Abstract
This study examines the associations between sensory responsiveness levels and biological age among midlife adults, providing insights into the aging process. A cross-sectional study was conducted from 2020 to 2021 on a subset of the Jerusalem Perinatal Study in Israel, including 96 midlife adults (aged 45.65 ± 0.66, 49% women). Biological age was estimated using physiological and blood biomarkers, following the Klemera-Doubal method. The Sensory Responsiveness Questionnaire Scale and the Social Engagement and Activities Questionnaire were employed, along with physical capacity measurements such as muscle strength, cardiorespiratory fitness, and gait analysis. The hypothesis suggested that levels of sensory responsiveness (high or low) would be associated with biological age. Results indicated that low sensory responsiveness was positively associated with older biological age (adjusted R²=0.25, p < 0.01), after controlling for age, sex, social engagement, education and physical capacity. Conversely, high sensory responsiveness showed no significant correlation with biological age. These findings suggest that sensory responsiveness may serve as a potential marker for biological age during midlife and should be considered when addressing early signs of aging, thereby contributing to a deeper understanding of the biological mechanisms underlying aging and age-related diseases.
Similar content being viewed by others
Introduction
Forecasts indicate increasing burdens of disease and disability over time, due to the progressive aging of the human population around the world1. Studies, therefore, place great emphasis on identifying aging-related hallmarks that can serve as risk factors for accelerated aging to develop and implement preventative intervention programs for at-risk populations2,3. Unfortunately, the literature body is largely focused on older adult population, typically those aged 65 and older, where various hallmarks of aging, such as genomic instability, telomere attrition, and cellular senescence, have been more clearly defined and linked to aging processes4,5. However, there remains a notable gap in the literature regarding the identification of such aging-related hallmarks for midlife individuals, specifically those in the age range of 40 to 64 years old5.
Biological age reflects the body’s overall status and inter-individual differences in aging6. This approach for age assessment better estimates health-related status than chronological age alone. Biological age is affected, to some extent, by genetic characteristics, but more significantly by the frequency and quality of healthy behaviors such as conducting regular physical activity that lead to improve physical capacity (i.e., a collection of markers associated with function and health), sleep, and social engagement7,8,9, as well as mental health (e.g., anxiety, depression)10. Healthy behaviors and effective emotional regulation have been reported to influence telomerase activity and control obesity, insulin resistance, and cardiovascular health11, and these factors significantly impact inflammation and oxidative stress, crucial elements in the modulation of aging and the prevention of age-related diseases10,12,13.
Healthy behaviors are byproducts of responses to perceived everyday environmental (external world) and internal (body) stimuli14,15. The process in which multi-sensory stimulation is translated to a robust perception of the world and body is termed multi-sensory integration16, enabling an efficient response to a given situation while filtering out irrelevant stimuli17. Furthermore, modulating the type and intensity of responses across sensory systems supports the maintenance of appropriate arousal14.
Adaptive social and emotional behaviors, critical for performing everyday activities and maintaining quality of life, are significantly influenced by appropriate sensory responses16,18,19. These responses shape interactions with the world and influence both functional independence and life satisfaction20. While much research on sensory response alterations has established their impact on daily functioning, it has largely concentrated on specific populations, including those with sensory modulation disorders, the elderly, and individuals experiencing chronic pain or disability18,21,22.
While multi-sensory responsiveness is an individual trait17,23, two widely conserved response patterns have been identified and were found to affect daily activities, routines, and quality-of-life: Low Sensory Responsiveness denotes requiring more intense signals to elicit behavioral responses which may explain why individuals characterized by low sensory responsiveness can appear unmotivated, apathetic, or even socially disengaged as well as exhibit reduced bodily awareness17,20. High Sensory Responsiveness, on the other hand, is characterized by heightened arousal, and ordinary environments may be perceived as overwhelmingly14. High sensory responsiveness is expressed behaviorally by enhanced responses to non-painful sensory input, with behaviors ranging from aggression to avoidance. Emotional effects include irritability and difficulty with socialization, and individuals may also display a preference for control and rigidity20,22,24.
