strongly significant with P-value (0.001). The students who used their stethoscopes to only examine patients were 101/293 (34.4%) of acceptable contamination rate while those who examined the patients and other students were 193/293 (65.8%), and the difference was significant with P-value (0.021) (Table 3).
The differences in the distribution of contamination (TSA means / Cm2) among different variables
There was a significant difference between the places of rotation due to bacterial contamination (TSA mean/ Cm2); the mean of hospital A was 50.8 with standard deviation (SD) 13 and the other hospitals (B, C) the mean were 27.5, 26.5 respectively. Therefore, we can conclude that the contamination in the hospital A was higher than B and C and this difference was significant due to P- value was 0.028. The contamination means between students, who underwent Online Learning During COVID-19 mean 37.5 with S.D 6.56, was lower than who don’t take that mean 2.47 S. D 0.66 with P- value 0.047. Therefore, the students who were learning online had contamination rate more than who didn’t learn online. (Table 4). Other variables did not show any significance (Table S3).
The differences in the distribution of Staphylococcal contamination of (MSA means / Cm2) among different variables
MSA contamination levels were highest in hospital A (12.52 ± 5.32), followed by hospital B (8.73 ± 5.19), and lowest in hospital C (3.43 ± 1.31), with a significant difference (P-value = 0.017). MSA contamination was highest in surgery rotation (mean 12.94 ± 2.98) among medical students, followed by others rotation (mean 10.95 ± 8.66) and pediatric (10.29 ± 8.63). The lowest two rotations were internal medicine and obs-gyna with mean of 3.7. These differences were significant with a P-value of (0.010).
Sixth-year medical students had the greatest MSA contamination mean (14.02 ± 5.38), followed by fifth-year medical students (7.60 ± 5.2) and fourth-year medical students with mean (3.1 ± 1.14). These differences were significant (P-value = 0.028). Students who disinfected their stethoscopes monthly had a higher contamination mean (12.38) than those who disinfected them weekly or daily (mean 11.5), while students who disinfected their stethoscopes hourly had the lowest contamination mean (9.39 ± 5.09). These differences were significant with a P-value of 0.005. The study unit with equipment disinfection reminders had a higher mean MSA contamination rate (14.32 ± 4.96) compared to those without (4.75 ± 2.76), with significant differences (P-value = 0.001). Students who believe hand hygiene is crucial for preventing or reducing stethoscope contamination had higher MSA contamination rates (8.30 ± 2.256) and (3.95 ± 0.52), respectively, with a P-value of 0.014 indicating significant differences (Table 5).
There was no significant difference between students who examined just patients with a stethoscope and those who examined other students as well. Students who cleaned their stethoscopes hourly had the lowest mean (0.93 ± 0.88), with significant differences (P-value = 0.031). Students who learned about disinfection in clinical research had lower contamination rates (7.23 ± 1.42) compared to those who did not. Contamination rates in hospitals and their rotations display considerable variability, affected by several variables like as hygiene standards and the availability of disinfection supplies, which subsequently influence infection control. Effective hand hygiene is essential; enhancements in water supply, sanitation, and handwashing facilities have shown a reduction in health-related illnesses, especially in resource-limited settings.
Discussion
This study highlights critical gaps in stethoscope hygiene among medical students and their implications for infection control. Despite widespread awareness of contamination risks, inconsistent disinfection practices persist. The finding that 78% of stethoscopes were contaminated—with multidrug-resistant organisms such as MRSA—raises serious concerns about transmission of HAIs within clinical training environments.
A notable and unexpected finding was that students enrolled in online courses had higher contamination rates than those in in-person programs. While reduced hands-on training is a plausible factor, further explanation is warranted. Limited supervision, absence of real-time feedback on hygiene behavior, and less exposure to clinical infection control protocols may contribute. Students might also have used the same stethoscopes without proper training in disinfection protocols before clinical rotations resumed25,26,27.
