Abstract
Combination therapy with dabrafenib and trametinib has been shown to improve response and survival outcomes in patients with BRAF V600E-mutated non–small cell lung cancer (NSCLC). However, safety and efficacy data in Japanese patients with unresectable NSCLC are limited in clinical trials. This post marketing surveillance study investigated the safety and effectiveness of dabrafenib and trametinib in Japan between March 2018 and August 2023. Most patients included in the safety analysis set (N = 76) had stage IV NSCLC (82.89%) and Eastern Cooperative Oncology Group performance status 0 or 1 (85.53%). Adverse events (AEs) occurred in 70 patients (92.11%); the most common AEs (≥ 5%) were pyrexia (46.05%), progression of NSCLC (38.16%), and rash (13.16%). Incidences of adverse drug reactions of safety specifications were pyrexia (46.05%), rhabdomyolysis (11.84%), and hepatic impairment and cardiac disorder (10.53% each). In the overall response rate (ORR) analysis set (n = 64), ORR was 67.19% (95% confidence interval [CI], 54.31-78.41%). Seventy-four patients were analyzed for progression-free survival (PFS); PFS rates (95% CI) at 182 days and 364 days were 71.07% (58.79-80.29%) and 49.64% (37.17-60.91%), respectively. Median PFS (95% CI) survival was 364 days (246.00 to not estimable). Real-world data observed in this surveillance study were similar to those reported previously.
Similar content being viewed by others
Introduction
BRAF mutations are reported across several different types of cancer, including melanoma, thyroid cancer, and non–small cell lung cancer (NSCLC) and account for approximately 3-5% of all NSCLC cases, with the majority having the adenocarcinoma histology1. BRAF V600E is the most common point mutation found in 90% of BRAF-mutated tumors (without any noticeable variation based on geographic location2), and overall, V600 mutations represent one-third of BRAF mutations in NSCLC1. In BRAF-mutated NSCLC, the V600 mutation in the BRAF oncogene constitutively activates the mitogen-activated protein kinase (MAPK) pathway, leading to unregulated signaling and tumor growth3.
In the past decade, BRAF inhibitors, either as monotherapy or in combination with a MEK inhibitor, have demonstrated improved response and survival outcomes in patients with BRAF V600-mutated melanoma4,5,6,7. Based on these successful outcomes, this approach has also been translated into NSCLC, where similar outcomes have been demonstrated1. Two therapies of growing interest are dabrafenib and trametinib. Dabrafenib is an inhibitor of the RAF proteins BRAF and CRAF through ATP-competitive binding to the active conformation of BRAF kinase8. Trametinib is an allosteric MEK1/2 inhibitor that inhibits MEK1/2 kinase activity and prevents RAF-dependent MEK phosphorylation3. In combination therapy, dabrafenib and trametinib block the MAP kinase pathway at 2 different levels, inhibiting downstream signaling and causing cell cycle arrest3. Dabrafenib and trametinib combination therapy has shown improved response and survival outcomes in patients with BRAF V600E-mutated NSCLC, regardless of pretreatment status in a global phase 2 trial (NCT01336634), which led to United States Food and Drug Administration approval for this indication9,10,11. This study reported the safety and efficacy of dabrafenib and trametinib combination therapy (hereafter referred to as the “DAB/TRA”) in patients with stage IV NSCLC who were positive for BRAF V600E mutations, including 57 previously treated patients (cohort B)9 and 36 previously untreated patients (cohort C)10.
In Japan, DAB/TRA was approved for the treatment of unresectable malignant melanoma with BRAF mutations in March 2016, after which an all-case surveillance was later initiated in June 2016 because of the limited number of patients in this country. An additional indication of “unresectable, advanced or recurrent NSCLC with BRAF mutations” was approved in March 2018, with a further all-case surveillance initiated on the same day. DAB/TRA was further approved in Japan in November 2023 as a tumor-agnostic approach for the treatment of unresectable or metastatic solid tumors with a BRAF V600E mutation (which is difficult to be treated with standard of care, except for colorectal cancer) and relapsed or refractory hairy cell leukemia with a BRAF V600E mutation. In this post marketing surveillance study, we evaluated the real-world safety and effectiveness of DAB/TRA in Japanese patients with unresectable advanced or recurrent NSCLC with BRAF mutations.
Methods
Study design and data collection
This was a multicenter, observational, surveillance study without a control group to evaluate the safety and effectiveness of DAB/TRA in clinical settings in Japanese patients with unresectable advanced or recurrent NSCLC with BRAF mutations, which was initiated on March 23, 2018. It was conducted in accordance with Good Post-marketing Study Practice (GPSP) Ordinance (Japan local regulation). The study protocol and the implementation guideline (summary of the study protocol) were approved by a scientific/ethical review committee of Novartis. This was an all-case surveillance based on the conditions for approval, and the surveillance was designed to collect information on all patients within the specified patient population who received DAB/TRA for a certain period after the market launch. Only case registration was performed in principle for patients who received DAB/TRA in April 2019 and onward.