The magnitude of sensory responsiveness and its impacts have been studied across various domains, including psychological state25,26, social interactions27, personality traits28, and pain perception24, as well as in behavioral aspects such as sleep quality29, and gait15. These studies have primarily focused on children, adolescents, young adults, and older adults and typically study specific populations, particularly those with developmental disorders such as autism spectrum disorders, attention deficit hyperactivity disorder, and early-onset schizophrenia. There is, however, a knowledge gap surrounding how sensory responsiveness is associated with biological age in healthy midlife populations; filling this gap could offer crucial insights regarding the aging process and support ameliorating accelerated aging.
Thus, this study aims to examine the associations between sensory responsiveness and biological age in a midlife population of healthy individuals. We hypothesized that both types of sensory responsiveness, low and high, would be positively associated with an increase in biological age in midlife adults even.
while accounting for major covariates associated with biological age (e.g., chronological age, sex, education, social engagement, and physical capacity).
Methods
Participants and study design
This research focuses on a subset of adults from the Jerusalem Perinatal Study, which includes all births from 1974 to 1976 in West Jerusalem30. The selected subset for the current study includes participants who engaged in the latest follow-up examination at ages 42–45, from 2016 to 202131, and were willing to take part in this research extension.
Inclusion criteria were: (1) participation in Jerusalem Perinatal Study follow-up at age 45, (2) providing a fasting blood sample at that follow-up point, and (3) completion of a battery of tests for objective physical capacity assessments. The exclusion criterion was a failure to answer more than four questions on the sensory responsiveness questionnaire.
This extension study took place between November 2020 and November 2021, when the pool of possible volunteers was exhausted; each participant received general information on the study aims and provided informed consent before testing. Consenting participants were provided with a link to online, self-administered questionnaires and were asked to complete them over the subsequent 10 days.
The study was approved by the Ethics Committee of the Faculty of Social Welfare and Health Sciences at the University of Haifa (#407/20), and Hadassah Hospital Institutional Review Board (#10-01.04.05) provided ethical approval for the performed extension of the Jerusalem Perinatal Study. All performed procedures were in accordance with approved guidelines and regulations.
Measures
Dependent variable: biological age
To calculate participants’ biological age, we applied Klemera and Doubal’s Method32, which is considered a robust biological age evaluation methodology6, predicting individual differences in morbidity and mortality in midlife and older adults33. Estimated Klemera and Doubal biological age reflects the chronological age at which the participants’ biomarkers would match the mean in the reference sample. Klemera and Doubal’s method was implemented within the BioAge R Package34, utilizing the third United States Health and Nutrition Examination Surveys III35. To estimate Klemera and Doubal’s biological age, we used the following fasting blood serum and physiological biomarkers, collected in Jerusalem Perinatal Study follow-up: (1) glucose; (2) insulin; (3) high-density lipoprotein; (4) low-density lipoprotein; (5) triglycerides; (6) total cholesterol; (7) body mass index; (8) diastolic resting blood pressure; (9) systolic resting blood pressure; (10) resting heart rate; and (11) waist circumference. Although these biomarkers mainly relate to participants’ cardio-metabolic status, they represent a fair estimation of biological age36.
Independent variable: sensory responsiveness questionnaire intensity scale
Participants’ sensory responsiveness levels were assessed using the Sensory Responsiveness Questionnaire Intensity Scale37, a self-reporting questionnaire for identifying sensory modulation difficulties in adults through behavioral responses to daily sensations. The Sensory Responsiveness Questionnaire Intensity Scale includes 58 scenarios reflective of common daily situations, each focusing on a specific sensory input: vestibular, auditory, olfactory, visual, gustatory, and somatosensory, while avoiding scenarios involving pain. These scenarios elicit either a positive (hedonic) or negative (aversive) perception of the sensory experience. Participants are asked to evaluate the intensity of their reaction to each scenario—whether it elicits a pleasurable (indicative of low sensory responsiveness) or adverse response (indicative of high sensory responsiveness)—using a Likert-like scale that ranges from 1 (not at all) to 5 (very much so).