Surgical rotations exhibited the highest contamination levels, reinforcing the need for stricter hygiene compliance in high-risk departments. Surprisingly, no significant differences in hygiene practices were found across gender, age, or clinical year—suggesting that stethoscope contamination is a systemic issue, not limited to any subgroup. This lack of variation underscores the necessity of incorporating standardized training across all clinical years14,27.
The association between students’ awareness and their disinfection behavior (p < 0.001) confirms the effectiveness of targeted education. Those trained in disinfection methods were more likely to clean their stethoscopes after each use (p = 0.004), in line with other studies emphasizing the role of education in infection prevention. However, one-third of students reported not sterilizing their stethoscopes at all, reinforcing the need for stronger institutional enforcement33,34.
This study also draws attention to regulatory compliance issues in hospitals, as contamination persisted despite infection control legislation. Institutional commitment must extend beyond policy into practice, including the provision of accessible disinfectants and regular audits.
Educational interventions, such as workshops and simulation-based learning, have shown promise in improving hygiene practices. For instance, a Nigerian study saw significant improvements following a disinfection campaign. Including such programs in the medical curriculum can cultivate a culture of hygiene accountability34.
This study aimed to assess the hygiene practices and contamination risks associated with stethoscope use among student doctors. Our findings reveal several key insights:
-
(1)
Association between knowledge and hygiene practices: The significance of good hygiene practices is highlighted by the null hypothesis’ rejection (p < 0.001) on the association between students’ awareness of infection dangers and how frequently they clean their stethoscopes. Students were far more likely to routinely disinfect their stethoscopes if they had a greater awareness of the risk of disease transmission through these devices. These results are consistent with earlier studies that highlight the necessity of focused instruction on infection control strategies.
-
(2)
The null hypothesis, which claimed that there was no connection between online learning and stethoscope contamination, was disproved (p = 0.047). This implies that stethoscope contamination was higher among students enrolled in online courses than among those undergoing in-person instructions. Less frequent hands-on training may have resulted from the COVID-19-induced shift to virtual education, which could have contributed to this outcome.
-
(3)
Rotation Speciality and Hygiene Practices: Surgical rotations had noticeably higher contamination levels, while there were no discernible variations in contamination levels between clinical years (p = 0.433). This emphasises the necessity of paying closer attention to cleanliness guidelines, particularly in high-risk settings like surgery where there is a greater chance of infection.
-
(4)
Gender, Age, and Hygiene Practices: In spite of earlier worries, there was no discernible link between stethoscope cleaning practices and either gender or age. Nevertheless, the results indicate that despite these considerations, there is a general lack of consistency in hygiene practices, with some students ignoring disinfection procedures even if they recognise how important they are.
Regarding the study limitations: Although this study offers insightful information, there are a few things to keep in mind. First, because the study was cross-sectional, data were only gathered once, which made it difficult to determine causative links or monitor how stethoscope contamination and cleaning procedures changed over time. Trends and causality could be better understood with a longitudinal research.
Second, because participants might not remember or describe their cleaning routines precisely, the use of self-reported disinfection methods raises the risk of bias. Future research could use more methods that are objective. Including direct observation or validation using biochemical markers, to lessen this.
Furthermore, there was insufficient attention paid to the variations in stethoscope use among various clinical settings or departments. Department-specific variations in stethoscope usage frequency and technique may have an impact on contamination rates. It would be easier to determine if particular environments lead to higher contamination levels if future research considered departmental differences.
Finally, the results may not be entirely generalizable to other healthcare practitioners or contexts because our sample was restricted to medical students in a specific area. The results’ external validity would be strengthened if the study were expanded to include a wider spectrum of participants from other healthcare settings.
Our study concludes by pointing out serious deficiencies in student physicians’ hygiene habits, which has important ramifications for infection prevention in medical facilities. We have reaffirmed the necessity of focused interventions to raise awareness and encourage adherence to appropriate disinfection procedures, especially in light of the ongoing epidemic, by rejecting the null hypothesis for important variables.
