Abstract
Parasites are important components of biodiversity and play multiple roles in natural systems. In aquatic birds, endoparasites are acquired mainly through the diet; thus, the environment is the main determinant of the parasitic community. The kelp gull is a widespread, generalist and opportunistic seabird species. Owing to their feeding plasticity, the aim of this study was to analyse the composition and structure of the helminth community of two kelp gull groups on the northern Patagonian coast: one group pecked on the backs of southern right whales, whereas the other fed on fishing discards. These results were compared with those of a previous study in which a kelp gull group fed on natural prey items in Península Valdés. Among the 95 analysed gulls, 92.6% were parasitized by at least one helminth species, including Cestoda, Trematoda, Nematoda and Acanthocephala, with trematodes being the most diverse. The pecking of gulls on whale backs resulted in a more depauperate parasitic community. The parasitological results suggest that altered feeding behavior in kelp gulls reduces natural prey consumption, emphasizing the importance of parasites as integral components of biodiversity and trophic markers. It also highlights the urgent need to manage urban waste and fishery discards on the northern Patagonian coast to reduce kelp gull–whale interactions.
Similar content being viewed by others
Introduction
Parasites are important components of biodiversity, as parasitism is one of the most widespread ecological interactions in nature1. They play multiple roles in natural systems, influencing host reproductive rates, altering host behavior and regulating the host population. In addition, parasites can affect trophic web structure and energy flow, modulate interactions between hosts and influence species composition in wild communities2. Parasites also provide information on environmental health and the phylogenetic relationships of hosts and are used to define protected areas and fisheries regulations2.
In particular, endoparasites of aquatic birds are acquired primarily through the diet, making the environment a key factor in determining the parasitic community. The availability of intermediate hosts, such as molluscs, crustaceans or fish, and the ecological conditions that support their abundance and distribution directly influence parasite transmission. Consequently, the range and composition of a host’s diet, shaped by habitat-specific resources, affect the number and diversity of endoparasites it harbours. Therefore, environmental characteristics, such as water quality and temperature, can explain the presence of a particular parasite in one host population and its absence in another3.
Seabirds are significantly affected by anthropogenic activities, particularly contamination from urban and industrial waste, which disrupts prey availability and trophic dynamics4,5,6,7. Furthermore, human-driven habitat modifications have promoted the expansion of gull populations8 facilitated by their trophic plasticity, which allows them to exploit alternative food sources such as fishery discards5,9 and garbage in landfills or open dumps10,11.
Fishing is one of the main economic activities in northern Patagonian gulfs. As a result, large amounts of waste, mainly shrimps (Crustacea: Decapoda), are disposed of in open garbage dumps such as the municipal bowls in Puerto Madryn city, Chubut, Argentina. Inadequate waste management generates unpleasant odors and leads to an increase in insects and rodents, affecting the quality of life of local residents (Pers. Observ.). In addition, a “free” food source is created for gulls, favouring their population growth, with adverse environmental effects7.
Among seabirds, the kelp gull Larus dominicanus Lichtenstein (Laridae) is widespread in the southern hemisphere and includes subantarctic islands and the Antarctica Peninsula12. In Argentina, this seabird breeds along the coast and inland environments13. They are generalist and opportunistic predators, feeding mainly on fish and intertidal invertebrates14. The exponential increase in the kelp gull population in northern and central Patagonia is believed to result from the plasticity of their feeding behavior, particularly in coastal areas with a relatively high availability of anthropogenic food in the form of fishery discards and urban waste13. This seabird species is listed as least concerning by the IUCN Red List of Threatened Species15.
One of the consequences of the increased number of kelp gulls in this area is their negative interaction with the southern right whale (Eubalaena australis) during its breeding season in Península Valdés, Chubut Province, Argentina. The skin and cyamides (Crustacea: Cyamidae) released by whales during swimming or jumping constitute food for kelp gulls16. However, since the 1970 s, there have been reports of kelp gulls attacking southern right whales and tearing pieces of skin and blubber from their backs16,17,18. The avoidance reactions of whales have a significant effect on their behavior and possibly on their distribution19,20. Recent studies have shown that stress due to injuries and increased energy requirements caused by gull harassment can contribute to the death of calves in this population21,22. In this context, the question arises as to whether gull attacks are merely aggressive behavior or whether whales are a real food source for these birds, resembling micropredation as defined by Piotto et al.23.
