Abstract
Nanoparticles (NPs) have gained increasing attention due to their unique physicochemical properties and broad applications. However, concerns about their potential toxicity, particularly reproductive toxicity, have emerged. Silica dioxide nanoparticles (SiO₂-NPs) are among the most commonly used NPs and have been linked to adverse effects on male reproductive health. This study aimed to evaluate the potential ameliorative effect of thymol, a natural monoterpene phenol with antioxidant and anti-inflammatory properties, against SiO₂-NPs-induced reproductive toxicity in male rats. Twenty-four adult male Sprague-Dawley rats were randomly assigned to four groups: control, SiO₂-NPs-treated (10 mg/kg body weight, intraperitoneally), thymol-treated (30 mg/kg body weight, orally), and SiO₂-NPs + thymol co-treated. Treatments were administered daily for 56 days. Male reproductive performance was evaluated through sexual behavior assessment, sperm characteristics, reproductive hormone levels, oxidative stress markers, inflammatory biomarkers, gene expression analysis, and histopathological examination of testicular tissue. Results revealed that SiO₂-NPs significantly impaired reproductive performance, indicated by reduced sperm motility, viability, and count, along with increased sperm abnormalities. Thymol co-administration significantly restored testosterone levels and partially normalized elevated LH and FSH levels caused by SiO₂-NPs, indicating endocrine protection. Moreover, SiO₂-NPs induced oxidative stress, elevated pro-inflammatory cytokines (TNF-α and IL-6), and disrupted the expression of key genes associated with oxidative stress response (NRF2), apoptosis (BAX, BCL-2), steroidogenesis (STAR, CYP11A1), and spermatogenesis (PRM1, GATA4). Thymol co-administration with SiO₂-NPs significantly mitigated these adverse effects by restoring antioxidant levels, reducing inflammation, and improving gene expression and the histological architecture of the testes. The findings suggest that thymol has a promising protective role against SiO₂-NPs-induced male reproductive toxicity through its antioxidant and anti-inflammatory actions.
Similar content being viewed by others
Introduction
Nanoparticles (NPs) have gained more interest in the last few decades due to their exceptional characteristics, including size, surface area, optical features, and biocompatibility1. One of the most widely used nanomaterials across various industries is Silica nanoparticles (Si-NPs). For example, in the construction field, consumer products, electronics, biomedicine, and pharmaceutical products are used2. This is because of their unique physico-chemical properties.
The toxicological effects of nanoparticles have become a growing concern, especially in relation to reproductive health. The reproductive system is particularly vulnerable to external stressors due to its role in transmitting genetic material3. Silica dioxide nanoparticles (SiO₂-NPs) are extensively used in biomedical and industrial applications, but growing evidence highlights their potential reproductive toxicity4. The risks associated with SiO₂-NPs on the reproductive system are receiving increased attention5. SiO₂-NPs were observed to produce sperm deformity and decrease the number of sperm and their motility in male rats6. It also harms Sertoli cells7 and causes a reduction in Leydig cell count of rats8. Moreover, it was reported to affect sperm maturation in the epididymis, causing decreased sperm count and quality and energy metabolism dysfunction in mice9. SiO₂-NPs can penetrate the blood-testis barrier, accumulate in testicular tissue, and induce oxidative stress through excessive ROS generation, leading to lipid peroxidation and DNA damage9. This oxidative damage disrupts spermatogenesis, reduces sperm count and motility, and increases abnormalities.
Additionally, SiO₂-NPs impair mitochondrial function and downregulate key genes involved in steroidogenesis (STAR, CYP11A1) and spermatogenesis (PRM1, GATA4), resulting in hormonal imbalance and germ cell apoptosis10,11,12. Histologically, they cause degenerative changes in seminiferous tubules and interstitial edema13. Due to the reported harmful impact of Silica nanoparticles on male reproductive function, identifying an effective agent that could ameliorate and/or mitigate their adverse effects on male reproductive health become a more critical issue. Recent studies have highlighted growing interest in natural antioxidants like thymol for their therapeutic potential14. Dietary phytochemicals like thymol have been in the spotlight recently due to their promising pharmacological, physicochemical, and pharmacokinetic properties14. An important component of thyme essential oil and a wide variety of plants, such as Thymus vulgaris, Nigella sativa, Thymus ciliates, and Origanum vulgarae, is thymol, a monoterpene phenolic chemical14. Thymol displays a wide range of biological functions, including antioxidant15 and anti-inflammatory properties16. Thymol’s antioxidant properties have been demonstrated in various models, including Chinese hamster fibroblast cells (V79)17, intestinal Caco-2 cell line18, and rodent models exposed to oxidative stressors such as titanium dioxide and methomyl19. The phenolic hydroxyl group, in its structure, could scavenge and neutralize free radicals14. Moreover, previous studies reported thymol’s ability to improve sperm quality evaluations20.
Güvenç et al.20 showed that thymol orally administered at dosages of (10 and 20 mg/kg BW) over 10 weeks in rats resulted in enhanced sperm concentration, motility, and viability while concurrently reducing sperm abnormalities in comparison to the control group. Moreover, Jafari et al.19 revealed that oral co-treatment of TiO2 nanoparticle-intoxicated rats with thymol (10 and 30 mg/kg BW/day) for 60 days increased the testicle weight in a dose-dependent manner compared to the group treated with TiO2 nanoparticles alone. In addition, thymol improved all sperm parameter abnormalities.
Accordingly, the current investigation set out to assess the potential ameliorative impact of Thymol against Silica dioxide nanoparticles (SiO2 NPs) induced reproductive toxicity in male rats through evaluation of sexual behavior, semen characteristics, reproductive hormones level, in addition to antioxidants, inflammatory markers and gene expression in testes, together with the testicular tissue histopathology.
Materials and methods
Chemicals
Silicon dioxide nanoparticles (SiO₂-NPs) were sourced from Sigma-Aldrich Co. (St. Louis, MO, USA), while Thymol was procured from Oxford Lab Fine Chem Co. (India).
SiO2- NPs characterization
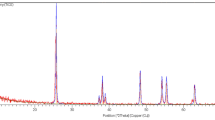
SiO₂ nanoparticles (SiO₂-NPs) were characterized at the Nanotechnology Center, Chemistry Department, Faculty of Science, Kafrelsheikh University, Egypt, using scanning electron microscopy (SEM), X-ray diffraction (XRD), and a zeta potential/particle size analyzer. The XRD analysis was carried out with a Shimadzu 6000–XRD diffractometer, employing Cu Kα radiation (λ = 1.54056 Å). The particle size and surface morphology were assessed using a JEOL (JSM-IT100) scanning electron microscope operating at 30 kV. Zeta potential values were determined using a Brookhaven zeta potential/particle size analyzer21.