The observation period for each patient was up to 1 year (52 weeks). The safety observation period was until 30 days after the completion of the observation period of the surveillance when treatment with DAB/TRA was continued, or until 30 days after the last dose of DAB/TRA, when DAB/TRA treatment was discontinued/completed before the completion of the observation period of the surveillance. Data were collected by the physicians at participating sites through surveillance using an electronic data capture (EDC) system on a central registration system at the start of treatment with DAB/TRA. Survey items included patient characteristics, administration status of DAB/TRA, prior medication history, history of surgery/radiotherapy, concomitant medications and therapies, laboratory investigations, adverse events (AEs), and effectiveness.
This study was conducted by Novartis Pharma K.K. in accordance with the Japan regulation (ministerial ordinance on GPSP), under which there is no description regarding requirement of informed consent for conducting post marketing surveillance studies in Japan. However, Novartis Pharma K.K. provided the investigators with an information sheet describing the objectives and content of this surveillance, protection of personal information, and disclosure of surveillance data, and the investigators explained the surveillance details to patients using the information sheet before the start of treatment. The investigators were then required to enter necessary information in the case report form (CRF) in the EDC system for all registered patients subject to CRF collection (including patients who discontinued or dropped out) and submitted the CRF promptly to the sponsor after completion of the observation period. Data of demographic characteristics such as age, sex, and other baseline characteristics were collected as baseline values, for which frequency and its proportion were calculated for categorical data, and summary statistics were calculated for continuous data.
Patient population
This study included Japanese patients with unresectable advanced or recurrent NSCLC with BRAF mutations (confirmed using approved in vitro diagnostics) who received DAB/TRA after approval for this indication. All patients who received DAB/TRA were included in this surveillance study. Patients could be enrolled even if they started DAB/TRA before or after concluding a contract with each study site.
Study endpoints
The safety endpoints were incidence of AEs and serious AEs, adverse drug reactions (ADRs), safety specifications (which are grouping terms for identified risks to either or both DAB/TRA), and laboratory findings. The effectiveness endpoints were overall response rate (ORR) and progression-free survival (PFS). ORR was defined as the percentage of patients with a best overall response of complete response (CR) or partial response (PR) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.112. PFS was defined as the time from the start date of DAB/TRA treatment to the date of the first documented disease progression or death due to any cause during the observation period. Patients without these events were censored at 30 days after the end of DAB/TRA treatment or the date of the last observation in this surveillance study.
Study assessments
Safety
An AE was defined as any untoward medical occurrence in a patient receiving DAB/TRA, which may not necessarily have a causal relationship with this treatment. An AE could therefore be any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of DAB/TRA, whether or not related to DAB/TRA. A serious AE was defined as follows: Fatal or life-threatening; results in persistent or significant disability/inability; constitutes a congenital anomaly/birth defect; and/or requires inpatient hospitalization or prolongation of existing hospitalization. Exceptions were as follows: routine treatment or monitoring of the studied indication; not associated with any deterioration in condition; elective or pre-planned treatment for a preexisting condition that is unrelated to the indication under study and has not worsened since the start of the investigational product; and/or social reasons and respite care in the absence of any deterioration in the patient’s general condition is medically significant, i.e., defined as an event that jeopardizes the patient or may require medical or surgical intervention to prevent one of the outcomes listed. An ADR was defined as an AE for which a causal relationship with DAB/TRA could not be ruled out by the investigator/sub-investigator based on the entries in the CRF.
To confirm potential safety issues, analyses were performed in the following subgroups: renal impairment (absent/present), elderly (< 65 years, ≥ 65 years), late elderly (< 75 years, ≥ 75 years), Japanese children [< 15 years, ≥ 15 years], European children [< 18 years, ≥ 18 years], pregnancy (yes/no), Eastern Cooperative Oncology Group Performance Status (ECOG PS; 0–2, ≥ 3), number of lines of therapy used before the start of treatment with DAB/TRA (0, ≥ 1), cardiac disease (absent/present), previous immune checkpoint inhibitor (ICI) medication (absent/present), and other factors for which the 2-sided 95% confidence interval (CI) of the adjusted odds ratio did not include 1 in the tables for incidences of ADRs by background factor (multivariate logistic regression). Analysis by the absence/presence of hepatic impairment was planned but not analyzed because no patients had hepatic impairment at baseline. Safety specifications included in this post marketing surveillance study were cutaneous squamous cell carcinoma, secondary malignancy other than cutaneous squamous carcinoma, eye disorders, pyrexia, hepatic dysfunction, cardiac disorders, and rhabdomyolysis, for which the number of patients with events and incidence was calculated. Laboratory evaluations included blood chemistry (aspartate transaminase [AST], alanine transaminase [ALT], lactate dehydrogenase, creatine phosphokinase [CPK], creatinine, serum amylase, lipase, and myoglobin) and urinalysis (urine amylase and myoglobin).