The parasite fauna of a host population is recognized to provide valuable insights into the position of the host within the food web and its involvement in various predator‒prey relationships24. In aquatic birds, the occurrence of a more diverse and richer helminth community indicates the consumption of a wider variety of natural prey items, whereas a less diverse parasite community is related to a restricted and altered diet. For example, Diaz et al.25 conducted a study on a kelp gull population in the intertidal zone of Península Valdés, Argentina. These findings suggest that the parasitic community of kelp gulls reflects a natural dietary pattern that includes a wide variety of natural prey. These findings are consistent with the concept of an interactive parasitic community (i.e., high species richness, species interactions), as described by Holmes and Price26 and Kennedy et al.27. On the other hand, studies on gulls that have altered their feeding behavior and use landfills or garbage dumps as a food source have shown that the parasitic community is depauperate (e.g28,29,30)
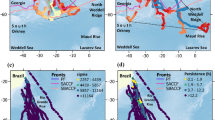
This study aimed to analyse the composition and structure of helminth communities in two kelp gull groups with different trophic behaviors along the northern Patagonian coast; one group pecked on whales’ backs, whereas the other fed on fishing discards. The results were compared with those previously reported for kelp gulls feeding on natural prey in the Península Valdés area25 (Fig. 1).
Given that most marine helminths are transmitted through trophic interactions, with invertebrates typically serving as intermediate hosts, the feeding behavior of kelp gulls plays a crucial role in their parasite community structure. Compared with those foraging in the intertidal zone, kelp gulls that attack whales or feed on fishery discards are less likely to consume these intermediate hosts. Consequently, these gulls are expected to present lower parasite abundance, prevalence, intensity, and/or richness. By exploiting a specialized food source (whale skin or blubber), they reduce their exposure to marine helminths, ultimately leading to lower parasite diversity.
Results
A total of 95 kelp gulls were analysed, 92.6% (88) of which were parasitized by at least one helminth species. Digenean trematodes represented the most diverse group of parasites, with 13 identified taxa. Table 1 shows the composition of helminth communities from the three groups of kelp gulls studied with different trophic behaviors. The parasitic richness was lower (13) in the group of gulls that pecked whales back than in the other two groups (feeding on fishing discard and natural trophic prey).
Kelp gull whales back (KGW)
A total of 2,373 helminths belonging to 13 parasite taxa (two cestodes, seven trematodes, three nematodes and one acanthocephalan) were found. Among the total gulls examined (N = 55), 48 were parasitized (P = 87.3) by at least one of the identified taxa (MI = 49, MA = 43). The most prevalent group was the cestodes, followed by nematodes, trematodes and acanthocephalans (see values in Table 1). Trematodes were the most abundant parasite group, with Microphallidae exhibiting the highest MI and MA, represented by three species: Maritrema madrynense Diaz & Cremonte, 2010; Maritrema formicae Diaz, Gilardoni & Cremonte, 2012; and Odhneria odhneri Travassos, 1921 (see Table 1). The site of infection, P, MI, and MA of each taxon are indicated in Table 1, and Fig. 2 illustrates the parasite diversity found. Although the component community comprised 13 taxa, the richness of the infracommunities varied between one and six parasite species. A total of 26 (47.2%) of the hosts were infected by only one or two parasite species (Fig. 3).
Parasite diversity of kelp gulls (Larus dominicanus) with different trophic behavior in the northern Patagonian coast, Argentina. a = Tetrabothrius (Culmenammniculus) cylindraceus, b = Anomotaenia dominicana, c = Maritrema madrynense, d = Levinseniella sp., e = Odhneria odhneri, f = Gymnophallus australis, g = Marinabilharzia patagonense, h = Stephanoprora podicipei, i = Himasthla escamosa, j = Parorchis trophoni, k = Brachylecithum sp., l = Renicola sp., m = Eucoleus sp., n = Cosmocephalus obvelatus, o = Paracuaria adunca, p = Profilicollis chasmagnathi. a, b,c, e,g, h,i, j,k, l,m = stained samples under a light microscope; d, f = drawings; n, p = scanning electron microscope. Maritrema formicae, Bartolius pierrei, Diplostomum sp. and Corynosoma sp. are not illustrated.