Ethical statement
All animal procedures and experimental protocols were conducted in accordance with the ARRIVE 2.0 guidelines for reporting in vivo experiments22. Ethical approval was granted by the Ethics Committee of Alexandria University Institutional Animal Care and Use Committee (ALEXU-IACUC, approval number 120/2022). All procedures were performed in compliance with the relevant institutional and international guidelines for the care and use of laboratory animals.
Animals
Forty-eight (Twenty-four male and twenty-four female) Sprague-Dawley adult rats, aged 3–4 months, with an average body weight ranging from 160 to 180 g, were sourced from the Medical Research Institute, Alexandria University, Egypt. The rats were housed at the animal facility of the Medical Research Institute and the Medical Technology Center for Research and Services, Alexandria University, Egypt, and maintained under a natural light/dark cycle. They were given unrestricted access to food and water. The diet provided was a commercial broiler starter (Al-Eman Co., Egypt) containing 21% crude protein, 4.11% fat, and 2.44% crude fiber, aligning with the NRC dietary recommendations23. Prior to the initiation of the treatment, all rats were acclimated for two weeks.
Experimental design
Twenty-four male rats were randomly assigned into four groups (6 each): (1) The control group received intraperitoneal injections of saline and oral administration of corn oil to account for the potential effects of both administration routes; (2) SiO2 -NPs treated group administered SiO2- NPs (10 mg/kg bwt) IP; (3) Thymol treated group received thymol (30 mg/kg bwt) orally via gavage, The selected dose of thymol (30 mg/kg bwt) was based on previously published studies demonstrating its efficacy and safety in rodent models without inducing systemic or reproductive toxicity19,24,25. Therefore, this dose was considered appropriate for assessing its protective role in the current study. The oral LD50 values in rats for thymol were 980 mg/kg body weight26 ; (4) SiO2 -NPs and Thymol treated group received both drugs. SiO2 -NPs were dispersed in normal saline and then sonicated for 5 min before use. Thymol was dissolved in corn oil. The treatment was administered daily for 56 days24,27; this experiment continued for 56 days to complete spermatogenesis and sperm maturation in the epididymis28. The doses and route of administration used for both drugs were according to24,27. The co-administration in the first and fourth groups was separated by time interval (30 min) with the start with the IP injection followed by the oral one. At the end of the treatment period, sexual behavior was determined by a fertility test. Afterward, rats were humanely euthanized by decapitation. Blood samples were collected for reproductive hormone level determination. Also, sperm characteristics and reproductive organ weights were evaluated. After organ weighting, one testicle was kept at − 80 ◦C for antioxidants, inflammatory biomarkers, and gene expression, whereas the second testicle was submerged in formalin solutions for histopathological analysis.
Sexual behavior (fertility test)
Twenty-four female rats received intraperitoneal injections of Lutalyse® (dinoprost tromethamine) at a dose of 0.1 mg/100 g body weight29 twice daily to synchronize the estrous cycle. Vaginal smears were taken to confirm estrous. Females in estrous were paired with males for mating within a plastic enclosure and documented on video for 15 min30. The following parameters were determined: mount frequency: mounts number till ejaculation; intromission frequency: intromissions number till ejaculation; mount latency: time from female entrance till the first mount; intromission latency: time from female entrance till the first intromission and ejaculatory latency: time from first intromission till ejaculation31.
Reproductive hormones and reproductive organ’s weight
The rats were anesthetized with sodium pentobarbital (60 mg/kg; Sigma-Aldrich Co., St. Louis, MO, USA) and subsequently euthanized by decapitation. Blood samples were collected through cardiac puncture into tubes without anticoagulants. The samples were left to clot at 4 °C and then centrifuged at 3000 rpm for 10 min to separate the serum. The obtained serum was stored at -20 °C until it was used for analysis. Serum levels of testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) were measured using rat-specific ELISA kits (Cusabio Biotech Co., Wuhan, China; Catalog Nos. CSB-E06869r for testosterone, CSB-E12654r for FSH, and CSB-E05100r for LH) following24. The sensitivity of the kits was 0.1 ng/mL for testosterone, 0.1 mIU/mL for FSH, and 0.05 mIU/mL for LH. Testicles, epididymis, prostate, and seminal vesicles were harvested from beheaded rats and weighed32,33.
Sperm characteristics
The Sperm Characteristics were performed as follows, according to34,35. Epididymal sperm were collected by slicing the epididymis in a sterile petri dish, allowing sperm to be released from the tubules. A drop of the suspension was placed on a clean slide, covered with a cover slip, and examined under a light microscope at 400x magnification. The motility percentage was assessed within 2–4 min by observing at least 10 fields and calculating the proportion of motile sperm. For viability analysis, an equal drop of 1% eosin Y and 5% nigrosine stain was mixed with an equal drop of epididymal content, incubated for 2 min at room temperature, and examined at 400x magnification. Live sperm remained colorless, while dead sperm appeared dark pink, with viability determined by counting 100 sperm per slide. Sperm abnormalities were assessed by preparing a smear from a mixture of the stain and epididymal content, observing 100 sperm cells per slide at 400x magnification, and recording any head or tail deformities. For sperm count, 5 µl of epididymal suspension was diluted with 95 µl of a solution containing 5 g NaCl and five drops of formalin in 100 ml distilled water. A drop of the diluted sample was placed on a hemocytometer coverslip and left in a moist chamber for five minutes for sedimentation. Sperm cells were counted under a light microscope at 400x magnification.
Antioxidants, oxidative stress, and inflammatory biomarkers
The testicular homogenate was centrifuged at 3000 rpm for 10 min at 4 °C36, after which it was utilized to extract testicular tissue from phosphate-buffered saline (PBS). The supernatant was collected for biomarker analysis. The assessed inflammatory markers included tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)36, antioxidants including glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), and malondialdehyde (MDA)37,38.
Histopathological examination
Testicular tissue was collected from all treated groups and instantly fixed in 10% formalin for at least 24 h for histological analysis by light microscopy. Specimens were dehydrated in ascending concentrations of alcohol, cleared in xylene, and embedded in paraffin wax. . Furthermore, the grading of testicular lesions was described as follows: negative (−), very mild (+), mild (++), mild to moderate (+++), moderate (++++), and severe (+++++).