The questionnaire is subdivided to provide a separate score for each sensory responsiveness type: (1) high sensory responsiveness, determined via the Sensory Responsiveness Questionnaire-Aversive subscale score (32 items, e.g., eating crunchy foods bothers me); and (2) low sensory responsiveness, determined via the Sensory Responsiveness Questionnaire-Hedonic subscale score (26 items, e.g., I enjoy eating sour foods)38. The Sensory Responsiveness Questionnaire-Intensity Scale has been reported to have content, construct, and criterion validity; internal consistency (Cronbach’s α = 0.90–0.93); and test-retest reliability (r = 0.71–0.84; p < 0.01)37.
Covariates
The study included the following covariates: (1) chronological age; (2) sex; (3) education; (4) physical capacity; and (5) social engagement.
For physical capacity, we relied on previously performed analyses establishing a composite score for physical capacity, based on fifteen physical functional tests (e.g., muscle strength, cardiorespiratory fitness, and gait analysis) which was calculated according to the algorithm described previously39; the composite scores ranged from minimal physical capacity (0) to a maximum of 640. Social engagement was measured using the Social Engagement and Activities Questionnaire, a self-reporting questionnaire that identifies social groups and interpersonal interactions in adults41. The Social Engagement and Activities Questionnaire presents a set of 6 items. Respondents rank each item on a 7-point scale ranging from 1 (never) to 7 (more than twice a week). Ranking characterizes the intensity of their social engagement, which is a sum of questionnaire items.
Data analysis
Statistical analyses were performed with SPSS V.27.0 (IBM Corp, Armonk, NY). The p-value was set at p ≤ 0.05 for statistical significance. To assure adequate analysis, the descriptive characteristics of the variables were examined; all variables (e.g., biological age, low sensory responsiveness, high sensory responsiveness, physical capacity, and social engagement) were regarded as continuous and checked for normality. Bivariate correlations were examined with Pearson’s tests, and linear regression models were used to investigate associations between the various sensory responsiveness variables and biological age. These associations were examined after controlling for chronological age, sex, education, physical capacity, and social engagement. A missing value analysis was conducted to assess the randomness of any unknown values, confirming that the data were missing at random. Subsequently, a stochastic regression imputation was used to estimate missing values42.
Results
Study sample
In the current study, a total of 96 adults were included (47 women; see Fig. 1). Among these participants, four required data imputation: two participants were missing a single blood biomarker (fasting insulin levels) imputed by matching participants’ values with similar anthropometric characteristics (same gender, similar body mass index, and blood pressure). Two additional subjects were missing values in the Social Engagement and Activities Questionnaire; a missing value analysis was conducted to assess the randomness of these unknown values. 2% missingness was found in the Social Engagement and Activities Questionnaire (Little’s test for Missing Completely at Random43 : χ2 = 6.81, df = 5, p = 0.24). Thus, one round of imputation was applied for missingness below 5%44. Table 1 presents cohort demographics and descriptives of the main variables examined in this study. Sensitivity analysis between the most recent Jerusalem Perinatal Study follow-up cohort and included participants was conducted, demonstrating no significant differences between cohorts (see supplementary data, Table S1).
Association between high/Low sensory responsiveness and biological age
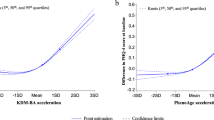
Bivariate Pearson’s correlations demonstrate a positive significant association between low sensory responsiveness and biological age (r = 0.27; p = 0.02); no correlation was found between high sensory responsiveness and biological age (r=−0.13; p = 0.21). To further examine the association between low and high sensory responsiveness and biological age, while controlling chronological age, physical capacity, education, sex, and social engagement, we used a hierarchical regression. Our analysis demonstrates that low sensory responsiveness was significantly associated with biological age (adjusted R2 = 0.25; p < 0.001), suggesting a positive relationship between low sensory responsiveness and older biological age (see Table 2, linear regression model). When examining the association of high SR with BA in a similar regression model, the contribution of high SR was statistically insignificant (B=−0.082, p = 0.38, 95% CI [−3.47, 1.32])
Discussion
This study aimed to examine the associations between sensory responsiveness and biological age in midlife adults, towards identifying aging-related hallmarks that may serve as risk factors for an accelerated biological aging process. Our hypothesis that both low and high sensory responsiveness would be associated with older biological age in midlife adults was partially supported. Our findings showed significant associations between low sensory responsiveness and older biological age, even after controlling for chronological age, sex, education, physical capacity, and social engagement. However, a similar link was not observed between high sensory responsiveness and biological age.