Conclusion
This study, involving 293 medical students, revealed widespread stethoscope contamination and inconsistent hygiene practices, despite high awareness of infection risks. The detection of multidrug-resistant organisms—including MRSA—underscores the urgent need for institutional and educational reforms to address these gaps.
To enhance patient safety and limit the spread of healthcare-associated infections, we recommend that medical schools integrate standardized disinfection protocols into clinical training, supported by hands-on workshops and regular reminders. Embedding infection control principles into the curriculum can foster a culture of hygiene accountability early in medical education. Hospitals must also ensure the availability of accessible disinfectants and enforce compliance through routine monitoring.
Furthermore, targeted interventions—such as peer-led hygiene campaigns and simulation-based learning—should be explored for their potential to improve long-term adherence. Future research should assess the effectiveness of these strategies and evaluate emerging technologies like UV-C disinfection and antimicrobial stethoscope materials.
By combining institutional commitment, educational innovation, and evidence-based practice, we can substantially reduce stethoscope-related contamination and strengthen defenses against antimicrobial resistance—ultimately advancing patient safety and the quality of clinical care.
Data availability
Data is provided within the manuscript and/or supplementary information files.
References
Zhu, J., Tan, Y., Huang, B., Zhu, Y. & Gao, X. H. Don’t throw the stethoscope away! Eur. Heart J. 42, 10–12. https://doi.org/10.1093/eurheartj/ehaa343 (2021).
Seah, J. J., Zhao, J., Wang, Y. & Lee, H. P. Review on the advancements of stethoscope types in chest auscultation. Diagnostics (Basel). 13. https://doi.org/10.3390/diagnostics13091545 (2023).
Nazari-Shafti, T. Z. et al. A clinical study to evaluate the safe and effective use of a new, single use stethoscope cover to enable reduction in pathogen transmission during auscultation. Front. Med. (Lausanne). 10, 1179145. https://doi.org/10.3389/fmed.2023.1179145 (2023).
Liu, V. X. et al. Comparison of early warning scoring systems for hospitalized patients with and without infection at risk for In-Hospital mortality and transfer to the intensive care unit. JAMA Netw. Open. 3, e205191. https://doi.org/10.1001/jamanetworkopen.2020.5191 (2020).
Zayed, A. R. et al. Whole-genome sequencing of the clinical isolate of Legionella pneumophila ALAW1 from the West bank allows high-resolution typing and determination of pathogenicity mechanisms. Eur. Clin. Respir J. 10, 2168346. https://doi.org/10.1080/20018525.2023.2168346 (2023).
Benenson, S. et al. Is it financially beneficial for hospitals to prevent nosocomial infections? BMC Health Serv. Res. 20, 653. https://doi.org/10.1186/s12913-020-05428-7 (2020).
Peacock, W. F. The hidden expense of stethoscope hygiene versus the real costs of failure. Clin. Exp. Emerg. Med. 11, 6–8. https://doi.org/10.15441/ceem.23.161 (2024).
Saleem, Z. et al. Point prevalence surveys of health-care-associated infections: a systematic review. Pathog Glob Health. 113, 191–205. https://doi.org/10.1080/20477724.2019.1632070 (2019).
Wissmann, J. et al. (ed, E.) Persistence of pathogens on inanimate surfaces: A narrative review. Microorganisms 9 https://doi.org/10.3390/microorganisms9020343 (2021).
Jablonska-Trypuc, A. et al. Inanimate surfaces as a source of hospital infections caused by fungi, Bacteria and viruses with particular emphasis on SARS-CoV-2. Int. J. Environ. Res. Public. Health. 19 https://doi.org/10.3390/ijerph19138121 (2022).
Katzenberger, R. H., Rosel, A. & Vonberg, R. P. Bacterial survival on inanimate surfaces: a field study. BMC Res. Notes. 14, 97. https://doi.org/10.1186/s13104-021-05492-0 (2021).
Datta, P. et al. The friendly foe - A study to evaluate bacterial contamination of stethoscopes and disinfection practices. J. Infect. Dev. Ctries. 12, 887–893. https://doi.org/10.3855/jidc.10128 (2018).