RT-PCR
The gene expression in the testicles was assessed using quantitative real-time polymerase chain reaction. Total RNA was extracted from about 100 mg of testicular tissue using TRIZOL Reagents (Invitrogen, Carlsbad, CA, USA). A Nanodrop spectrophotometer was used to measure the quantities of RNA. A cDNA synthesis kit (Fermentas, Waltham, MA, USA) was used for complementary DNA (cDNA) synthesis, with only RNA samples showing an A260/A280 ratio of 1.8 or above being deemed appropriate. Table 1 shows the particular primers and SYBR Green Master Mix that were used to amplify the cDNA that was obtained. An internal reference gene used to normalize the expression levels was the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene. In order to find the relative quantification of gene expression, the 2^(-ΔΔCt) calculation method was employed39.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS, version 25) was used for all statistical analyses. To explore relationships among multiple measured parameters (e.g., sexual behavior, sperm characteristics, and reproductive organ weights), Principal Component Analysis (PCA) was employed as an exploratory multivariate technique. PCA reduced the dimensionality of correlated variables by transforming them into a smaller set of uncorrelated variables—principal components—that retained most of the original variance. This method allowed for a clearer interpretation of interrelationships among outcomes while minimizing multicollinearity. PCA was not used for inferential hypothesis testing but rather to summarize data structure and guide subsequent analyses. Components with eigenvalues greater than 1 were retained, and variables with loadings greater than 0.60 were considered significant contributors.
To assess treatment effects on measured parameters, a one-way General Linear Model (GLM) was used. Data were presented as means ± standard error (SEM), and p-values < 0.05 were considered statistically significant. Post hoc comparisons between treatment groups were conducted using Duncan’s multiple range test to identify significant differences.
Results
Characterization of the SiO2 NPs
Scanning electron microscopy (SEM), X-ray diffractometer, and zeta potential/particle size analysis showed that the average diameter of SiO2 NPs was < 50 nm (Fig. 1).
Sexual behavior
The association between the five sexual behavior items of adult male rats (mount frequency, intromission frequency, mount latency, intromission latency, and ejaculatory latency) was tested using Principal component analysis. Two components had an eigenvalue greater than 1, explaining 79.82% of the total variance. After observing component coefficients, one item (ejaculatory latency) was removed as having a coefficient less than 0.60, and four items were retained in the two components, as shown in Table 2. Component 1 contains mount latency, intromission latency, and intromission frequency, whereas mount frequency comes in component 2.
Figure 2A showed a non-significant difference between groups for the mount and intromission frequencies. However, there was a noteworthy increase in mount and intromission latencies in rats administered SiO2-NPs compared to control rats (Fig. 2B). Moreover, longer mount and intromission latencies were found in rats administered SiO2-NPs plus thymol than the control group, but this was shorter than SiO2-NPs treated group, although non-significant. Figure 2C, ejaculatory latency demonstrated a notable reduction in the rats that received SiO2- NPs plus thymol compared to the control and SiO2- NPs treated group.
Reproductive organs weight
The association between the four reproductive organs’ absolute weight (Testicles, epididymis, seminal vesicles, and prostate glands) of adult male rats was examined using principal component analysis. Two components had an eigenvalue greater than 1, explaining 72.52% of the total variance. After observing the components matrix, the two components with the four items were retained because they had coefficients > 0.60, as shown in Table 3. Testicles and epididymis weight come in one component, whereas seminal vesicles and prostate glands come in separate components. Figure 3A revealed an increment in testicle absolute weight in rats administered SiO2- NPs plus thymol compared to the control group, whereas it was elevated than SiO2- NPs group, although non-significant. Epididymis, seminal vesicles, and prostate gland absolute weights revealed a nonsignificant difference between all treated groups (Fig. 3A, B). Furthermore, the relative organ weight to body weight showed a nonsignificant difference between treated groups for all organs, testicles (P = 0.925), epididymis (P = 0.101), seminal vesicles (P = 0.719), and prostate gland (P = 0.458). (Figure S1)
Sperm characteristics and the reproductive hormones
The association between the four measurements of sperm characteristics (motility, viability, abnormalities, and count) of adult male rats was tested using Principal component analysis. One component had an eigenvalue greater than 1, explaining 74.68% of the total variance. After observing the component matrix, all items in the element were retained (Table 4). However, all items are positively related except sperm abnormalities % were negatively associated with other items.
In comparison to the other groups, the SiO2-NPs group showed a markedly lower percentage of sperm motility and sperm viability (Fig. 4A). Rats given SiO2- NPs had a much lower sperm cell count than the other treatment groups (Fig. 4B). Figure 4C showed that rats given a combination of SiO2-NPs and thymol had a substantially lower percentage of sperm abnormalities than rats given SiO2-NPs alone.
Exposure to SiO₂ nanoparticles (SiO₂-NPs) resulted in significant hormonal disruptions in male rats, as evidenced by the marked decrease in serum testosterone levels and the significant elevation of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) compared to the control group (p < 0.05). Thymol treatment alone maintained hormone levels comparable to those of the control group, indicating no adverse endocrine effects. Notably, co-administration of thymol with SiO₂-NPs significantly mitigated these alterations, with testosterone levels increasing and LH and FSH levels decreasing relative to the SiO₂-NPs group, although not fully returning to baseline. These findings suggest that thymol offers a partial protective effect against SiO₂-NPs-induced endocrine disruption (Fig. 5).
Histopathology
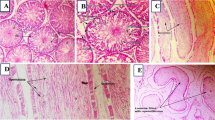
Testicular specimens from the control and thymol-treated groups (Fig. 6a and h) exhibited the typical anatomical structure of fully developed, functional seminiferous tubules (Sts), characterized by a complete and well-organized spermatogenic series and normal histological appearance of interstitial connective tissue and Leydig cells.
In contrast, rats treated with SiO₂-NPs displayed extensive histopathological alterations. These included severe vacuolar degeneration of the germinal epithelium, moderate sloughing of germinal cells into the lumen (Fig. 6b), and shrunken, buckled, and disorganized seminiferous tubules (Fig. 6c). Additional findings included small-sized tubules with absent or sparse germinal cell layers (Fig. 6d), hyalinization of luminal contents (Fig. 6e), and necrotic seminiferous tubules (Fig. 6f). The interstitial tissue also showed dilated and congested blood vessels and moderate, faint eosinophilic albuminous edema (Fig. 6g), indicating inflammatory damage.