Kelp gull fishing discards (KGF)
A total of 2,679 parasites belonging to 17 taxa (two cestodes, 11 trematodes, three nematodes and one acanthocephalan) were identified. All gulls examined (N = 40) were parasitized by at least one of the identified taxa (P = 100, MI = 67, MA = 67). Trematodes were the most prevalent group, followed by cestodes, nematodes and acanthocephalans (see values in Table 1). Among the trematodes of the family Microphallidae, 4 species (M. madrynense, M. formicae, Levinseniella sp. and O. odhneri) and trematodes of the family Gymnophallidae, represented by one species (Gymnophallus australis Szidat, 1962), had the highest MI and MA. The site of infection, P, MI, and MA of each taxon are indicated in Table 1, and Fig. 2 illustrates the parasite diversity found. Although the component community comprised 17 taxa, the richness of the infracommunities varied between one and 11 parasite species. A total of 9 (22.5%) of the hosts were infected by three parasite species (Fig. 3). Importantly, nematode larvae (family Anisakidae) were found in both studied groups of kelp gulls. Owing to their larval stage and the fact that they do not develop into adults, they were not considered part of the helminthic community and were therefore excluded from the comparative analysis.
Comparison of parasite communities
The component communities of KGW, KGF and KGN (kelp gull natural feeding behavior) presented similar richness (13, 17 and 17, respectively). Although fewer hosts were analysed (55, 40 and 28, respectively), the total abundance of parasites reported by Diaz et al.24 (KGN) was greater than those reported by KGW and KGF (i.e., 89,726 vs. 2,373 and 2,679, respectively). Importantly, one KGN sample, which presented the highest parasite abundance, was excluded from the analysis, as noted by Diaz et al.25. At the infracommunity level, KGF and KGN presented similar richness (from one to 11 and from one to 9, respectively), but KGW presented the lowest richness (from one to six). The main difference in the richness of the observed communities was that KGW (54%) was parasitized by one or two taxa, while most KGF hosts (23%) were parasitized by three parasite taxa, and KGN had the greatest number of hosts (29%) parasitized by five parasite taxa (Fig. 3). The analysis of infracommunity richness revealed significant differences (F = 14.152, num df = 2.000, denom df = 61.469, P < 0.001). Post hoc comparisons revealed significant differences between KGW and KGN (P < 0.001) and between KGF and KGW (P < 0.001), whereas no significant differences were detected between KGF and KGN (P = 0.56).
Parasite infracommunity richness in the three kelp gull groups with different trophic behaviors on the northern Patagonian coast. Boxplots represent the number of parasite species (S = richness) parasitizing each host within the different kelp gull groups. The same asterisk color corresponds to a significant difference (pairwise t test with Holm post hoc methods, P < 0.05). KGW = kelp gull pecking whales; KGF = kelp gull feeding on fishing discards; KGN = kelp gull feeding on natural prey.
When statistical comparisons were possible among parasitic species present in all three gull groups and infecting five or more hosts in each group (Table 1), differences were found only in the prevalence of Paracuaria adunca (Creplin, 1846) (Nematoda) (X-squared = 15.78, df = 2, P < 0.001), with a significant difference between KGW and KGN (P < 0.001) and between KGF and KGN (P = 0.001). There was no difference between KGW and KGF. The other taxa that could be compared showed no differences in their prevalence: Anomotaenia sp. (Cestoda) (X-squared = 3.96, df = 2, P = 0.14), Tetrabothrius (Culmenamniculus) cylindraceus Rudolphi, 1819 (Cestoda) (X-squared = 0.73, df = 2, P = 0.69), Microphallidae (Trematoda) (X-squared = 2.27, df = 2, P = 0.32) and Parochis trophoni Diaz, Capasso, Gilardoni, Lorenti, Tkach & Cremonte, 2023 (Trematoda) (X-squared = 3.86, df = 2, P = 0.14).
In terms of the mean intensity, there was no difference between the nematode P. adunca in the three groups of kelp gulls analysed (F = 1.62, num df = 2, denom df = 35, P = 0.21); the cestode Anomotaenia sp. differed (F = 4.53, num df = 2.0, denom df = 29.851, P = 0.019), with differences between KGW and KGN (P < 0.001), and between KGF and KGN (P < 0.001), but there was no difference between KGW and KGF; the cestode T. (C.) cylindraceus showed no difference in the general test (F = 3.06, num df = 2.0, denom df = 17.818, P = 0.07), and the trematodes Microphallidae and P. trophoni showed no difference (F = 2.8, num df = 2, denom df = 44, P = 0.06; F = 0.67, num df = 2, denom df = 35, P = 0.51, respectively).
Discussion
The present study describes the parasite communities of two groups of kelp gulls with different trophic behaviors on the northern Patagonian coast: one group pecked whales back (KGW), while the other group fed on fishing discards (KGF). Additionally, the parasite communities of these two groups were compared with those reported by Diaz et al.25 in which a kelp gull group was fed natural prey (KGN).
