Abstract
The aim of this study was to investigate the effects and mechanisms of ANGPTL4 on cognitive impairment in vascular dementia rats. 36 SD rats were randomly divided into Sham(n= 9), VaD(n= 9), VaD + ANGPTL4 OE(n= 9), and VaD + ANGPTL4 KD(n= 9). A bilateral carotid artery ligation (2-VO) rat VaD was established to study the effects of ANGPTL4. Spatial memory was tested in rats using the Morris water maze. Morphological changes of neurons were detected in the CA1 region of the hippocampus by hematoxylin-eosin staining. The expression of ANGPTL4, p-Syk in the cells of hippocampal CA1 area was also detected by immunohistochemistry. Afterwards, protein expression of ANGPTL4, p-Syk, p-JNK, BNIP3 was detected by Western blot (WB). Afterwards, the mechanism of ANGPTL4 effect on cognitive impairment in vascular dementia rats was further explored by ANGPTL4 OE hippocampal cells 1%O2 low-serum low-glucose stimulation with Syk inhibitor, JNK inhibitor. ANGPTL4 OE aggravated cognitive dysfunction in 2VO rats. ANGPTL4 KD treatment improved the memory performance of 2VO rats.Hippocampal tissue damage was obvious in the VaD group.Hippocampal tissue damage was aggravated in the ANGPTL4 OE group (P < 0.001).ANGPTL4 KD group Pathological features were significantly improved(P < 0.001). WB assay showed that the expression of ANGPTL4, p-Syk, p-JNK, and BNIP3 proteins was increased in 2VO rats(P < 0.05), which was further up-regulated by ANGPTL4 OE treatment, and significantly inhibited by ANGPTL4 KD treatment in 2VO rats(P < 0.05). In vitro cellular experiments revealed that ANGPTL4 OE treatment again up-regulated ANGPTL4, integrin, p-Syk, and p-JNK protein expression consistent with the in vivo results(P < 0.05), where the Syk inhibitor suppressed both p-Syk, and p-JNK protein expression(P < 0.05). It is also worth noting that the mitochondrial autophagy-related proteins BNIP3, PINK1, Parkin and JC-1 mitochondrial membrane potential assayed by wb revealed that ANGPTL4 OE treatment exacerbated mitochondrial stress(P < 0.05) and apoptosis(P < 0.05) in hippocampal cells, and that Syk inhibitor, and JNK inhibitor significantly inhibited the modulation of ANGPTL4 OE(P < 0.05). ANGPTL4 promotes mitochondrial autophagy and apoptosis in the hippocampal CA1 region by activating the integrin/p-Syk signalling pathway, thus aggravating cognitive impairment in vascular dementia rats.
Similar content being viewed by others
Introduction
Dementia is a serious neurological disorder characterised by significant impairment of memory, thinking skills, behaviour and daily activities1. According to the World Health Organization’s latest dementia data for 2023, more than 55 million people worldwide are currently living with dementia2. Vascular dementia (VaD) is the second most common type of dementia after Alzheimer’s disease, which is mainly caused by lesions in the cerebrovascular system and manifests as a gradual decline in cognitive function3. Vascular dementia accounts for about 20–30% of all dementia cases.
With the acceleration of the global aging process, the prevalence of dementia is increasing year by year, bringing a heavy burden to the society and healthcare system. The etiology of vascular dementia is complex and is usually closely associated with cerebrovascular diseases, such as stroke, atherosclerosis and microvascular lesions4. These vascular lesions lead to insufficient blood supply to the brain, resulting in neuronal damage and death, which in turn causes cognitive dysfunction5. Although research on vascular dementia has increased in recent years, its exact pathogenesis has not been fully elucidated, and effective prevention and treatment are still lacking.
Recent studies have shown that mitochondrial dysfunction plays a role in the pathogenesis of vascular dementia6. Mitochondria are the main energy-producing factories in cells, and their health status directly affects cell survival and function. Mitochondrial autophagy (mitophagy) is an important mechanism by which cells remove damaged mitochondria to maintain normal mitochondrial function, but abnormal mitochondrial autophagy may lead to apoptosis and exacerbate neurodegenerative diseases such as vascular dementia7,8.
ANGPTL4 is a multifunctional secreted protein that plays important roles in different physiological and pathological processes. For example, ANGPTL4 has shown significant regulatory roles in metabolic regulation, inflammatory response, angiogenesis and tumour progression9,10,11. In particular, in the central nervous system, ANGPTL4 has been shown to have an effect on blood-brain barrier permeability, as well as the ability to regulate neuroinflammation11,12,13. However, the specific role of ANGPTL4 in vascular dementia and its molecular mechanisms are still in their infancy and have not been systematically investigated.