The association between sensory responsiveness and biological age has not yet been studied among healthy, midlife adults. To address this gap, we designed a cross-sectional study examining the relationship between these variables. While these factors likely influence each other bidirectionally45,46, we treated biological age as the dependent variable. Our primary research aim was to identify risk factors associated with accelerated aging that could inform the development of new, personalized interventions. This objective guided our analytical approach and supported our decision to examine sensory responsiveness as a potential predictor of biological age.
Several shared behavioral and physiological mechanisms may explain the link between sensory responsiveness and biological age. These mechanisms might encompass health-impeding behaviors such as heightened emotionality47,48,49, and increased pain sensitivity24,38. Indeed, extensive research links these mechanisms with low-grade systemic inflammation10,12,50,51, a biological marker associated with aging and included in the calculation of Klemera and Doubal’s biological age. This phenomenon, also referred to as “inflammaging,” is characterized by elevated levels of proinflammatory cytokines constantly maintaining low level activation of the immune system3, which in turn accelerates the aging process3,10. Our findings demonstrate a possible link between biological aging mechanisms and low sensory responsiveness.Low sensory responsiveness, namely the diminished perception of environmental stimuli and reduced awareness of surroundings, may result in missing critical information. This, in turn, can foster uncertainty in various situations, potentially leading to increased anxiety22, and compromised social interactions14. In cases of environmental uncertainty, crucial areas in the prefrontal cortex turn hypoactive and have been linked to disinhibition of the sympathoexcitatory circuits, essential in energy mobilization52. Under prolonged uncertainty, the system components develop excess wear and tear, termed allostatic load53, which is associated with overstimulation or aberrant activation of neural, neuroendocrine and neuroendocrine-immune mechanisms53, and has been linked to accelerated aging54. While research is limited, low sensory responsiveness has been previously linked with higher levels of somatization, negative self-esteem, distress, problems at work, social deviance, poignancy, and social introversion27,48.
Low sensory responsiveness has also been associated with unresponsiveness to bodily sensory stimuli, and thus individuals may overlook visceral or other physical signals (e.g., interoceptive, tactile, or pain stimuli) and ignore bodily hazards, possibly leading to health care neglect and posing physical health risks which in turn can affect biological age22. Indeed, we previously found that patients characterized by low sensory responsiveness had a higher probability of developing complex regional pain syndrome after limb injury.
Basic human functions, such as voiding the bladder and eating when hungry, are initiated by internal sensory signals, illustrating the fundamental role of the body’s sensory systems in everyday behaviors55. The viscerosensory system, which monitors autonomic, immunological, and metabolic processes within cortex regions like the insula, anterior cingulate cortex, and prefrontal cortex, is hypothesized to play a critical role in stabilizing allostatic effects through top-down projections to the brainstem areas56,57. This maintenance of internal physiological homeostasis is crucial for modulating the biological aging process. Our findings indicate a significant association between lower sensory responsiveness and older biological age, highlighting the crucial role of efficient interoceptive processing in aging.