In the present research, variations in the composition of parasite communities were found, indicating a lower consumption of natural prey items in groups of kelp gulls with altered feeding behaviors, especially those that peck on whales. This is evidenced by the lower parasite abundance and mean intensity of infection in KGW and KGF than in KGN, as well as a decrease in parasite richness at both the component community and infracommunity levels in KGW compared with those in KGF and KGN. Figure 3 shows the distribution of the parasite richness of the infracommunities, indicating a reduction in the consumption of intertidal prey in kelp gulls with altered feeding behaviour. This suggests that each gull within the observed population prey primarily on the available trophic element and exhibits selective feeding behavior that is consistent across the studied group.
In addition, the prevalence and mean intensity were significantly different for some parasite taxa, as expected (Table 1). The observed difference in the prevalence of the nematode Paracuaria adunca and in the mean intensity of the cestode Anomotaenia sp. suggests lower fish consumption in KGW and in KGF than in KGN. Although no differences were found in the mean intensity of the cestode Tetrabothrius (C.) cylindraceus among kelp gull groups, the value in KGW is notably lower than the mean intensity in KGN (see Table 1). The nematode Eucoleus sp. parasitized fewer than five hosts in the KGF and KGW groups, whereas only a few immature specimens were reported in the KGN group. Although comparisons were not possible, the mean intensity of Cosmocephalus obvelatus (Creplin, 1825) was lower in KGW than in KGN, which also suggests reduced natural fish consumption. Similarly, the mean intensity of the trematode microphallids and gymnophallids was greater than 1,000 in the KGN, whereas it was much lower in the KGW and KGF, although the difference was not statistically significant. This suggests a lower consumption of the crab Cyrtograpsus altimanus Rathbun and mussels (bivalvs), which are the respective intermediate hosts for both species of trematodes31,32. In particular, the absence of the trematode Bartolius pierrei Cremonte, 2001, in the KGW and KWF would indicate less or no consumption of Darina solenoides King & Broderip, the clam that serves as an intermediate host for this gymnophallid species33. The absence of trematodes from the family Echinostomatidae (Stephanoprora podicipei Etchegoin & Martorelli, 1997 and Himasthla escamosa Diaz & Cremonte, 2004) in KGW suggests a decrease in the consumption of their second intermediate hosts, such as some gastropods, bivalves, crustaceans or fish34,35.
Although there was no significant difference, the lower mean intensity of the trematode Parorchis trophoni in KGW and KGF than in KGN suggests a lower consumption of shelled invertebrates such as molluscs or crabs, on which the metacercariae encyst36. Since the trematode Brachylecithum sp. uses insects such as beetles (Coleoptera) as intermediate hosts, it is not surprising that only two specimens were counted in one infected gull in KGW, whereas 23 and 17 specimens were found in KGF and KGN, respectively. These findings indicate that insects are not preferred when kelp gulls feed on whales. The mean intensity of the acanthocephalan Profilicollis chasmagnathi (Holcman-Spector, Mañé-Garzón & Dei-Cas, 1977) was lower in KGW than in the other two groups, suggesting that this group consumes fewer crustaceans, such as the crab C. altimanus, than does KGN37.
The trematodes Marinabilharzia patagonense Lorenti, Brant, Gilardoni, Diaz & Cremote, 2022 and Renicola sp. were not detected in any of the groups of kelp gulls analysed. This could be due to errors in the fixation and processing of the KGN (J. I. Diaz pers. com.). Marinabilharzia patagonense is a thin and elongated worm species that parasitizes the blood vessels of the gut mesentery. It is only visible in fresh organs, and obtaining entire samples is particularly difficult38. Similarly, Renicola sp. parasitizes the kidneys and is difficult to find if the organ is not immediately processed fresh. Figure 4 illustrates the amount of natural prey items consumed by the individual groups of kelp gulls studied (KGN, KGF, and KGW) according to the composition and structure of their helminth communities.
A nonspecific, schematic representation of the amount of natural items consumed by the different kelp gull groups on the basis of the composition and structure of their helminth communities. The large blue circle indicates the availability of natural items (a general representation of major intermediate host groups). The size of the circles represents the amount of natural prey consumed according to the parasitological results.