Sebastian Marcos, P., Hermes, D. & Sharman, M. Comparative assessment of the effectiveness of three disinfection protocols for reducing bacterial contamination of stethoscopes. Infect. Control Hosp. Epidemiol. 41, 120–123. https://doi.org/10.1017/ice.2019.308 (2020).
Alali, S. A. et al. Community hospital stethoscope cleaning practices and contamination rates. Am. J. Infect. Control. 48, 1365–1369. https://doi.org/10.1016/j.ajic.2020.04.019 (2020).
Tahir, M. J. et al. Microbiological impacts of decontamination of stethoscopes and assessment of disinfecting practices among physicians in pakistan: A quality improvement survey. Am. J. Trop. Med. Hyg. 107, 52–58. https://doi.org/10.4269/ajtmh.21-1283 (2022).
O’Flaherty, N. & Fenelon, L. The stethoscope and healthcare-associated infection: a snake in the grass or innocent bystander? J. Hosp. Infect. 91, 1–7. https://doi.org/10.1016/j.jhin.2015.04.010 (2015).
Zehra, D. et al. Awareness among healthcare professionals regarding contaminated stethoscopes as a source of nosocomial infections. Cureus 11, e5968. https://doi.org/10.7759/cureus.5968 (2019).
Vasudevan, R. S., Amin, A., Hannula, D. L. & Maisel, A. S. Stethoscope hygiene: A legal consideration for cardiologists practicing in a new era of infection control (COVID-19). Am. Heart J. Plus. 7, 100039. https://doi.org/10.1016/j.ahjo.2021.100039 (2021).
Bradwell, H. L. et al. Microbial contamination and efficacy of disinfection procedures of companion robots in care homes. PLoS One. 15, e0237069. https://doi.org/10.1371/journal.pone.0237069 (2020).
Sahiledengle, B., Tekalegn, Y., Bekele, K., Tesemma, A. & Edward Quisido, B. J. Disinfection of stethoscope and Non-Infrared thermometer: practices of physicians in Ethiopia in the era of COVID-19. Risk Manag Healthc. Policy. 13, 3245–3257. https://doi.org/10.2147/RMHP.S289125 (2020).
Vasudevan, R. S. et al. Persistent value of the stethoscope in the age of COVID-19. Am. J. Med. 133, 1143–1150. https://doi.org/10.1016/j.amjmed.2020.05.018 (2020).
Bansal, A., Bhan, R. S. S., Gupta, B. D., Purwar, S. & K. & To assess the stethoscope cleaning practices, microbial load and efficacy of cleaning stethoscopes with alcohol-based disinfectant in a tertiary care hospital. J. Infect. Prev. 20, 46–50. https://doi.org/10.1177/1757177418802353 (2019).
Napolitani, M., Bezzini, D., Moirano, F., Bedogni, C. & Messina, G. Methods of disinfecting stethoscopes: systematic review. Int. J. Environ. Res. Public. Health. 17 https://doi.org/10.3390/ijerph17061856 (2020).
Kalra, S. et al. Stethoscope hygiene: A call to action. Recommendations to update the CDC guidelines. Infect. Control Hosp. Epidemiol. 42, 740–742. https://doi.org/10.1017/ice.2021.115 (2021).
Gazibara, T. et al. Stethoscope hygiene: practice and attitude of medical students. Med. Princ Pract. 24, 509–514. https://doi.org/10.1159/000434753 (2015).
Holleck, J. L., Merchant, N., Lin, S. & Gupta, S. Can education influence stethoscope hygiene? Am. J. Infect. Control. 45, 811–812. https://doi.org/10.1016/j.ajic.2017.02.004 (2017).
Boulee, D., Kalra, S., Haddock, A., Johnson, T. D. & Peacock, W. F. Contemporary stethoscope cleaning practices: what we haven’t learned in 150 years. Am. J. Infect. Control. 47, 238–242. https://doi.org/10.1016/j.ajic.2018.08.005 (2019).