Interoceptive coding, the top-down viscerosensory cortical projections to the brainstem, facilitates the anticipation of interoceptive input based on past experiences with similar demands14,56. This anticipatory mechanism is essential for effective physiological regulation and is intricately linked to the aging process, as evidenced by our findings that low sensory responsiveness correlates with an older biological age. Interoception, or the perception of bodily signals and states, enables the clustering of sensory experiences to extract meaningful information, influencing both bodily self-awareness and the perception of others22,49,58,59. This interoceptive awareness is foundational to overall health and well-being, affecting the aging process22. Ignoring bodily sensations can lead to chronic physiological imbalances, stressing bodily systems and potentially accelerating biological aging, thereby increasing health risks. This perspective could elucidate the observed non-significant association between high sensory responsiveness and accelerated aging in our study. Individuals with high sensory responsiveness are more attuned to their bodily needs, which could mitigate the risk of abnormal aging processes.
Several research limitations should be addressed. Although this study offers a temporal perspective of aging by estimating biological age from measures taken simultaneously, causal associations between low sensory responsiveness and biological age cannot be drawn. In addition, the relatively small sample size may limit the generalizability of our findings. Lastly, brain mechanisms such as electrophysiology were not tested, limiting our ability to understand this link’s neurobiological underpinnings deeply. These limitations highlight the preliminary nature of our findings and underscore the need for larger-scale, longitudinal investigations.
Conclusions
This study identifies low sensory responsiveness as a potential risk factor for accelerated biological aging in midlife adults. The significant association persisted after controlling confounding factors, suggesting low sensory responsiveness may directly influence biological aging rates. These findings have implications for both early detection and intervention: sensory responsiveness patterns could serve as non-invasive indicators of aging risk, while targeted sensory interventions might support healthy aging trajectories. Longitudinal research is needed to confirm these cross-sectional results and explore the bidirectional relationship between sensory responsiveness and aging processes. Despite limitations, our findings establish promising research directions for early detection of aging acceleration and development of preventive interventions.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are in controlled access in the Zenodo repository DOI 10.5281/zenodo.13797333.
References
Xi, J. Y., Lin, X. & Hao, Y. T. Measurement and projection of the burden of disease attributable to population aging in 188 countries, 1990–2050: A population-based study. J. Glob. Health 12, 04093. https://doi.org/10.7189/jogh.12.04093 (2022).
Jylhävä, J., Pedersen, N. L. & Hägg, S. Biological age predictors. EBioMedicine 21, 29–36. https://doi.org/10.1016/j.ebiom.2017.03.046 (2017).
Fougère, B., Boulanger, E., Nourhashémi, F., Guyonnet, S. & Cesari, M. Chronic inflammation: Accelerator of biological aging. J. Gerontol. A. Biol. Sci. Med. Sci. 72(9), 1218–1225. https://doi.org/10.1093/gerona/glw240 (2017).
Zhang, L. et al. Cellular senescence: a key therapeutic target in aging and diseases. J. Clin. Investig. 132(15), e158450. https://doi.org/10.1172/JCI158450 (2022).
Guerville, F. et al. Revisiting the hallmarks of aging to identify markers of biological age. J. Prev. Alzheimer’s Disease. 7, 56–64. https://doi.org/10.14283/jpad.2019.50 (2020).
Zhong, X. et al. Estimating biological age in the Singapore longitudinal aging study. Journals Gerontology: Ser. A. 75 (10), 1913–1920. https://doi.org/10.1093/gerona/glz146 (2020).
Lin, Y., Chen, Y., Tseng, Y., Tsai, S. & Tseng, Y. Physical activity and successful aging among middle-aged and older adults: a systematic review and meta-analysis of cohort studies,. Aging 12(9), 7704–7716. https://doi.org/10.18632/aging.103057 (2020).
Ryan, J., Wrigglesworth, J., Loong, J., Fransquet, P. D. & Woods, R. L. A systematic review and meta-analysis of environmental, lifestyle, and health factors associated with DNA methylation age. Journals Gerontol. - Ser. Biol. Sci. Med. Sci. 75 (3), 481–494. https://doi.org/10.1093/gerona/glz099 (2020).
Ng, T. P. et al. Socio-Environmental, Lifestyle, Behavioural, and Psychological Determinants of Biological Ageing: The Singapore Longitudinal Ageing Study. Gerontology 66(6), 603–613. https://doi.org/10.1159/000511211 (2020).