Previous studies on gulls attacking whales could not confirm whether this behavior represents the trophic specialization of a particular gull population39. This means that any kelp gulls arriving in the area can learn and attack whales. A recent study demonstrated that kelp gull attacks increased from 2005 to 2019, contributing to calf deaths in Península Valdés23. The available parasitological results indicate that gulls not only attack, harass, and injure whales during their breeding season. They also use their skin and blubber as a food source (micropredation), which implies a reduction in their consumption of prey from the intertidal zone.
The results obtained in this study agree with those reported by Labriola and Suriano29who reported a low parasite richness (S = 8) for kelp gulls feeding at a rubbish dump in Mar del Plata city, Buenos Aires Province. Similarly, Lorenti et al. (unpublished data) studied a kelp gull population frequenting a household waste landfill in Ensenada city, Buenos Aires Province, and their observations also revealed low parasite richness (S = 10). These observations are consistent with studies on gulls of the genus Larus in Europe. For example, Bosch et al.28 reported ten taxa in yellow-legged gulls (Larus michaellis, Naumann), describing a depauperate parasitic community, which they attributed to the dietary reliance of the gulls on a rubbish dump of urban waste and fishing discards. Hervias-Parejo et al.30 studied another colony of yellow-legged gulls in the southern Mediterranean and reported a low specific richness (S = 10), attributing this value to the use of landfills as a food source.
In an ecosystem where natural trophic relationships are in equilibrium, a rich and diverse parasite community acts as a natural control, limiting the abundance of certain parasite species and maintaining their impact at a tolerable level for gulls. In contrast, a reduction in the consumption of natural prey in altered environments creates available parasite niches, increasing the susceptibility of birds to new parasite infections, which can be pathogenic40. Moreover, the lowest parasite diversity has significant implications for the intertidal ecosystems where gulls reside. For example, it can reduce the availability of larvae, which serve as prey for other invertebrates and play important roles in the dynamics of invertebrate hosts. This reduction may lead to cascading effects throughout the ecosystem40,41.
In summary, this study emphasizes the importance of parasites as integral components of ecosystem biodiversity and highlights their utility as biological and trophic markers. Additionally, the findings underscore the importance and urgency of implementing measures to control urban waste and fishery waste in the coastal area of Puerto Madryn, as these factors contribute to the growth of kelp gull populations and, consequently, to increased attacks on southern right whales along the northern Patagonian coast.
Methods
Fifty-five (55) kelp gulls that peck on whales’ backs (KGW) were captured in Puerto Pirámides, Península Valdés, Chubut, Argentina (42°34’29” S, 64°16’46” W) (Fig. 1), during October 2012, October 2013 and September 2015 through the provincial action plan implemented to mitigate the interactions between gulls and whales. Forty (40) kelp gulls were captured in the fishing garbage dump (Municipal Bowls) of Puerto Madryn, Chubut, Argentina (42°43′52″ S, 65°8′16″ W) (Fig. 1) during the autumn‒winter and spring seasons of 2016, 2017, 2018 and 2019 while feeding on fishing discard (KGF).
All the gull samples were collected during dedicated culling campaigns under permits issued by the provincial government (Decreto N°1106; Disposiciones N°15/16, N°60/17, N°03/18 and N°78/19) and under the supervision of the authors. No kelp gulls were gifted or purchased, and no experiments on live vertebrates were performed. Seabird carcasses were sent to the parasitology laboratory (LAPA-IBIOMAR-CONICET) and examined for endoparasites. The gastrointestinal tract was divided into the esophagus, stomach and intestine. The latter was divided into three equal sections (anterior, middle, and posterior). The body cavity and all the viscera were also examined under a stereomicroscope (Leica M60, Leica DM6). Helminths recovered from each section were counted and preserved in 96% ethanol for future molecular studies or fixed with 10% hot formalin and preserved in 70% ethanol for morphological studies. For this purpose, cestodes and trematodes were stained with hydrochloric or acetic carmine, dehydrated in a graded ethanol series, cleared in methyl salicylate and mounted in Canada balsam. Nematodes and acanthocephalans were cleared in lactophenol or 25% glycerin alcohol. All the samples were examined via light microscopy (Olympus BX51® and Leica DMC 280®). Drawings were made with a lucid camera, and photographs were taken via digital cameras attached to the aforementioned microscopes. In addition, some samples were dried via the critical point method for scanning electron microscopy (JEOL 6360LV®, JEOL, Tokyo, Japan) and photographed. The taxonomic identifications were made following42,43,44,45,46,47,48,49 and the specific bibliography. The terms prevalence (P), mean intensity (MI), mean abundance (MA), and richness (S) of the component community and infracommunity were calculated and interpreted according to Bush et al.50.