Remarkably, testicular sections from the SiO₂-NPs + thymol co-treated group (Fig. 6i) showed significant histological improvement. Most seminiferous tubules regained a near-normal structure, containing elongated spermatids and spermatozoa, with only minor interstitial edema and slight vacuolization of the germinal epithelium remaining. These findings point to a notable restoration of spermatogenesis and architectural integrity.
A more detailed comparison between Fig. 6a (control) and Fig. 6i (SiO₂-NPs + thymol) reveals that although both groups display generally preserved seminiferous tubules, subtle distinctions are present. The control group shows densely packed, highly organized germinal epithelium, while the co-treated group exhibits mild disorganization and slightly reduced germ cell density in some tubules.
These observations suggest that thymol co-treatment substantially mitigated the testicular damage induced by SiO₂-NPs. While complete histological recovery was not achieved, likely due to residual nanoparticle toxicity, the improvement supports the conclusion that thymol provides a partial but significant protective effect against SiO₂-NPs-induced oxidative and inflammatory testicular injury.
For histopathological scoring, please refer to Table 5.
Testicular oxidative stress and lipid peroxidation
The results in Fig. 7 illustrate the effects of SiO₂-NPs and thymol on testicular tissue biomarkers linked to inflammation, oxidative injury, and lipid peroxidation in male rats. IL-6 and TNF-α levels were substantially elevated in the SiO₂-NPs group compared to the control and thymol-treated groups (p < 0.05). Conversely, co-administration of thymol with SiO₂-NPs led to a marked reduction in these inflammatory markers compared to the SiO₂-NPs group, though levels remained higher than in controls. Regarding oxidative injury indicators, (GSH), (CAT), and (SOD) levels were significantly decreased in the SiO₂-NPs group relative to controls, indicating compromised antioxidant defense. Thymol administration significantly enhanced these antioxidant parameters compared to the SiO₂-NPs group, suggesting a protective effect, while the combined SiO₂-NPs + thymol group showed partial restoration toward control levels. MDA, a lipid peroxidation indicator, was substantially heightened in the SiO₂-NPs group, reflecting elevated oxidative damage. Thymol treatment alone maintained MDA levels similar to control, and co-administration of thymol with SiO₂-NPs significantly reduced MDA levels compared to the SiO₂-NPs group, though not to baseline levels. These findings demonstrate that thymol mitigates SiO₂-NPs-induced oxidative injury, inflammation, and lipid peroxidation in testicular tissue.
Gene expression
Figure 8 presents the mRNA expression levels of critical genes involved in oxidative stress response, apoptosis, inflammation, steroidogenesis, and spermatogenesis in testicular tissues from the four experimental groups: control, SiO₂-NPs-treated, thymol-treated, and SiO₂-NPs + thymol-treated rats. Panel A showed a significant (p < 0.05) reduction in NRF2 expression in the SiO₂-NPs group compared to the control. In contrast, thymol treatment alone significantly (p < 0.05) increased NRF2 levels, and the co-administration of thymol with SiO₂-NPs partially restored NRF2 expression, showing a significant (p < 0.05) improvement compared to the SiO₂-NPs group. In panel B, BAX expression, a pro-apoptotic gene, was extensively (p < 0.05) upregulated in the SiO₂-NPs-treated group. Thymol treatment meaningfully (p < 0.05) reduced BAX expression, and the combined treatment group showed intermediate levels that were significantly (p < 0.05) different from both the SiO₂-NPs and control groups. BCL-2, an anti-apoptotic gene, had drastically (p < 0.05) lower expression in the SiO₂-NPs group. However, thymol administration led to a significant (p < 0.05) restoration of BCL-2 levels, with the co-treated group showing partial but significant (p < 0.05) recovery. TNF-α and IL-6 levels, markers of inflammation, were significantly (p < 0.05) elevated in the SiO₂-NPs-treated group. Thymol treatment, both alone and in combination with SiO₂-NPs, significantly (p < 0.05) reduced the expression of these inflammatory markers, indicating thymol’s notable anti-inflammatory effects. The antioxidant enzyme SOD2 (panel F) was significantly (p < 0.05) downregulated by SiO₂-NPs but significantly restored (p < 0.05) by thymol, with moderate but significant (p < 0.05) recovery in the combined group. Panels G and H display significant (p < 0.05) downregulation of STAR and CYP11A1, essential genes for steroidogenesis, in the SiO₂-NPs group, while thymol significantly improved (p < 0.05) their expression, suggesting restored steroidogenic capacity. Finally, spermatogenesis-related genes PRM1 and GATA4 (panels I and J) were significantly (p < 0.05) suppressed by SiO₂-NPs, with thymol treatment significantly (p < 0.05) restoring their expression. At the same time, co-treatment led to partial but significant (p < 0.05) normalization. These results highlight thymol’s significant (p < 0.05) potential in mitigating SiO₂-NPs-induced testicular gene expression disruptions.
Discussion
With the advancement of engineered nanoparticles (ENPs), a variety of ENPs, including metal nanoparticles (NPs) like Silica nanoparticles, have found extensive applications in drug delivery, therapeutics, diagnostics, vaccines, and food products40,41. The widespread incorporation of NPs into daily life has drawn significant attention to their potential risks. Research has indicated that SiO₂-NPs adversely impact the male reproductive system, reducing sperm quantity and quality in rodent studies10,12. In the extant study, we aimed to investigate the potential defensive impacts of thymol against the SiO₂-NPs induced reproductive toxicity in male rats. The principal component analysis indicates a good association between measured parameters in sexual behavior, semen characteristics, and absolute organ weight, which are used to assess the protective efficiency of thymol against induced reproductive performance toxicity of SiO₂-NPs. Results revealed that SiO₂-NPs have a negative impact on the ability of mature male rats to reproduce as the SiO₂-NPs group showed an increase in sperm abnormalities percentage, mount latency, intromission latency, and ejaculatory latency, whereas decreased sperm motility, sperm viability, and sperm cell count. This was confirmed by histopathological examination.
These results agreed with Zhang et al.42, who revealed that the administration of Silica nanoparticles at the dose of (10 mg/kg BW) interferes with the sexual behavior of male rats. Furthermore, Lin et al.43 stated that SiO₂-NPs caused a decrease in the mating rate in male rats. The results of the semen characteristics are consistent with previous studies, which stated that SiO₂-NPs at the doses of (10 and 40 mg/kg BW) led to a reduction in sperm motility and sperm cell count and augmented sperm abnormalities in mice13,27. Moreover, Lin et al.6 deduced that silica dioxide nanoparticles by tracheal administration at the dose of 7.5 mg/kg BW every two days for 5 weeks in male rats reduced sperm count and motility while increasing sperm deformity.