Squassina, A., Pisanu, C. & Vanni, R. Mood disorders, accelerated aging, and inflammation: is the link hidden in telomeres?. Cells 8(1), 52–52. https://doi.org/10.3390/cells8010052 (2019).
Schellnegger, M., Lin, A. C., Hammer, N. & Kamolz, L. P. Physical activity on telomere length as a biomarker for aging: a systematic review. Sports medicine-open 8(1), 111. https://doi.org/10.1186/s40798-022-00503-1 (2022).
Rajado, A. T. et al. How can we modulate aging through nutrition and physical exercise? An epigenetic approach. Aging (Albany NY) 15(8), 3191. https://doi.org/10.18632/aging.204668 (2023).
Harvanek, Z. M., Fogelman, N., Xu, K. & Sinha, R. Psychological and biological resilience modulates the effects of stress on epigenetic aging. Translational Psychiatry. 11 (1), 601. https://doi.org/10.1038/s41398-021-01735-7 (2021).
Harricharan, S., McKinnon, M. C. & Lanius, R. A. How processing of sensory information from the internal and external worlds shape the perception and engagement with the world in the aftermath of trauma: implications for PTSD. Front. Neurosci. 15, 1–20. https://doi.org/10.3389/fnins.2021.625490 (2021).
Agmon, M., Bar-Shalita, T. & Kizony, R. High sensory responsiveness in older adults is associated with walking outside but not inside: proof of concept study. Clin. Interv. Aging 16, 1651–1657. https://doi.org/10.2147/cia.s322728 (2021).
Chandrasekaran, C. ScienceDirect computational principles and models of multisensory integration. Curr. Opin. Neurobiol. 43, 25–34. https://doi.org/10.1016/j.conb.2016.11.002 (2017).
Miller, L. J., Anzalone, M. E., Lane, S. J., Cermak, S. A. & Osten, E. T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Therapy. 61 (2), 135. https://doi.org/10.5014/ajot.61.2.135 (2007).
Dunn, W., Little, L., Dean, E., Robertson, S. & Evans, B. The state of the science on sensory factors and their impact on daily life for children: A scoping review. OTJR: Occupation Participation Health 36(2_suppl), 3S-26S. https://doi.org/10.1177/1539449215617923 (2016).
Sievers, B., Lee, C., Haslett, W. & Wheatley, T. T. A multi-sensory code for emotional arousal. Proceedings of the Royal Society B 286(1906), 20190513. https://doi.org/10.1098/rspb.2019.0513 (1906).
Costa-lópez, B., Ferrer‐cascales, R., Ruiz‐robledillo, N., Albaladejo‐blázquez, N. & Baryła‐matejczuk, M. Relationship between sensory processing and quality of life: A systematic review. J. Clin. Med. 10(17), 3961. https://doi.org/10.3390/jcm10173961 (2021).
Bar-shalita, T., Deutsch, L., Honigman, L. & Weissman-fogel, I. Research in developmental disabilities ecological aspects of pain in sensory modulation disorder. Res. Dev. Disabil. 45–46, 45–46. https://doi.org/10.1016/j.ridd.2015.07.028 (2015).
Schmitt, C. M. & Schoen, S. Interoception: A multi-sensory foundation of participation in daily life. Front. NeuroSci. 16, 875200. https://doi.org/10.3389/fnins.2022.875200 (2022).
Dunn, W. The sensations of everyday life: empirical, theoretical, and pragmatic considerations. Am. J. Occup. Ther. 55 (6), 608–620. https://doi.org/10.5014/ajot.55.6.608 (2001).
Bar-Shalita, T., Granovsky, Y., Parush, S. & Weissman-Fogel, I. Sensory modulation disorder (SMD) and pain: a new perspective. Front. Integr. Nuerosci. 13, 27. https://doi.org/10.3389/fnint.2019.00027/full (2019).
Bar-Shalita, T. & Cermak, S. A. Atypical sensory modulation and psychological distress in the general population. Am. J. Occup. Ther. 70(4), 7004250010p1-7004250010p9. https://doi.org/10.5014/ajot.2016.018648 (2016).