For comparative analysis, we used data obtained from a kelp gull population with natural trophic behavior (KGN)25,51. The P and MI values of KGW, KGF, and KGN were compared. To determine significant differences, the proportions differences test and one-way test were employed as general tests for P and MI, respectively. After detecting differences with these general tests, the Benjamini‒Hochberg and Holm methods were applied as post hoc analyses to determine the specific pairwise differences in P and MI, respectively. For this purpose, we considered only those parasite species that were present in the three gull groups studied and that parasitized more than five hosts. Species belonging to the families Trematoda Microphallidae and Gymnophallidae were considered together, and Trematoda Marinabilharzia patagonense was considered only for P, as it could not be counted. Furthermore, a one-way test was employed to compare the infracommunity richness, and post hoc analyses (pairwise t tests with Holm methods) were applied to determine the specific pairwise differences. Tests were performed via RStudio version 4.3.252 and all P values < 0.05 were considered significant.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on responsable request.
References
Poulin, R. & Morand, S. The diversity of parasites. Q. Rev. Biol. 75, 277–293 (2000).
Timi, J. T. & Poulin, R. Why ignoring parasites in fish ecology is a mistake. Int. J. Parasitol. 50, 755–761 (2020).
Poulin, R. & Morand, S. Parasite Biodiversity. Smithsonian Institution Books 216 pWashington D.C. (2004).
Bost, C. A. & Le Maho, Y. Seabirds as bioindicators of changing marine ecosystems: new perspectives. Acta Oecologica –. Int. J. Ecol. 14, 463–470 (1993).
Bertellotti, M. & Yorio, P. Spatial and Temporal patterns in the diet of the Kelp gull in patagonia. Condor 101, 790–798. https://doi.org/10.2307/1370066 (1999).
Parsons, M. et al. Seabirds as indicators of the marine environment. ICES J Mar. Sci. 65, 1520–1526. https://doi.org/10.1093/icesjms/fsn155 (2008).
Frixione, M. G., D’Amico, V., Adami, M. A. & Bertellotti, M. Urbanity as a source of genotoxicity in the synanthropic Kelp gull (Larus dominicanus). Sci. Total Environ. 850, 157958. https://doi.org/10.1016/j.scitotenv.2022.157958 (2022).
Anderson, J. G. T., Shlepr, K. R., Bond, A. L. & Ronconi, R. A. Introduction: a historical perspective on trends in some gulls in Eastern North america, with reference to other regions. Waterbirds 39, 1–9. https://doi.org/10.1675/063.039.sp106 (2016).
Yorio, P., Bertellotti, M., Gandini, P. & Frere, E. Kelp gulls Larus dominicanus breeding on the Argentine coast: population status and relationship with coastal management and conservation. Mar. Ornithol. 26, 11–18 (1998).
Bertellotti, M., Yorio, P., Blanco, G. & Giaccardi, M. Use of tips by nesting Kelp gulls at a growing colony in patagonia. J. Field Ornithol. 72, 338–348. https://doi.org/10.1648/0273-8570-72.3.338 (2001).
Giaccardi, M. & Yorio, P. Temporal patterns of abundance and waste use by Kelp gulls (Larus dominicanus) at an urban and fishery waste site in Northern coastal patagonia, Argentina. Ornitol Neotrop. 15, 93–102 (2004).
Burger, J., Gochfeld, M., Garcia, E. F. J. Kirwan, G. M. Kelp Gull(Larus dominicanus)version 1.0. In del Hoyo J., Elliott A., Sargatal J., Christie D. A., de Juana E. (eds) Birds of the World Cornell Lab of Ornithology, Ithaca, NY, USA (2020)https://doi.org/10.2173/bow.kelgul.01
Yorio, P., Olinto Branco, J., Lenzi, J., Luna-Jorquera, G. & Zavalaga, C. Distribution and trends in Kelp gull (Larus dominicanus) coastal breeding populations in South America. Waterbirds 39, 114–135 (2016).
Lisnizer, N. et al. Winter consumption of the introduced green crab Carcinus maenas by Kelp gulls Larus dominicanus. N Z. J. Mar. Freshw. Res. 58, 635–648 (2024).
, B. L. I. Larus dominicanus. IUCN Red List. Threatened Species. https://doi.org/10.2305/IUCN.UK.20182.RLTS.T22694329A132542863.en (2018). Accessed on 23 July 2024.