The integrin receptor is involved in the regulation of a variety of cellular functions, including survival, differentiation and migration, by interacting with multiple ligands and activating the downstream Syk kinase (Syk)14. In addition, the Syk kinase signalling pathway plays a key role in the onset and progression of several neurodegenerative diseases, and its aberrant activation may be closely related to mitochondrial autophagy and apoptosis15,16. Therefore, we speculate that ANGPTL4 may exacerbate cognitive impairment in vascular dementia by affecting mitochondrial autophagy and apoptosis through the integrin/Syk signalling pathway.
The aim of this study was to investigate the role of ANGPTL4 in vascular dementia and its underlying mechanisms, and to elucidate whether ANGPTL4 causes mitochondrial autophagy and apoptosis in the hippocampal region (especially the CA1 region) via the integrin/Syk signalling pathway, thereby contributing to cognitive dysfunction, through a systematic study. By understanding the specific mechanism of action of ANGPTL4 in vascular dementia, we hope to provide a theoretical basis and potential targets for the development of novel therapeutic strategies for vascular dementia.
Materials and methods
Bioinformatics
The dataset GSE122063 was downloaded from the Gene Expression Omnibus (GEO) database, which contains 18 VaD-FrontalCortex samples and 22 healthy control samples, and 18 VaD-TemporalCortex samples and 22 healthy control samples. The data were analysed for differentially expressed genes using the limma package, with a threshold set at |log2 Fold Change|>1 and a p-value of < 0.05. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were carried out using the ClusterProfiler package, with the GO analyses covering cellular components (CC), molecular functions ( MF) and biological processes (BP), and KEGG analysis was used to reveal key biological pathways. Relationships between differentially expressed genes were analysed by plotting correlation scatter plots.
Animal grouping and vadling
Male Sprague-Dawley rats were purchased from Henan Skibbes Biotechnology Co. The rats were housed in a hospital experimental centre at a temperature of 25 ± 1 °C with a light-dark cycle of 12:12 h. Rats were randomly divided into four groups: Sham group (n = 9), VaD (2VO rats) group (n = 9), VaD + ANGPTL4 OE group (n = 9), and VaD + ANGPTL4 KD group (n = 9).
2-vessel occlusions (2-VOs) was performed to rats as previously described17. After induction of anaesthesia by inhalation of 4% isoflurane for 3–5 min, anaesthesia was maintained in rats using 2% isoflurane (0.5 L / min). After the rats were in the supine position, a 2-cm incision was made in the middle of the rat’s neck region. Next, the muscles and fascia were bluntly dissected. The carotid artery was isolated and exposed within the carotid sheath. The carotid artery was permanently ligated using a 4 − 0 silk suture.After 30 min, the other carotid artery was ligated in the same manner.The Sham group underwent the same procedure without carotid artery ligation. Nude mice are placed in the IVC cage box. After putting the animals into the cage, fasten the lid of the cage, access the CO2 gas pipe at the entrance where the water bottle is placed, open the valve of the gas bottle, and fill CO2 into the box at the rate of replacing 10%~30% of the euthanasia box’s volume per minute, so as to allow CO2 to fill the cage box. After the nude mice fainted and lost the ability to move, increase the gas flow rate, the maximum flow rate should not exceed 0.5KPa, make sure that the nude mice do not move, do not breathe, and the pupils are dilated, then turn off the CO2 and observe for two minutes to make sure that the nude mice are dead. After euthanasia of the nude mice, close the gas valve, remove the body and store it in the designated freezer. All methods in this experiment were performed in accordance with the relevant guidelines and regulations and conformed to THE RULES OF 3R and ARRIVE guidelines.
Corresponding lentiviral constructs from the VaD + ANGPTL4 OE group and the VaD + ANGPTL4 KD group were injected stereotactically into the bilateral hippocampus and bilateral ventricles to overexpress or knock down the expression of the ANGPTL4 gene in the hippocampus and some other brain regions. the VaD group, as a negative control, was injected with NC lentivirus, and the Sham group underwent the same procedure without carotid artery ligation injection of equal amounts of saline. Demented rats were induced by 2-VO 1 week after stereotactic injection.Spatial learning and memory performances were evaluated four weeks after 2-VO via the Morris Water Maze (MWM) test.