Summary statistics and time-course plots (mean ± standard deviation [SD]) were created for the observed values and changes from baseline (at the start of treatment) at each scheduled time point. Patient factors were sex, age, disease duration, medical history, comorbidity, ECOG PS, lung cancer staging, histological type, brain metastasis, smoking history, number of lines of therapy used before the start of treatment with DAB/TRA, history of surgery for the primary disease, history of radiotherapy for the primary disease, presence or absence of prior treatment for the primary disease, presence or absence of prior treatment with ICI immediately before DAB/TRA, and presence or absence of pregnancy.
Effectiveness
ORR and its 2-sided 95% CI were calculated. Two-sided 95% CIs were calculated using the Clopper-Pearson method13. The number and proportion of patients were calculated by response assessment. For PFS, the Kaplan-Meier method was used to determine the distribution and number of patients in the at-risk group at each time point. The 2-sided 95% CI of the PFS rate was estimated using the Greenwood’s formula14. Analyses were performed in the following subgroups: renal impairment (absent/present), hepatic impairment (absent/present), elderly (< 65 years, ≥ 65 years), late elderly (< 75 years, ≥ 75 years), Japanese children [< 15 years, ≥ 15 years], European children [< 18 years, ≥ 18 years], ECOG PS (0–2, ≥ 3), number of lines of therapy used before the start of treatment with DAB/TRA (0, ≥ 1), cardiac disease (absent/present), and previous ICI medication (absent/present).
Methods with statistical analyses
All statistical analyses for safety and effectiveness were performed at CMIC Co., Ltd, using SAS version 9.4 (SAS Institute Japan Ltd, Tokyo, Japan) and were mainly descriptive. Further details of methods with statistical analyses are described in aforementioned “Study endpoints” and “Study assessments” parts of Methods section.
Results
Patient disposition
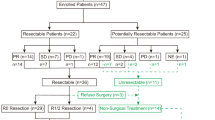
A flowchart on patient disposition is shown in Fig. 1. During the surveillance period (March 23, 2018, to August 3, 2023), a total of 644 Japanese patients were registered from 354 sites, of which 77 had CRFs locked. After lifting the approval condition, 567 patients with CRFs were not required to be collected. Seventy-six patients were included in the safety analysis set after excluding 1 patient from the safety analysis set (an off-label case due to the confirmation of a BRAF mutation using a method other than the approved in vitro diagnostics). Of the 76 patients included in the safety analysis set, 64 patients were included in the ORR analysis set after excluding 12 patients undeterminable for effectiveness (best overall response was not assessed). After excluding 2 patients undeterminable for effectiveness (presence or absence of disease progression was unknown), 74 were included in the PFS analysis set.
Patient baseline characteristics
Table 1 summarizes the demographic and disease characteristics for patients included in the safety analysis population. Of the 76 patients in the safety analysis set, more than half were male (49 patients, 64.47%). The mean ± SD age at the start of treatment was 68.0 ± 10.2 years, and most patients were aged ≥ 65 years (56 patients, 73.68%). The mean ± SD duration of disease was 114.3 ± 153.0 weeks. Notably, most patients had stage IV disease (63 patients, 82.89%), were former or current smokers (54 patients, 71.05%), and had ECOG PS 0 or 1 (65 patients, 85.53%). Twenty-four patients (31.58%) had not received any prior therapy, whereas 50 patients (65.79%) had received ≥ 1 line of therapy before the start of treatment with DAB/TRA.
Administration status of DAB/TRA
Of the 76 patients treated with DAB/TRA, approximately 90% of patients received DAB/TRA for at least 28 days. The median (range) duration of total exposure was 252.0 (2-364) days for dabrafenib and 243.5 (2-364) days for trametinib. The starting daily dose for dabrafenib for the majority of patients (72 patients, 94.74%) was 300 mg, while that for trametinib for the majority of patients (73 patients, 96.05%,) was 2 mg. Treatment compliance for DAB/TRA is shown in Supplementary Table 1. Of the 76 patients, more than half discontinued this surveillance study (42 patients, 55.26%). The most common reason for discontinuation was AEs (including progression of primary disease), which occurred in 39.47% of patients (30 patients), followed by death due to AEs (including death due to primary disease) in 7.89% of patients (6 patients), not visiting the site mid-course in 3.95% of patients (3 patients), patient and/or family decision in 2.63% of patients (2 patients), and physician decision in 1.32% of patients (1 patient).