Fazio, A., Bertellotti, M. & Villanueva, C. Kelp gulls attack Southern right whales: a conservation concern? Mar. Biol. 159, 1981–1990 (2012).
Rowntree, V. et al. Increased harassment of right whales (E. australis) by Kelp gulls (L. dominicanus) at Península valdés, Argentina. Mar. Mamm. Sci. 14, 99–115 (1998).
Frixione, M., Casaux, R., Villanueva, C. & Alarcón, P. A recently established Kelp gull colony in a freshwater environment supported by an inland refuse dump in patagonia. Emu 112, 174–178 (2012).
Sironi, M., Rowntree, V., Snowdon, C., Valenzuela, L. & Marón, C. Kelp Gulls (Larus dominicanus) Feeding on Southern Right Whales (Eubalaena australis) at Península Valdés, Argentina: Updated Estimates and Conservation ImplicationsSC/61/BRG19 (Scientific Committee of International Whaling Commission, 2008).
Fazio, A., Argüelles, B. & Bertellotti, M. Change in Southern right Whale breathing behavior in response to gull attacks. Mar. Biol. 162, 267–273 (2015).
Fernández Ajó, A. A. et al. Lifetime glucocorticoid profiles in Baleen of right Whale calves: potential relationships to chronic stress of repeated wounding by Kelp gulls. Conserv. Physiol. 6, coy045 (2018).
Fernández Ajó, A. A. et al. Retrospective analysis of the lifetime endocrine response of Southern right Whale calves to gull wounding and harassment: a Baleen hormone approach. Gen. Comp. Endocrinol. 296, 113536 (2020).
Piotto, M. et al. Seabird attacks contribute to calf mortality in a Whale population. Mar. Ecol. Prog Ser. 746, 1–16 (2024).
Marcogliese, D. J. Parasites: small players with crucial roles in the ecological theater. EcoHealth J. 1, 151–164 (2004).
Diaz, J. I., Cremonte, F. & Navone, G. T. Helminths of the Kelp gull, Larus dominicanus, from the Northern Patagonian Coast. Parasitol. Res. 109, 1555–1562 (2011).
Holmes, J. C. & Price, R. D. Communities of Parasites. In Community Ecology: Patterns and Processes (eds. Andersen & Kikkawa) 87–213Blackwell Scientific Publications, Oxford, (1986).
Kennedy, C. R., Bush, A. O. & Aho, J. M. Patterns in helminths communities: why are birds and fish different? Parasitol 93, 205–215 (1986).
Bosch, M., Torres, J. & Figuerola, J. A helminth community in breeding Yellow-legged gulls (Larus cachinnans): pattern of association and its effect on host fitness. Can. J. Zool. 78, 777–786 (2000).
Labriola, J. & Suriano, D. M. Community structure of parasitic helminths of birds of the genus Larus from Mar Del plata, Argentina. Vie Et Milieu. 51, 67–76 (2001).
Hervías-Parejo, S. H. et al. Parasitic fauna of a yellow-legged gull colony in the Island of Escombreras (South-eastern Mediterranean) in close proximity to a landfill site: potential effects on cohabiting species. Acta Parasitol. 60, 290–297 (2015).
Cremonte, F., Vázquez, N. & Ituarte, C. The development of Gymnophallus australis szidat, 1962 (Digenea: Gymnophallidae) from the Patagonian Coast (Argentina) from metacercaria to adult, with an amended diagnosis of Gymnophallus odhner, 1905. Syst. Parasitol. 69, 23–31 (2008). (2008).
Diaz, J. I. & Cremonte, F. Development from metacercaria to adult of a new species of Maritrema (Digenea: Microphallidae) parasitic in the Kelp gull, Larus dominicanus, from the Patagonian coast, Argentina. J. Parasitol. 96, 740–745 (2010).
Cremonte, F. Life cycle and geographic distribution of the gymnophallid Bartolius pierrei (Digenea) on the Patagonian coast, Argentina. J. Nat. Hist. 38, 1591–1604 (2004).
Ostrowski de Nuñez, M., Flores, V., Viozzi, G. & Kreiter, A. Stephanoprora Uruguayense Holcman-Spector et olagüe, 1989 (Digenea, Echinostomatidae) from argentina, and comments on species of Stephanoprora from birds of the Neotropical region. Acta Parasitol. 49, 292–299 (2004).