In the present study, the SiO₂-NPs-treated group exhibited prolonged ejaculatory latency, a finding that, while potentially counterintuitive at first glance, aligns with toxicological impairment of sexual behavior. Ejaculatory latency is considered a behavioral biomarker of sexual desire, and longer latencies are indicative of reduced sexual motivation or arousal, which may result from neurobehavioral or endocrine disruptions31,44. This delay may also be associated with decreased sensitivity in achieving the ejaculation threshold, likely due to SiO₂-NPs-induced alterations in dopaminergic or serotonergic pathways, both of which are crucial for sexual reflex control45.
These effects are further supported by other findings in our study, including prolonged mount and intromission latencies, decreased sperm quality, and disrupted reproductive hormone levels. The co-administration of thymol significantly reversed the increase in ejaculatory latency. This ameliorative effect is likely due to thymol’s antioxidant, anti-inflammatory, and neuroprotective properties, which have been shown to restore redox balance, modulate neurotransmitter function, and improve sexual performance under toxicant exposure19,46. Collectively, these results reinforce the protective potential of thymol against SiO₂-NPs-induced reproductive and behavioral toxicity.
Silica nanoparticles notably elevated the levels of reactive oxygen species. Therefore, the reduction in sperm quality and number may be attributed to alterations in the redox system caused by Si-NPs13. Excessive ROS are thought to be harmful to sperm, as they can oxidize polyunsaturated fatty acids in the plasma membrane of sperm, damage amino acids and proteins, trigger DNA damage, and lead to apoptosis47. Moreover, the blood-epididymal barrier plays a vital role in maintaining the microenvironment within the duct, facilitating sperm maturation and movement, similar to the blood testicular barrier48. Thus, the observed effects may also be due to structural damage or dysfunction of the blood epididymal barrier induced by Silica nanoparticles13. Furthermore, Silica nanoparticles can negatively impact epididymal sperm quality and quantity by inducing oxidative stress and damaging mitochondrial structures, leading to disruptions in energy metabolism9.
On the other hand, this study showed that thymol can ameliorate reproductive toxicity of SiO₂-NPs and improve male reproductive performance as rats treated with both SiO₂-NPs and thymol exhibited a decrease in ejaculatory latency and sperm abnormalities percentage, along with increased sperm motility, viability, and sperm count in comparison with rats treated with SiO₂-NPs alone. These results are supported by Güvenç et al.20, who revealed that thymol (10 and 20 mg/kg BW) led to decreased sperm abnormalities and enhanced sperm motility and viability in rats. Moreover, Jafari et al.19 recounted that thymol at doses of (10 and 30 mg/kg body weight) improved all sperm parameters negatively affected by TiO2 nanoparticles in rats. Furthermore, Aboushouk et al.49 demonstrated that thymol at a dose of (100 mg/kg BW) reduced sperm abnormalities induced by methomyl toxicity in rats. Saber et al.24 found that co-administration of thymol at a dose of (30 mg/kg BW) in imidacloprid-intoxicated rats enhanced sperm count, motility, and viability while reducing sperm abnormalities. In addition, Tijani et al.25, reported that administering thymol orally at (30 mg/kg BW) improved sperm parameters that were adversely affected by hexachlorobenzene in rats. This effect could be ascribed to thymol’s ability to scavenge free radicals, reduce lipid peroxidation, and increase antioxidant activities19,25.
The current study demonstrated that exposure to SiO₂ nanoparticles (SiO₂-NPs) significantly disrupted the reproductive endocrine axis in male rats, as evidenced by decreased serum testosterone levels and elevated concentrations of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormonal alterations are consistent with previous findings in which imidacloprid (IMI), a structurally and functionally similar environmental toxicant, induced comparable endocrine disruptions24. The reduction in testosterone may result from direct oxidative damage to Leydig cells, which impairs steroidogenesis and reduces testosterone biosynthesis. This effect is often accompanied by a compensatory increase in LH and FSH levels, driven by negative feedback mechanisms within the hypothalamic-pituitary-gonadal (HPG) axis due to insufficient androgen signaling.
Notably, co-administration of thymol effectively mitigated these hormonal disturbances. Testosterone levels were significantly restored, while LH and FSH levels were reduced compared to the SiO₂-NPs-only group, indicating a partial normalization of endocrine function. These protective effects align with the reported antioxidant and steroidogenesis-supporting properties of thymol, which has been shown to preserve Leydig cell integrity, reduce lipid peroxidation, and upregulate steroidogenic gene expression (e.g., StAR, CYP11A1) under toxicant-induced stress conditions19,24. Thus, the ability of thymol to counteract SiO₂-NPs-induced hormonal imbalance likely stems from its modulation of oxidative stress and support of key molecular pathways involved in testosterone production.
The present study demonstrates the detrimental impact of SiO₂-NPs on testicular tissue, as evidenced by significant alterations in inflammatory, oxidative stress, lipid peroxidation biomarkers, and key gene expressions associated with reproductive function. The co-administration of thymol significantly mitigated these adverse effects, highlighting its therapeutic potential. SiO₂-NPs exposure led to elevated levels of pro-inflammatory cytokines IL-6 and TNF-α, indicating enhanced inflammatory responses44. These cytokines are critical in testicular apoptosis and impaired spermatogenesis45. Thymol administration significantly reduced IL-6 and TNF-α levels, underscoring its anti-inflammatory properties, likely through the suppression of NF-κB signaling. This observation aligns with previous studies that reported similar anti-inflammatory effects of thymol24. Furthermore, the reduction in these inflammatory markers upon thymol treatment suggests its potential role in maintaining testicular immune homeostasis.