Safety
Incidence of AEs
Of the 76 patients assessed in the safety analysis set, 70 experienced AEs (92.11%; Supplementary Table 2). The most common AEs (≥ 5%) were pyrexia (35 patients, 46.05%); progression of NSCLC (29 patients, 38.16%); rash (10 patients, 13.16%); pneumonia, rhabdomyolysis, ALT increased, AST increased, and blood CPK increased (5 patients each, 6.58% each); stomatitis, abnormal hepatic function, and decreased appetite (4 patients each, 5.26% each). In the post marketing surveillance study, progression of NSCLC is reported as an AE. Thirty-eight patients experienced serious AEs (50.00%). The major serious AEs (≥ 2%) were progression of NSCLC (19 patients, 25.00%); pneumonia and rhabdomyolysis (4 patients each, 5.26% each); infection (3 patients, 3.95%); and pyrexia, abnormal hepatic function, and disseminated intravascular coagulation (2 patients each, 2.63% each; Supplementary Table 2).
Incidence of ADRs
Data of the incidence of ADRs and serious ADRs are summarized in Table 2. The overall incidence of ADRs was 65.79% (50 of 76 patients). The most common ADRs (≥ 5%) were pyrexia (35 patients, 46.05%); rash (9 patients, 11.84%); rhabdomyolysis and blood CPK increased (5 patients each, 6.58% each); and progression of NSCLC, decreased appetite, stomatitis, ALT increased, and AST increased (4 patients each, 5.26% each). The incidence of grade ≥ 3 ADRs was 27.63% (21 patients). Events occurring in ≥ 2 patients were rhabdomyolysis (4 patients, 5.26%); progression of NSCLC, pyrexia, and AST increased (3 patients each, 3.95% each); and infection, decreased appetite, abnormal hepatic function, ALT increased, and blood CPK increased (2 patients each, 2.63% each). The incidence of serious ADRs was 15.79% (12 of 76 patients). The most common serious ADRs (≥ 2 patients) included rhabdomyolysis (4 patients, 5.26%), progression of NSCLC (3 patients, 3.95%), and infection and pyrexia (2 patients each, 2.63% each).
Death
Twenty patients died due to AEs, and 3 of them died due to ADRs (2 cases of progression of NSCLC and 1 case of pneumonia).
Incidence of ADRs of safety specifications
The incidences of each reported ADR of safety specifications were pyrexia (35 patients, 46.05%), rhabdomyolysis (9 patients, 11.84%), hepatic impairment and cardiac disorder (8 patients each, 10.53% each), and eye disorder (1 patient, 1.32%). No cutaneous squamous carcinoma and secondary malignancy other than cutaneous squamous cell carcinoma was observed (Table 3). The incidence of grade 3 ADRs was reported in 13 patients (17.11%); the ADRs included rhabdomyolysis (4 patients, 5.26%); pyrexia and increased AST (3 patients each, 3.95% each); ALT increased, abnormal hepatic function, and blood CPK increased (2 patients each, 2.63% each); and increased gamma-glutamyl transferase, liver disorder, and hemoptysis (1 patient each, 1.32% each). No grade ≥ 4 events were observed.
Safety analysis by patient factor
To examine the contributing factors that may affect safety, the odds ratio and CI for the incidence of ADRs were analyzed based on patient factors (Table 4). The contributing factor for which the 95% CI of the odds ratio did not include 1 was “comorbidity” (the incidence of ADRs was 50.00% [14 of 28 patients] in patients without comorbidity and 75.00% [38 of 48 patients]) in patients with comorbidity (odds ratio [95% CI]: 3.0 [1.117-8.055]). Adjusted analysis using factors suspected to be clinically relevant (late elderly [aged ≥ 75 years] and ECOG PS) as covariates in addition to “comorbidity” showed that the 95% CI of the adjusted odds ratio did not include 1 (adjusted odds ratio [95% CI]: 2.952 [1.095-7.958]). For the presence or absence of prior ICI medication immediately before the administration of DAB/TRA, the 95% CI of the odds ratio included 1; however, the incidence of ADRs was determined by presence or absence (Supplementary Table 3). The incidence of ADRs in the presence or absence of prior ICI medication immediately before the administration of DAB/TRA was 73.33% (11 of 15 patients) in patients who received prior ICI medication immediately before the administration of DAB/TRA and 63.93% (39 of 61 patients) in those who did not receive prior ICI medication immediately before the administration of DAB/TRA (Supplementary Table 3). ADRs that occurred at an incidence of ≥ 10% in patients with prior ICI medication immediately before the administration of DAB/TRA and at an incidence of more than twice that in patients without prior ICI medication immediately before the administration of DAB/TRA were rash and platelet count decreased.