La Sala, L. F., Martorelli, S. R., Alda, P. & Marcotegui, P. Some digeneans from olrog’s gull Larus atlanticus olrog, 1958 (Aves: Laridae) from the Bahía Blanca estuary, Argentina. Comp. Parasitol. 76, 113–116 (2009).
Diaz, J. I. et al. Complete life cycle of Parorchis trophoni sp. Nov. (Digenea: Philophthalmidae) from the Southwestern Atlantic coast, argentina, revealed by morphological and molecular data. Polar Biol. 46, 737–748 (2023).
Lorenti, E., Rodríguez, S. M., Cremonte, F., D’Elía, G. & Diaz, J. I. Life cycle of the parasite Profilicollis chasmagnathi (Acanthocephala) on the Patagonian Coast of Argentina based on morphological and molecular data. J. Parasitol. 104, 479–485 (2018).
Lorenti, E., Brant, S. V., Gilardoni, C., Diaz, J. I. & Cremonte, F. Two new genera and species of avian schistosomes from Argentina with proposed recommendations and discussion of the polyphyletic genus Gigantobilharzia (Trematoda, Schistosomatidae). Parasitol 149, 675–694 (2022).
Fazio, A. Alimentación De Gaviotas Cocineras (Larus dominicanus) De Piel Y Grasa De Ballenas Francas Del Sur (Eubalaena australis) En Península Valdés (FCEyN, UBA,, 2012).
Mouritsen, K. N. & Poulin, R. Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitol 124, 101–117 (2002).
Fredensborg, B. L., Mouritsen, K. N. & Poulin, R. Impact of trematodes on host survival and population density in the intertidal gastropod Zeacumantus subcarinatus. Mar. Ecol. Prog Ser. 290, 109–117 (2005).
Yamaguti, S. Systema Helminthum Vol. III: the Nematodes of Vertebrates (Interscience, 1961).
Yamaguti, S. Systema Helminthum. Parte V: AcanthocephalaInterscience Publishers), 423 (New York, 1963).
McDonald, M. E. Key to trematodes reported in waterfowl. Resource Publication US Fish. Wildl. Service. 142, 1–157 (1981).
Khalil, L. F., Jones, A. spsampsps Bray, R. A. Keys to the cestode parasites of vertebratesCAB international (1994).
Anderson, R. C. Nematode Parasites of Vertebrates: their Development and Transmission, 650 (CABI Publishing Wallingford, 2000). Oxon (UK)).
Anderson, R. C., Chabaud, A. G. spsampsps Willmott, S. (eds) Keys To the Nematode Parasites of Vertebrates: Archival Volume463 (CABI Publishing, 2009).
Gibson, I. D., Jones, A. spsampsps Bray, R. A. Keys To the Trematoda, Vol 1 (CABI. The Natural History Museum, 2002).
Amin, O. M. Classification of the Acanthocephala. Folia Parasitol. 60, 273–305 (2013).
Bush, A. O., Lafferty, K. D., Lotz, J. M. & Shostak, A. W. Parasitology Meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 83, 575–583 (1997).
Diaz, J. I. Las comunidades parasitarias como expresión de distinto comportamiento trófico en aves del Mar Argentino (Facultad de Ciencias Naturales y Museo, 2006).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2023). https://www.Rproject.org/(
Acknowledgements
The authors are grateful to all their colleagues at CEPAVE and LAPA for their field and laboratory assistance. We also thank Mariano Dorrego, María Laura Agüero, Florencia Soto, María Irazábal and Matías di Martino for their assistance in the Municipal Bowls and Andrés da Costa Faro, María Laura Agüero and Edgardo Kabe Solas for their graphical contributions. We thank the flora and fauna secretary and the tourism and protected areas secretary of Chubut Province for granting the necessary permits.
Funding
This work was supported by CONICET to J.I.D. (PIP 0698) and Universidad Nacional de La Plata to J.I.D. (N859 and N996).
Author information
Authors and Affiliations
Contributions
L.E. and D.J.I. wrote the main manuscript text. L.E., D.J.I., C.F. and N.G. participated in conceptualization, methodology and investigation. L.E. prepared Figs. 1, 2 and 3. D.J.I. prepared Figure 4. L.E. prepared Table 1. M.G. provided statistical analysis. B.M. supplied an important portion of the studied material. All the authors reviewed and edited the main manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lorenti, E., Cremonte, F., Minardi, G. et al. Trophic behavior and parasite communities in kelp gulls from the northern Patagonian coast, Argentina. Sci Rep 15, 20422 (2025). https://doi.org/10.1038/s41598-025-07544-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07544-y