McMahon, K., Anand, D., Morris-Jones, M. & Rosenthal, M. Z. A path from childhood sensory processing disorder to anxiety disorders: the mediating role of emotion dysregulation and adult sensory processing disorder symptoms. Front. Integr. Nuerosci. 13, 452078. https://doi.org/10.3389/fnint.2019.00022 (2019).
Ben-Avi, N., Almagor, M. & Engel-Yeger, B. Sensory processing difficulties and interpersonal relationships in adults: an exploratory study. Psychology 3(1), 70–70. https://doi.org/10.4236/psych.2012.31012 (2012).
Lionetti, F. et al. Sensory processing sensitivity and its association with personality traits and affect: A meta-analysis. J. Res. Pers. 81, 138–152. https://doi.org/10.1016/j.jrp.2019.05.013 (2019).
Engel-Yeger, B. & Shochat, T. The relationship between sensory processing patterns and sleep quality in healthy adults. Can. J. Occup. Ther. 79 (3), 134–141. https://doi.org/10.2182/cjot.2012.79.3.2 (2012).
Harlap, S. et al. The Jerusalem perinatal study cohort, 1964–2005: methods and a review of the main results. Paediatr. Perinat. Epidemiol. 21 (3), 256–273. https://doi.org/10.1111/j.1365-3016.2007.00799.x (2007).
Talisman, S., Friedlander, Y. & Hochner, H. Perinatal socio-behavioral and obstetric predictors of metabolically healthy and unhealthy obesity in adult offspring. Obesity 30(1), 209–220. https://doi.org/10.1002/oby.23288 (2021).
Klemera, P. & Doubal, S. A new approach to the concept and computation of biological age. Mech. Ageing Dev. 127 (3), 240–248. https://doi.org/10.1016/j.mad.2005.10.004 (2006).
Shapiro, I. et al. Familial aggregation of the aging process: biological age measured in young adult offspring as a predictor of parental mortality. GeroScience 45(2), 901–913. https://doi.org/10.1007/s11357-022-00687-0 (2023).
Kwon, D. & Belsky, D. W. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. GeroScience 43(6), 2795–2808. https://doi.org/10.1007/s11357-021-00480-5 (2021).
CDC. National Health and Nutrition Examination Survey Data (NHANESIII), Url: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx
Lind, L., Ingelsson, E., Sundström, J., Siegbahn, A. & Lampa, E. Methylation-based estimated biological age and cardiovascular disease. Eur. J. Clin. Invest. 48 (2), e12872 (2018).
Bar-Shalita, T., Seltzer, Z., Vatine, J. J., Yochman, A. & Parush, S. Development and psychometric properties of the sensory responsiveness questionnaire (SRQ). Disabil. Rehabil. 31 (3), 189–201. https://doi.org/10.1080/09638280801903096 (2009).
Assayag, N. et al. Perceived sensitivity to pain and responsiveness to non-noxious sensation in substance use disorder. Pain Med. 21 (9), 1902–1912. https://doi.org/10.1093/pm/pnz292 (2020).
Cooper, R., Strand, B. H., Hardy, R., Patel, K. V. & Kuh, D. Physical capability in mid-life and survival over 13 years of follow-up: British birth cohort study. BMJ (Online). 348, 1–12. https://doi.org/10.1136/bmj.g2219 (2014).
Tzemah-Shahar, R. & Agmon, M. Physical capacity as marker for rate of aging in mid life. Innov. Aging, 7(Suppl 1), 274–275 (2023).
Marti, C. N. & Choi, N. G. Measuring social engagement among low-income, depressed homebound older adults: validation of the social engagement and activities questionnaire. Clin. Gerontologist. 45 (3), 548–561. https://doi.org/10.1080/07317115.2020.1753275 (2022).
Van Buuren, S. Flexible Imputation of Missing Data (CRC, 2018).
Little, R. J. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 83 (404), 1198–1202. https://doi.org/10.1080/01621459.1988.10478722 (1988).