Safety analysis by special characteristics
In safety analysis by special characteristics, 56 of 76 patients (73.68%) were elderly (aged ≥ 65 years), and ADRs were observed in 66.07% (37 patients) of those elderly patients. The incidence of ADRs in non-elderly patients (aged < 65 years) was 65.00% (13 of 20 patients), showing a similar incidence between elderly and non-elderly patients. The most common ADR in both elderly and non-elderly patients was pyrexia, with an incidence of 42.86% (24 of 56 patients) in elderly patients and 55.00% (11 of 20 patients) in non-elderly patients. All serious ADRs listed in Table 2 occurred in elderly patients (aged ≥ 65 years) except for 2 cases. Although the incidence of ADRs in elderly patients was similar to that in non-elderly patients, serious ADRs occurred more frequently. Many of the outcomes of serious events were resolved or resolving, and no safety concerns requiring special attention were observed. Twenty patients were aged ≥ 75 years (26.32%), and ADRs were observed in 70.00% (14 of 20 patients) of these patients. The incidence of ADRs in patients aged < 75 years was 64.29% (36 of 56 patients). Little difference was observed in the incidence of ADRs between patients aged ≥ 75 years and those aged < 75 years. Serious ADRs that occurred in the late elderly aged ≥ 75 years were 2 cases of progression of NSCLC and 1 case each of decreased appetite, gastric ulcer, sepsis, pneumonia, and infection. The outcomes were death for 1 event of progression of NSCLC and 1 event of pneumonia, not resolved for 1 event each of progression of NSCLC and infection and resolved or resolving for the other events.
Of 76 patients, 1 (1.32%) had renal impairment; however, no serious ADRs were observed. Patients with cardiac disease accounted for 6.58% (5 of 76 patients). Serious ADRs that were observed in 5 patients with cardiac disease were abnormal hepatic function, decreased appetite, pyrexia, increased AST, rhabdomyolysis, increased ALT, increased gamma-glutamyl transferase, and increased blood alkaline phosphatase in 1 patient each, which either resolved or were resolving for all these events. One patient who had decreased appetite did not experience other ADRs but experienced myocardial ischemia (serious) unrelated to DAB/TRA treatment, and the outcome was fatal.
Effectiveness
Sixty-four patients had tumor assessment results available during the observation period (Table 5). The ORR (95% CI) based on the investigator’s assessment in accordance with RECIST version 1.1 was 67.19% (54.31-78.41%).
Effectiveness (ORR) by patient factors
To investigate factors that may affect the effectiveness of DAB/TRA, the odds ratio and the CI for ORR were analyzed by patient factors including age, sex, smoking history, and prior treatments, among others (Table 6). For the 13 and 51 patients with and without prior ICI treatment (nivolumab or pembrolizumab), respectively, immediately before DAB/TRA therapy, the ORRs (95% CI) were 69.23% (38.57-90.91%) and 66.67% (52.08-79.24%), respectively. For patients with ECOG PS 0–2 (n = 62) or ECOG PS ≥ 3 (n = 2), the ORRs (95% CI) were 67.74% (54.66-79.06%) and 50.00% (1.26-98.74%), respectively; for those patients without or with lines of therapy used before the start of DAB/TRA treatment (0 [n = 18]; or ≥ 1 [n = 45]), the ORRs (95% CI) were 77.78% (52.36-93.59%) and 62.22% (46.54-76.23%), respectively (1 patient with unknown/not specified number of lines of therapy used before the start of treatment with DAB/TRA experienced a PR). The 95% CI of the odds ratio between categories included 1 for all factors, and no factor showed a statistical difference.
Effectiveness (ORR) by special characteristics
The ORRs for patients with special characteristics, including the elderly (aged ≥ 65 years), late elderly (aged ≥ 75 years), those with renal impairment, and those with cardiac disease, are shown in Table 5.
Progression-free survival analyses
Seventy-four patients were analyzed for PFS. The PFS (95% CI) rates at 182 days (6 months) and 364 days (12 months) were 71.07% (58.79-80.29%) and 49.64% (37.17-60.91%), respectively. The median PFS (95% CI) was 364 days (12 months; 246.00 days to not estimable (Supplementary Fig. 1).
Progression-free survival analyses by patient factors
To investigate the factors that may affect the effectiveness of DAB/TRA, PFS was analyzed by patient factors including lines of prior therapy (0 or ≥ 1) [Figure 2], with or without prior ICI medication immediately before the administration of DAB/TRA (Supplementary Fig. 2) and ECOG PS (Supplementary Fig. 3). The PFS (95% CI) rates in patients with 0 line of prior therapy (n = 24) at 182 days (6 months) were 62.72% (38.94-79.40%) and at 364 days (12 months) was 57.89% (34.51-75.52%), respectively (Fig. 2). In patients with ≥ 1 prior line of therapy (n = 48), the PFS rates (95% CI) were 74.21% (59.04-84.46%) and 46.66% (31.58-60.40%), respectively (Fig. 2). The PFS (95% CI) rates in patients with a history of prior ICI medication immediately before the administration of DAB/TRA (n = 14) at 182 days (6 months) and 364 days (12 months) were 83.33% (48.17-95.55%) and 66.67% (33.70-85.97%), respectively (Supplementary Fig. 2). In patients without prior ICI medication (n = 60) immediately before the administration of DAB/TRA, the PFS (95% CI) rates were 68.21% (54.35-78.65%) and 45.77% (32.25-58.29%) at 182 days and 364 days, respectively (Supplementary Fig. 2).