The oxidative stress markers GSH, CAT, and SOD were significantly depleted in the SiO₂-NPs group, reflecting oxidative damage resulting from elevated ROS production50. This depletion compromises the antioxidant defense system, leading to lipid peroxidation, as elevated MDA level indicates. Thymol effectively restored antioxidant levels and reduced MDA concentrations, confirming its potent antioxidant activity. The phenolic hydroxyl group of thymol is likely responsible for scavenging free radicals, consistent with the findings of20,51. Gene expression analysis further supports these biochemical findings. The oxidative stress-responsive gene NRF2 and the mitochondrial antioxidant gene SOD2 were significantly downregulated in the SiO₂-NPs group, while thymol treatment restored their expression. This suggests thymol’s role in activating the NRF2 pathway, which is essential for combating oxidative stress52. Apoptosis-related genes followed a similar pattern: BAX (pro-apoptotic) expression was elevated, and BCL-2 (anti-apoptotic) expression was reduced by SiO₂-NPs, leading to cellular apoptosis. Thymol reversed these effects, indicating its anti-apoptotic potential46. Steroidogenesis and spermatogenesis were also affected by SiO₂-NPs, as evidenced by the downregulation of STAR and CYP11A1, essential for testosterone biosynthesis11.
Additionally, spermatogenesis-related genes PRM1 and GATA4 were significantly suppressed. PRM1 (Protamine 1) and GATA4 (GATA Binding Protein 4) are essential genes for spermatogenesis and male fertility. PRM1 plays a critical role in sperm chromatin condensation by replacing histones with protamines, ensuring DNA protection, genomic stability, and sperm motility11. Deficiencies in PRM1 expression are linked to sperm DNA fragmentation, abnormal morphology, and infertility53.
In contrast, GATA4 is pivotal for testicular development, regulating Sertoli and Leydig cell differentiation, testosterone synthesis through genes like STAR and CYP11A1, and germ cell survival54. Disruptions in GATA4 expression can lead to testicular dysgenesis and reproductive dysfunction55. These genes are key biomarkers and potential therapeutic targets for addressing male reproductive disorders.
While thymol has been previously investigated for its protective effects against various toxicants such as titanium dioxide (TiO₂) nanoparticles, imidacloprid, and methomyl19,24, the present study is among the first to comprehensively evaluate thymol’s role in counteracting reproductive toxicity induced by silica dioxide nanoparticles (SiO₂-NPs). Unlike TiO₂, which primarily induces oxidative stress through ROS generation and membrane lipid peroxidation, SiO₂-NPs have been shown to disrupt testicular architecture and function more profoundly, penetrating the blood-testis barrier, causing epithelial sloughing, germ cell loss, and inflammatory infiltration9.
Our findings reveal that thymol significantly mitigated these SiO₂-NPs-specific effects, restoring sperm quality, reducing inflammatory markers (IL-6, TNF-α), and normalizing key genes related to steroidogenesis and spermatogenesis (e.g., STAR, CYP11A1, PRM1, and GATA4). These outcomes suggest that thymol’s protective mechanism extends beyond general antioxidant activity and may involve targeted modulation of testicular gene expression and immune responses specific to silica nanoparticle exposure.
This study, therefore, adds novel evidence to the field by establishing thymol as a promising candidate for mitigating SiO₂-NP-induced reproductive dysfunction, distinguishing its effects from those reported in models of other toxicants.
Thymol significantly restored the expression of these genes, highlighting its role in supporting endocrine and reproductive functions. These results align with the observations of24,25, who reported similar results. Therefore, thymol can help protect against reproductive damage.
This study provides important insights into the protective role of thymol against SiO₂-NP-induced reproductive toxicity in male rats; however, several limitations should be acknowledged. The use of a short-term exposure model and restriction to male animals may limit the broader applicability of the findings. Future studies should explore chronic exposure, include female subjects, and adopt sex-comparative designs to provide a more comprehensive toxicological profile. While the observed protective effects of thymol are primarily attributed to its antioxidant, anti-inflammatory, and anti-apoptotic properties, the possibility of direct physicochemical interaction with SiO₂-NPs cannot be ruled out. However, it remains speculative without molecular-level evidence. Advanced approaches such as molecular docking, nanoparticle surface analyses, and binding assays are warranted to investigate this potential mechanism. Furthermore, broader transcriptomic or proteomic profiling could deepen mechanistic understanding and help identify novel molecular targets of both SiO₂-NP toxicity and thymol-mediated protection.
Conclusion
The present study demonstrated that exposure to silica dioxide nanoparticles (SiO₂-NPs) induces significant reproductive performance toxicity in male rats, as evidenced by impaired sexual behavior, sperm characteristics, altered reproductive hormone levels, increased oxidative stress, elevated inflammatory biomarkers, disrupted gene expression related to spermatogenesis and steroidogenesis, and notable histopathological damage in testicular tissue. However, thymol administration exhibited a remarkable protective effect against these adverse outcomes. Thymol effectively restored sperm characteristics, antioxidant enzyme levels, reduced pro-inflammatory cytokines (TNF-α and IL-6), normalized the expression of critical genes (NRF2, BAX, BCL-2, STAR, CYP11A1, PRM1, and GATA4), and improved testicular histological architecture. It also significantly restored serum testosterone levels while partially normalizing elevated LH and FSH levels, suggesting endocrine system recovery. These findings suggest that thymol’s antioxidant and anti-inflammatory properties play a crucial role in mitigating SiO₂-NPs-induced reproductive performance toxicity. Consequently, thymol could be considered a potential therapeutic agent for preventing or alleviating nanoparticle-induced reproductive dysfunction. Further studies are recommended to explore the underlying molecular mechanisms and evaluate the long-term protective effects of thymol in different models of nanoparticle exposure.
Effect of SiO₂-NPs and thymol on adult male rats’ sexual behavior during fertility test: (A) mount and intromission frequencies; (B) mount and intromission latencies (seconds); and (C) ejaculatory latency (seconds). All the values are expressed as the mean ± SEM. Different small letters(a-c) indicate significance at p < 0.05.
Effects of SiO₂-NPs and thymol on serum reproductive hormone levels in male rats.
(A) Testosterone concentration (ng/ml). (B) Luteinizing hormone (LH) levels (mIU/ml). (C) Follicle-stimulating hormone (FSH) levels (mIU/ml). Data are presented as mean ± SEM (n = 6). Bars with different superscript letters (a–c) indicate statistically significant differences between groups (p < 0.05).
Photomicrographs of the testicle of rats stained by HE (bar = 100 μm) (a) Control group showing the normal histological structure of active mature functioning seminiferous tubules (Sts) associated with complete spermatogenic series. (b, c, d, e, f, g) SiO₂-NPs-treated group showing vacuolar degeneration of the germinal epithelium (red arrows), sloughing of the germinal epithelium into the lumen of seminiferous tubules (black arrows) beside shrunken, buckled, disorganized seminiferous tubules (black arrowheads), small-sized seminiferous tubules with no - or single - germinal cell layers (black star), hyalinization of the luminal contents (yellow star), necrotic seminiferous tubules (red stars) and faint eosinophilic albuminous interstitial edema (blue stars). (h) Thymol-treated group showing the normal histological structure of seminiferous tubules (Sts) (i) SiO₂-NPs + Thymol-treated group showing the normal histological structure of seminiferous tubules (Sts).