Holleck, J. L. et al. Stethoscope hygiene: using cultures and real-time feedback with bioluminescence-based adenosine triphosphate technology to change behavior. Am. J. Infect. Control. 48, 380–385. https://doi.org/10.1016/j.ajic.2019.10.005 (2020).
Weinstein, M. P. & Lewis, J. S. 2 The clinical and laboratory standards Institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J. Clin. Microbiol. 58 https://doi.org/10.1128/JCM.01864-19 (2020).
Qadi, M. et al. Microbes on the mobile phones of healthcare workers in palestine: identification, characterization, and comparison. Can. J. Infect. Dis. Med. Microbiol. 2021 (8845879). https://doi.org/10.1155/2021/8845879 (2021).
Odeh, Z., Abatli, S. & Qadi, M. Radiology department: A potential source of Multidrug-Resistant microorganisms: A Cross-Sectional study at tertiary hospital, Palestine. Can. J. Infect. Dis. Med. Microbiol. 2023 (4441338). https://doi.org/10.1155/2023/4441338 (2023).
Aiesh, B. M. et al. Epidemiology and clinical characteristics of patients with healthcare-acquired multidrug-resistant Gram-negative bacilli: a retrospective study from a tertiary care hospital. Sci. Rep. 14, 3022. https://doi.org/10.1038/s41598-024-53596-x (2024).
Sahan, S., Guler, S. & Korkmaz, E. Implementation of stethoscope disinfection: an observational study on nursing staff practice and knowledge. GMS Hyg. Infect. Control. 19 (Doc30). https://doi.org/10.3205/dgkh000485 (2024).
Uneke, C. J. et al. Stethoscope disinfection campaign in a Nigerian teaching hospital:results of a before-and-after study. J. Infect. Dev. Ctries. 8, 86–93. https://doi.org/10.3855/jidc.2696 (2014).
Acknowledgements
ACKNOWLEDGEMENTSThe authors would like to thank An-Najah National University (www.najah.edu) for the technical support provided to publish the present manuscript.
Funding
This research did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Contributions
Mo. A, M.Aw, M.E, A.J and F.A.T performed sample acquisition, strain isolation and contributed to drafting of the manuscript.A.Z and A.M performed strain isolation and data analysis. M.Q critically reviewed the manuscript, interpreted the results A.R.Z and A.A. designed the work, contributed to data evaluation and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
We confirm that informed consent was obtained from all subjects and/or their legal guardians. Ethical approval (Ref. Med. 2022/14) was obtained from the Institutional Review Board (IRB) at An-Najah National University in Nablus, the West Bank, Palestine.
Ethical approval
Ethical approval for this study was obtained from the Institutional Review Board (IRB) prior to data collection. To ensure that participants were fully informed and their rights were protected, the following steps were taken to obtain informed consent: 1. Explanation of the Study: One of the authors visited with prospective volunteers to provide a comprehensive explanation of the study’s goals, methods, and purpose. Prior to their decision to participate, participants had the chance to clarify any confusion and ask questions. 2. Confidentiality Measures: All acquired data was anonymised to preserve participant privacy. To identify their paper questionnaire and any related data, participants was given a unique code. This function made sure that no names or other identifying information was connected to the data. No one else was able to link the data to the participant’s identity, and only they had access to their code. 3. Voluntary Participation and Written Consent: Study participation was optional. In order to affirm their acceptance to participate, participants had to sign a consent box in the last portion of the questionnaire. The participant’s data was not included in the study if the consent box was not signed. These measures ensured that the study adhered to ethical standards and that participants’ autonomy, privacy, and confidentiality were respected at all times.
These measures ensured that the study adhered to ethical standards and that participants’ autonomy, privacy, and confidentiality were respected at all times.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ali, M., Awwad, M., Eid, M. et al. Investigating stethoscope hygiene practices and bacterial contamination among medical trainees: an educational perspective. Sci Rep 15, 23444 (2025). https://doi.org/10.1038/s41598-025-07231-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07231-y