PFS was also analyzed by special characteristics including elderly [≥ 65 years and < 65 years], late elderly [≥ 75 years and < 75 years]), presence and absence of renal impairment, and presence and absence of cardiac disease, as shown in Supplementary Figs. 4–7.
PFS estimated using the Kaplan-Meier method by number of lines of therapy used before the start of treatment with DAB/TRA (PFS analysis set). CI confidence interval, DAB dabrafenib, NE not estimable, PFS progression-free survival, TRA trametinib. Two patients were categorized as unknown/ not specified.
Discussion
Positioning of this surveillance
The previous global phase 2 study demonstrated the safety and efficacy of DAB/TRA in BRAF V600E-mutated NSCLC in pretreated patients (cohort B, 57 patients) and treatment-naive patients (cohort C, 36 patients)9,10. However, the study had limited representation of Japanese patients, with only 3 patients being enrolled (2 in cohort A [dabrafenib monotherapy], 1 patient in cohort B, and none in cohort C received DAB/TRA) [data not published]. Therefore, to further understand the appropriate use of this combination therapy, additional real-world data were collected from 76 Japanese patients (pretreated: 52 patients; treatment-naive: 24 patients) in this post marketing surveillance study, in accordance with the approval conditions.
Status of DAB/TRA therapy
Of the 76 patients, approximately 90% of patients taking the combination therapy used DAB/TRA for a minimum of 28 days. Most patients (94.74%) were treated with a starting daily dose of dabrafenib (300 mg), and 96.05% were treated with trametinib (2 mg) in this surveillance study. The high compliance with the specified dosage range for each drug, per the package insert, indicates that the treatment was administered appropriately in clinical practice in Japan.
Safety results
ADRs were reported in 65.79% of patients (50 of 76 patients), of which most of the cases were not serious. The most frequent ADR was pyrexia (35 patients, 46.05%). Other common events were rash (9 patients, 11.84%); rhabdomyolysis and increased blood creatine phosphokinase (5 patients each, 6.58% each); and progression of NSCLC, decreased appetite, stomatitis, increased alanine aminotransferase, and increased aspartate aminotransferase (4 patients each, 5.26% each). The incidence of ADRs in the global phase 2 study was 89.25% (83 of 93 patients) [data not published]. The most common ADRs (≥ 5%) observed were pyrexia (46 patients, 49.46%), nausea (36 patients, 38.71%), and vomiting and dry skin (25 patients, 26.88%) [data not published]. Therefore, pyrexia was the most common ADR observed in this surveillance study, consistent with that observed in the global phase 2 study. Pyrexia is a well-documented AE associated with DAB/TRA treatment, and clinicians should continue to consider the risk profile when administering DAB/TRA and perform close monitoring. The incidence of nausea and vomiting (2.63% and 1.32%, respectively) was lower in this surveillance study than in the global phase 2 study. In this surveillance study, information on prophylaxis use of medication was not collected; only information on the drugs prescribed for the primary disease, drugs prescribed for the treatment of AEs, and use of CYP3A inhibitors were collected for concomitant medications. Based on this, it is not possible to provide a definitive explanation for this, however one possible reason is that, as nausea and vomiting may be expected in patients treated with DAB/TRA, prophylactic treatment may have been administered for these events, resulting in a lower rate of reported ADRs.
The incidences of ADRs of safety specifications in this surveillance study were 46.05% (35 patients) for pyrexia, 11.84% (9 patients) for rhabdomyolysis (including blood CPK increased and rhabdomyolysis [MedDRA preferred term)], 10.53% (8 patients) each for hepatic impairment and cardiac disorder, and 1.32% (1 patient) for eye disorder. No ADRs of cutaneous squamous cell carcinoma or secondary malignancy other than cutaneous squamous cell carcinoma occurred. As the outcome of many of the ADRs was recovered or recovering, and precautions are already provided in the package inserts of DAB/TRA, no special measures are considered necessary. In the global phase 2 study, the incidences of safety specifications (ADRs) for cohorts B and C (combined) were 46.46% (46 of 93 patients) for pyrexia (grade ≥ 3: 4 patients, 4.30%), 10.75% (10 of 93 patients) for hepatic impairment (grade ≥ 3: 5 patients, 5.38%), 32.26% (30 of 93 patients) for cardiac disorders (grade ≥ 3: 5 patients, 5.38%), and 13.98% (13 of 93 patients) for eye disorders (grade ≥ 3: 1 patient, 1.08%) [data not published]. Cutaneous squamous cell carcinoma was reported in 2.15% of patients (2 of 93 patients), of which 1 patient had a grade ≥ 3 ADR. No ADRs of secondary malignancy other than cutaneous squamous cell carcinoma occurred.