Enders, C. K. Applied Missing Data Analysis (Guilford Press, New York, 2010).
Paraskevoudi, N., Balcı, F. & Vatakis, A. Walking through the sensory, cognitive, and Temporal degradations of healthy aging. Ann. N. Y. Acad. Sci. 1426 (1), 72–92 (2018).
Elliott, M. L. et al. Disparities in the Pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat. Aging. 1 (3), 295–308 (2021).
Delahunt, J. Z. & Lawson, L. A. M. Relationships between adolescent body mass index, physical activity, and sensory-processing patterns. Can. J. Occup. Ther. 84(3), 189–198. https://doi.org/10.1177/0008417417711459 (2017).
Engel-Yeger, B. & Dunn, W. Exploring the relationship between affect and sensory processing patterns in adults. Br. J. Occup. Therapy. 74 (10), 456–464. https://doi.org/10.4276/030802211X13182481841868 (2011).
Hebert, K. The association between impulsivity and sensory processing patterns in healthy adults. Br. J. Occup. Therapy. 78 (4), 232–240. https://doi.org/10.1177/0308022615575670 (2015).
Frank, M. et al. Association between systemic inflammation and individual symptoms of depression: a pooled analysis of 15 population-based cohort studies. Am. J. Psychiatry. 178 (12), 1107–1118. https://doi.org/10.1176/appi.ajp.2021.20121776 (2021).
Kira, K., Hayley, A., Catchlove, S., Savage, K. & Stough, C. Is poor self-rated sleep quality associated with elevated systemic inflammation in healthy older adults?. Mech. Ageing Dev. 192, 111388–111388. https://doi.org/10.1016/j.mad.2020.11138 (2020).
Thayer, J. F. & Ruiz-Padial, E. Neurovisceral integration, emotions and health: An update, in International Congress Series, vol. 1287: Elsevier, pp. 122–127, https://doi.org/10.1016/j.ics.2005.12.018 (2006).
McEwen, B. S. Stress, adaptation, and disease: Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 840(1), 33–44. https://doi.org/10.1111/j.1749-6632.1998.tb09546.x (2006).
Bobba-alves, N. et al. Psychoneuroendocrinology cellular allostatic load is linked to increased energy expenditure and accelerated biological aging. No January. 155 https://doi.org/10.1016/j.psyneuen.2023.106322 (2023).
Stevenson, R. J., Mahmut, M. & Rooney, K. Individual differences in the interoceptive states of hunger, fullness and thirst. Appetite 95, 44–57. https://doi.org/10.1016/j.appet.2015.06.008 (2015).
Barrett, L. F. & Simmons, W. K. Interoceptive predictions in the brain. Nat. Publishing Group. 16, https://doi.org/10.1038/nrn3950 (2025).
Seth, A. K. & Friston, K. J. Active interoceptive inference and the emotional brain. Philosophical Trans. Royal Soc. B: Biol. Sci. 371 (1708), 20160007–20160007. https://doi.org/10.1098/rstb.2016.0007 (2016).
Khalsa, S. S. et al. Interoception and mental health: a roadmap. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging. 3 (6), 501–513 (2018).
Quigley, K. S., Kanoski, S., Grill, W. M., Barrett, L. F. & Tsakiris, M. Neurosciences functions of interoception: from energy regulation to experience of the self. Trends Neurosci. 44 (1), 29–38. https://doi.org/10.1016/j.tins.2020.09.008 (2020).
Acknowledgements
We thank the study participants for their contribution and time invested in making this study possible.
Funding
No funding was received for the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
M.Asher: conceptualization, methodology, original draft preparation. M.Agmon and TBS: conceptualization, original draft preparation, review & editing. RTS: methodology, review & editing. HH and IS: analysis, review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Asher, M., Tzemah-Shahar, R., Hochner, H. et al. Low sensory responsiveness is associated with accelerated aging in midlife. Sci Rep 15, 26024 (2025). https://doi.org/10.1038/s41598-025-07596-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07596-0