Effects of SiO₂-NPsand thymol on inflammatory cytokines, antioxidant markers, and lipid peroxidation levels in testicular tissue of male rats. (A) Interleukin-6 (IL-6) levels (pg/mg tissue). (B) Tumor necrosis factor-alpha (TNF-α) levels (pg/mg tissue). (C) Glutathione (GSH) concentration (µmol/g tissue). (D) Catalase (CAT) activity (U/mg protein). (E) Superoxide dismutase (SOD) activity (U/mg protein). (F) Malondialdehyde (MDA) levels (nmol/mg tissue). Data are presented as mean ± SEM (n = 6). Bars with different superscript letters (a–c) indicate statistically significant differences between groups (p < 0.05).
Relative mRNA expression levels (fold change) of genes involved in oxidative stress, apoptosis, inflammation, steroidogenesis, and spermatogenesis in testicular tissue of male rats. (A) NrF2 (B) Bax (C) Bcl-2 (D) TNF-α (E) IL-6 (F) SOD2 (G) STAR (H) CYP11A1 (I) PRM1(J) GATA4. Data are expressed as mean ± SEM (n = 6). Different superscript letters (a–d) indicate statistically significant differences between groups (p < 0.05).
Data availability
The data available from the corresponding author on reasonable request.
References
Sarkar, S. et al. Nanoparticle-sensitized photodegradation of bilirubin and potential therapeutic application. J. Phys. Chem. C. 116, 9608–9615 (2012).
Metilli, L. et al. Latest advances in imaging techniques for characterizing soft, multiphasic food materials. Adv. Colloid Interface Sci. 279, 102154 (2020).
Zhou, Q., Yue, Z., Li, Q., Zhou, R. & Liu, L. Exposure to PbSe nanoparticles and male reproductive damage in a rat model. Environ. Sci. Technol. 53, 13408–13416 (2019).
Ali, A. et al. Comparative study of silica and silica-decorated ZnO and ag nanocomposites for antimicrobial and photocatalytic applications. Sci. Rep. 15, 5010 (2025).
Morishita, Y. et al. Distribution and histologic effects of intravenously administered amorphous Nanosilica particles in the testes of mice. Biochem. Biophys. Res. Commun. 420, 297–301 (2012).
Lin, B., XI, Z. & Zhang, Y. Oxidative damage on testicles of male rats induced by Micro-nano-scale sio_2. Journal Environ. Health (2007).
Fan, Y-O. et al. Comparative study of nanosized and microsized silicon dioxide on spermatogenesis function of male rats. Wei Sheng Yan jiu = J. Hygiene Res. 35, 549–553 (2006).
Baki, M. E. et al. Effects of silver nano-particles on sperm parameters, number of Leydig cells and sex hormones in rats. Iran. J. Reproductive Med. 12, 139 (2014).
Xu, Y. et al. Sun Z-W: exposure to silica nanoparticles causes reversible damage of the spermatogenic process in mice. PLoS One. 9, e101572 (2014).
Zhang, J. et al. Silica nanoparticles induce start Inhibition of meiosis and cell cycle arrest via down-regulating meiotic relevant factors. Toxicol. Res. 5, 1453–1464 (2016).
Hu, Y. et al. VDR promotes testosterone synthesis in mouse Leydig cells via regulation of cholesterol side chain cleavage cytochrome P450 (Cyp11a1) expression. Genes Genomics. 45, 1377–1387 (2023).
Ren, L. et al. Silica nanoparticles induce reversible damage of spermatogenic cells via RIPK1 signal pathways in C57 mice. International J. Nanomedicine 2016:2251–2264 .
Sun, F. et al. Reproductive toxicity investigation of silica nanoparticles in male pubertal mice. Environ. Sci. Pollut. Res. 29, 36640–36654 (2022).
Nagoor Meeran, M. F., Javed, H., Al Taee, H., Azimullah, S. & Ojha, S. K. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 8, 380 (2017).
Liu, Y. et al. Protective effects of natural antioxidants on inflammatory bowel disease: thymol and its Pharmacological properties. Antioxidants 11, 1947 (2022).
de Cássia da Silveira e Sá R, Andrade, L. N. & De Sousa, D. P. A review on anti-inflammatory activity of monoterpenes. Molecules 18, 1227–1254 (2013).
Archana, P., Rao, B. N. & Rao, B. S. In vivo radioprotective potential of thymol, a monoterpene phenol derivative of Cymene. Mutat. Research/Genetic Toxicol. Environ. Mutagen. 726, 136–145 (2011).
Llana-Ruiz-Cabello, M. et al. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. Vitro. 29, 647–656 (2015).
Jafari, A., Karimipour, M., Khaksar, M. R. & Ghasemnejad-Berenji, M. Protective effects of orally administered thymol against titanium dioxide nanoparticle–induced testicular damage. Environ. Sci. Pollut. Res. 27, 2353–2360 (2020).
Güvenç, M., Cellat, M., Gökçek, İ., Yavaş, İ. & Yurdagül Özsoy, Ş. Effects of thymol and carvacrol on sperm quality and oxidant/antioxidant balance in rats. Arch. Physiol. Biochem. 125, 396–403 (2019).
Boukholda, K. et al. Subacute silica nanoparticle exposure induced oxidative stress and inflammation in rat hippocampus combined with disruption of cholinergic system and behavioral functions. NanoImpact 24, 100358 (2021).
McGrath, J. C., Drummond, G., McLachlan, E., Kilkenny, C. & Wainwright, C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br. J. Pharmacol. 160, 1573–1576 (2010).
Council, N. R. & Nutrition, S. P. Nutrient Requirements of Poultry: 1994 (National Academies, 1994).
Saber, T. M. et al. Thymol alleviates imidacloprid-induced testicular toxicity by modulating oxidative stress and expression of steroidogenesis and apoptosis-related genes in adult male rats. Ecotoxicol. Environ. Saf. 221, 112435 (2021).