Overall, the incidences of ADRs specified in the safety specifications in this surveillance study were comparable to or lower than those in the global phase 2 study except for rhabdomyolysis. The incidence of rhabdomyolysis was higher in this surveillance, as well as time to onset of rhabdomyolysis was earlier, than that in the global phase 2 study. The incidences of rhabdomyolysis (safety specification [ADRs]) and the median number of days to onset (minimum to maximum) for DAB/TRA were, respectively, 10.53% (8 of 76 patients) and 23.5 days (8-209 days) for dabrafenib and 11.84% (9 of 76 patients) and 25.0 days (8-209 days) for trametinib in this surveillance study. In the global phase 2 study, the incidence and the median number of days to onset (minimum to maximum) were, respectively, 8.77% (5 of 57 patients) and 589.0 days (297-715 days) for dabrafenib in cohort B, 8.77% (5 of 57 patients) and 589.0 days (297-715 days) for trametinib in cohort B, and 2.78% (1 of 36 patients) and 545.0 days for dabrafenib in cohort C, and 2.78% (1 of 36 patients) and 545.0 days for trametinib in cohort C (combined cohort B and C; 6.45%, 6 patients of 93 patients). Grade ≥ 3 rhabdomyolysis events were observed only in this surveillance study (dabrafenib: 6.58%, trametinib: 7.89%), but the outcome was resolved or resolving in all patients. It is important to note that rhabdomyolysis was not considered a safety risk, and the laboratory test for blood CPK was not mandatory in the global phase 2 study; therefore, there may have been fewer reports of rhabdomyolysis including blood CPK increased. On the contrary, after the marketing approval of the use of DAB/TRA combination was granted, laboratory tests such as blood CPK might have been proactively performed because rhabdomyolysis was recognized as an ADR of MEK inhibitors. As a result, rhabdomyolysis including blood CPK increased may have been frequently reported as an ADR in this surveillance. Precautions for rhabdomyolysis are provided in the package insert for each drug.
Effectiveness results
For the 64 patients who had tumor response assessment results during the observation period, the ORR (95% CI) was 67.19% (54.31-78.41%), with CR achieved in 1 patient (1.56%) and PR achieved in 42 patients (65.63%). When analyzing ORR by lines of prior therapy in this surveillance study, the ORR was 77.78% in treatment-naïve patients and 62.22% in patients with ≥ 1 lines of therapy used before the start of treatment with DAB/TRA. In the global phase 2 study, the investigator-assessed ORR (95% CI) for treatment-naïve patients was 64% (46.0-79.0%)10 and that for patients previously treated (2-4 lines) with the combination therapy was 63.2% (49.3-75.6%)9. When analyzing the effectiveness (as measured by ORR) by patient factor in this surveillance study, no factor had the 95% CI of the odds ratio between subgroups as 1, highlighting that no special attention was required, even in patients with special characteristics. Furthermore, in this surveillance study, the median PFS (95% CI) in the PFS analysis set of 74 patients was 364 days (12 months; 246.00 [8.1 months to not estimable]). The PFS rates were 71.07% and 49.64% at 182 days (6 months) and 364 days (12 months), respectively. The median PFS (95% CI) in the global phase 2 study for patients previously treated (2–4 lines) with DAB/TRA was 9.7 months (6.9-19.6) (9). At the 5-year data cutoff, median PFS (95% CI) rates were 10.2 (6.9-16.7) and 10.8 (7.0-14.5) months for previously treated and treatment-naïve patients, respectively11.
Noteworthy, among the patient demographics in this surveillance study compared with those in the global phase 2 study, some differences in age and ECOG PS were observed. The proportion of patients aged ≥ 65 years included in the surveillance study tended to be higher than that of patients in the global phase 2 study; 73.68% compared with 49% and 61% in previously treated patients (cohort B) and treatment-naïve patients (cohort C), respectively9,10. For ECOG PS, patients with ECOG PS ≥ 3 were excluded from the global phase 2 study. The proportion of patients with ECOG PS 2 was 9% in the previously treated cohort (cohort B) and 3% in the treatment-naïve cohort (cohort C)9,10. In this surveillance study, the proportion of patients with ECOG PS of 2 and ≥ 3 was 11.84% and 2.63%, respectively. Although some differences existed in terms of patient background characteristics and evaluation methods between this surveillance study and the global phase 2 study, no major differences were observed in Japanese patients in a real-world clinical setting from the results of the global phase 2 study before approval of DAB/TRA.
When interpreting these findings, this surveillance included patients with a poor prognosis (such as those who are late elderly or those with poor ECOG PS) who were excluded from the clinical studies, which mirrors real-world scenarios. Therefore, it can be inferred that comparable levels of effectiveness and safety are likely to be attained in clinical practice in Japan. Furthermore, the Japanese Lung Cancer Society guidelines also strongly recommend the use of DAB/TRA as first-line treatment for patients with NSCLC harboring BRAF V600E mutation (https://www.haigan.gr.jp/guideline/2023/).
Limitations
This was a single-arm surveillance study without a control group (patients not treated with DAB/TRA); therefore, the relationship between the results obtained and the effects of exposure to DAB/TRA cannot be clarified. Although the incidence rate of ADRs listed in the package insert is less than 1%, it is difficult to fully evaluate these ADRs in this surveillance study because only 76 patients were included in the safety analysis. While the small sample size is acknowledged in this post-marketing surveillance study, this is due to the requirement of patients to be registered only until the condition for approval was lifted, therefore CRFs for 567 patients were not collected. This study was conducted in compliance with the Japanese regulations GPSP Ordinance, and source data verification not specified in GPSP was not performed.
Conclusion
No new safety concerns with regards to DAB/TRA treatment were identified in this surveillance study of Japanese patients with unresectable advanced or recurrent NSCLC with BRAF mutations. Additionally, the effectiveness of DAB/TRA comparable to the efficacy reported in clinical trials was demonstrated in clinical practice in this patient population.
Data availability
Novartis is committed to sharing access to anonymized patient-level data and study reports from eligible studies with qualified external researchers. All data provided are anonymized to respect the privacy of patients who have participated in the trials in line with applicable laws and regulations. All requests for access should be addressed directly to Tomoaki Kaizuka <tomoaki.kaizuka@novartis.com> and Naoko Suenaga <naoko.suenaga@novartis.com>.
References
Guaitoli, G. et al. Non-small-cell lung cancer: how to manage BRAF-mutated disease. Drugs Context. 12 https://doi.org/10.7573/dic.2022-11-3 (2023).
Tan, A. C. & Tan, D. S. W. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J. Clin. Oncol. 40 (6), 611–625. https://doi.org/10.1200/JCO.21.01626 (2022).
Khunger, A., Khunger, M. & Velcheti, V. Dabrafenib in combination with Trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: clinical evidence and experience. Ther. Adv. Respir Dis. 12, 1753466618767611. https://doi.org/10.1177/1753466618767611 (2018).
Long, G. V. et al. Dabrafenib and Trametinib versus Dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386 (9992), 444–451. https://doi.org/10.1016/S0140-6736(15)60898-4 (2015).
Hauschild, A. et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380 (9839), 358–365. https://doi.org/10.1016/S0140-6736(12)60868-X (2012).
McArthur, G. A. et al. Safety and efficacy of Vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 15 (3), 323–332. https://doi.org/10.1016/S1470-2045(14)70012-9 (2014).
Ascierto, P. A. et al. Cobimetinib combined with Vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 17 (9), 1248–1260. https://doi.org/10.1016/S1470-2045(16)30122-X (2016).
Bowyer, S., Lee, R., Fusi, A. & Lorigan, P. Dabrafenib and its use in the treatment of metastatic melanoma. Melanoma Manag. 2 (3), 199–208. https://doi.org/10.2217/mmt.15.21 (2015).
Planchard, D. et al. Dabrafenib plus Trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 17 (7), 984–993. https://doi.org/10.1016/S1470-2045(16)30146-2 (2016).
Planchard, D. et al. Dabrafenib plus Trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 18 (10), 1307–1316. https://doi.org/10.1016/S1470-2045(17)30679-4 (2017).
Planchard, D. et al. Phase 2 study of Dabrafenib plus Trametinib in patients with BRAF V600E-Mutant metastatic NSCLC: updated 5-Year survival rates and genomic analysis. J. Thorac. Oncol. 17 (1), 103–115. https://doi.org/10.1016/j.jtho.2021.08.011 (2022).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer. 45 (2), 228–247. https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
Clopper, C. & Pearson, E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26 (4), 404–413 (1938).
Collett, D. Modelling Survival Data in Medical Research (In. Chapman & Hall, 1994).
Acknowledgements
Medical writing assistance was provided by Emma Richards-Sirianni, PhD, Novartis UK Ltd, and project management was provided by Ayako Honda, Novartis Pharma K.K.
Funding
This study was funded by Novartis Pharma K.K. (Japan) in accordance with Good Publication Practice (GPP2022) guidelines (https://www.ismpp.org/gpp-2022).
Author information
Authors and Affiliations
Contributions
TK was responsible for conceptualization, investigation, methodology, project administration, writing – original draft, and writing – review and editing. RK was responsible for conceptualization, formal analysis, methodology, writing – original draft, and writing – review and editing. YY was responsible for conceptualization, writing – original draft, and writing – review and editing. NS was responsible for conceptualization, supervision, writing – original draft, and writing – review and editing.
Corresponding authors
Ethics declarations
Competing interests
All authors are employees of Novartis.
Ethical approval
Consent to participate is not applicable, as informed consent is not required in post marketing surveillance under the Japan regulation (Good Post-Marketing Study Practice). All treatments were given in actual clinical practice and no interventions were made for the purpose of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kaizuka, T., Kurihara, R., Yokochi, Y. et al. A post-marketing surveillance study of dabrafenib and trametinib combination in Japanese patients with unresectable advanced or recurrent BRAF-mutated NSCLC. Sci Rep 15, 23116 (2025). https://doi.org/10.1038/s41598-025-07467-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07467-8