Tijani, A. S., Farombi, E. O. & Olori, D. O. Thymol co-administration abrogates hexachlorobenzene-induced reproductive toxicities in male rats. Hum. Exp. Toxicol. 42, 09603271221149201 (2023).
Can Baser, K. Biological and Pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 14, 3106–3119 (2008).
Guo, Z. et al. Silica nanoparticles cause spermatogenesis dysfunction in mice via inducing cell cycle arrest and apoptosis. Ecotoxicol. Environ. Saf. 231, 113210 (2022).
Kolasa, A. et al. The expression of inducible nitric oxide synthase (iNOS) in the testis and epididymis of rats with a dihydrotestosterone (DHT) deficiency. Cell. Mol. Biology Lett. 14, 511–527 (2009).
Tso, E-F. & Tam, W. The effect of continuous treatment with prostaglandin F-2α on oestrous cycle length and corpus luteum regression in hysterectomized guinea-pigs. Reproduction 50, 335–336 (1977).
Luna, L. G. Manual of histologic staining methods of the Armed Forces Institute of Pathology. In Manual of histologic staining methods of the Armed Forces Institute of Pathology. : xii, 258-xii, 258 (1968).
Yakubu, M. T., Akanji, M. A. & Oladiji, A. T. Male sexual dysfunction and methods used in assessing medicinal plants with aphrodisiac potentials. (2007).
Amini, A. & Kamkar, F. The effects of gossypol on spermatogenesis in NMRI mice. Iran. J. Sci. 29, 123–133 (2005).
Thakur, M. & Dixit, V. Effect of Chlorophytum borivilianum on androgenic & sexual behavior of male rats. INDIAN DRUGS-BOMBAY-. 43, 300 (2006).
Wyrobek, A. J. et al. An evaluation of the mouse sperm morphology test and other sperm tests in nonhuman mammals: A report of the US environmental protection agency Gene-Tox program. Mutat. Research/Reviews Genetic Toxicol. 115, 1–72 (1983).
Rezvanfar, M. et al. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum. Exp. Toxicol. 27, 901–910 (2008).
Goma, A. A. et al. Examining the influence of zinc oxide nanoparticles and bulk zinc oxide on rat brain functions: a comprehensive neurobehavioral, antioxidant, gene expression, and histopathological investigation. Biol. Trace Elem. Res. 202, 4654–4673 (2024).
Ghasemnejad-Berenji, M. et al. Rapamycin protects testes against germ cell apoptosis and oxidative stress induced by testicular ischemia-reperfusion. Iran. J. Basic. Med. Sci. 20, 905 (2017).
Yazdani, I., Majdani, R., Ghasemnejad-Berenji, M. & Dehpour, A. R. Comparison of multiple doses of cyclosporine A on germ cell apoptosis and epididymal sperm parameters after testicular ischemia/reperfusion in rats. Exp. Mol. Pathol. 110, 104271 (2019).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods 25, 402–408 (2001).
Bitar, A., Ahmad, N. M., Fessi, H. & Elaissari, A. Silica-based nanoparticles for biomedical applications. Drug Discovery Today. 17, 1147–1154 (2012).
Parveen, S., Misra, R. & Sahoo, S. K. Nanoparticles: a Boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine cancer 2017:47–98 .
Zhang, L. et al. Silica nanoparticles exacerbates reproductive toxicity development in high-fat diet-treated Wistar rats. J. Hazard. Mater. 384, 121361 (2020).
Lin, B. et al. Experimental study on the reproductive damage of male rats induced by micro-nano-scale SiO2. Asian J. Ecotoxicol. 2, 195–201 (2007).
Park, E-J. & Park, K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol. Lett. 184, 18–25 (2009).
Solomon, R. et al. Involvement of cytokines and hormones in the development of spermatogenesis in vitro from spermatogonial cells of cyclophosphamide-treated immature mice. Int. J. Mol. Sci. 22, 1672 (2021).
Herrera-Bravo, J. et al. Thymol as adjuvant in oncology: molecular mechanisms, therapeutic potentials, and prospects for integration in cancer management. Naunyn. Schmiedebergs Arch. Pharmacol. 397, 8259–8284 (2024).
Ryu, D-Y., Kim, K-U., Kwon, W-S., Rahman, M. S. & Khatun, A. Pang M-G: Peroxiredoxin activity is a major landmark of male fertility. Sci. Rep. 7, 17174 (2017).
Mital, P., Hinton, B. T. & Dufour, J. M. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol. Reprod. 84, 851–858 (2011).
Aboushouk, A., Mehana, E-S., Oda, S., Hashem, M. & El-Karim, D. The protective role of thymol against methomyl-induced toxicity in male rats: clinico-biochemical, histopathological and immuno-histochemical studies. (2021).
Hu, H. et al. Silicon dioxide nanoparticles induce insulin resistance through Endoplasmic reticulum stress and generation of reactive oxygen species. Part. Fibre Toxicol. 16, 1–18 (2019).
Jafari, A., Rasmi, Y., Hajaghazadeh, M. & Karimipour, M. Hepatoprotective effect of thymol against subchronic toxicity of titanium dioxide nanoparticles: biochemical and histological evidences. Environ. Toxicol. Pharmacol. 58, 29–36 (2018).
Peng, X., Zhang, X., Sharma, G. & Dai, C. Thymol as a potential neuroprotective agent: mechanisms, efficacy, and future prospects. J. Agric. Food Chem. 72, 6803–6814 (2024).
Aoki, V. W., Liu, L. & Carrell, D. T. A novel mechanism of Protamine expression deregulation highlighted by abnormal Protamine transcript retention in infertile human males with sperm Protamine deficiency. Mol. Hum. Reprod. 12, 41–50 (2006).
Viger, R. S., Guittot, S. M., Anttonen, M., Wilson, D. B. & Heikinheimo, M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol. Endocrinol. 22, 781–798 (2008).
Manuylov, N. et al. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev. Biol. 353, 229–241 (2011).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mahmoud A. Khedr, Amira A. Goma, Sara E. El-Kazaz and Rashed R. Rashed. The first draft of the manuscript was written by Mahmoud A. Khedr, Amira A. Goma, Mustafa Shukry, and Hossam G. Tohamy, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khedr, M.A., Goma, A.A., Rashed, R.R. et al. Thymol alleviates silica dioxide nanoparticle-induced reproductive performance toxicity via antioxidant and anti-inflammatory mechanisms in male rats. Sci Rep 15, 23913 (2025). https://doi.org/10.1038/s41598-025-07769-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07769-x