Morris water maze
The Morris water maze experiment was performed as previously described18. In the Morris water maze experiment, a circular pool of approximately 2.0 m in diameter is first prepared and a hidden circular platform is placed in the pool, ensuring that the platform is 1–2 cm underwater. Visual markers should be placed on the wall of the pool as references. Rats are placed in the pool from different quadrants and allowed to swim to find the hidden platform. Training usually lasts for 5 days, with each session lasting 60 s, or less if the animal finds the platform earlier. At the end of the training, a test phase was conducted, which included a platform removal test and a reversal task. In the platform removal test, the hidden platform is removed and the time the animal spends in the target area is recorded to assess its memory. Finally, a camera system was used to record the animal’s movement trajectory and behavioural data, and the equipment was cleaned and disinfected after each experiment. The experimental environment was ensured to be quiet and the water temperature was stable to ensure the reliability of the experimental data.
HE and immunohistochemistry
Rats were executed after the behavioural test for pathological examination. Hippocampal tissues were dissected from the brain and placed in 4% paraformaldehyde, cut into pieces, and the tissues were sectioned for pathological experiments using paraffin embedding.HE staining: hematoxylin was used to stain the nucleus chromatin while eosin was used to stain the cytoplasm. All samples were observed under an Olympus BX53 microscope at 400x magnification. Images of the same CA1 region were captured from three sections of each animal using image analysis software (Olympus Stream). Immunohistochemistry: antigen repair was performed by heating in citrate buffer. Sections were then closed with 10% goat serum TBS to prevent non-specific binding. Incubation with primary antibodies ANGPTL4 and p-Syk (ANGPTL4, 1:100, ab206420; p-Syk, 1:100, ab62338) was performed overnight at 4 °C. Subsequently, secondary antibodies were applied and incubated for 30 min at room temperature. Afterwards, the affinobiotin-biotin complex (ABC) was added and incubated for 30 min. Colour was developed using 3,3′-diaminobenzidine (DAB) solution and incubated for 10 min. Finally, the sections were restained with hematoxylin for nuclear staining. The results were observed under a microscope and recorded.
Western blot
First, hippocampal tissues were lysed using RIPA buffer (P0013B; Beyotime Biotechnology, Shanghai, China) and PMSF (ST505; Beyotime Biotechnology, Shanghai, China). The lysed homogenate was centrifuged at 4 °C for 10 min, and the supernatant was collected into EP tubes and the protein was quantified using the BCA Protein Assay Kit (P0012S; Beyotime Biotechnology, Shanghai, China). Then, the samples were heated at 95–100 °C for 5 min to denature the proteins. Next, 20 µg of proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane (IPVH00010; Milford, MA, USA). The PVDF membrane was closed with 5% skimmed milk for 30 min at room temperature to block non-specific protein binding. Subsequently, the membranes were incubated with primary antibodies at 4 °C overnight. Next, horseradish peroxidase-coupled secondary antibody (1:10,000; SA00001-2; Proteintech, Wuhan, China) was diluted with 0.1% TBST and incubated for 1 h at room temperature. Finally, the expression of target proteins was visualised and semi-quantitatively analysed using an enhanced chemiluminescence system (Tanon-5200, Shanghai, China). Primary antibodies used: ANGPTL4 (1:1000, ab206420), p-Syk (1:1000,ab62338), p-JNK (1:1000,ab307802), BNIP3 (1:1000,ab109362), Integrin (1:500, ab227154), PINK1 (1:1000,ab216144),Parkin (1:2000,ab77924) GAPDH (1:1000,ab8245).
Hippocampal cell culture
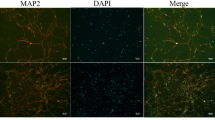
Hippocampal cells(HT22; Zhong Qiao Xin Zhou Biotechnology Co., Ltd, Shanghai, China) were divided into five groups: the NC group, where cells were cultured in normal medium without any treatment; the 1%O2 low serum low glucose stimulation group, where cells were cultured in 1% FBS and 1 g/L glucose and incubated in a 1%O2 cell culture incubator; the 1%O2 low serum low glucose stimulation + ANGPTL4 OE group, where hippocampal cells transfected with ANGPTL4 OE were cultured in 1% FBS and 1 g/L glucose and cultured in 1% O2 cell culture incubator; 1% O2 low blood and low glucose stimulation + ANGPTL4 OE + Syk inhibitor (R788 disodium; Selleckchem) group, hippocampal cells transfected with ANGPTL4 OE were cultured in 1% FBS and 1 g/L glucose and cultured in a 1% O2 cell culture incubator, and hippocampal cells were stimulated using an additional R788 disodium (10 µM, 1 h); for the 1% O2 hypoglycaemic and hypoglycaemic stimulation + ANGPTL4 OE + JNK inhibitor (SP600125 ; MedChemExpress) group, the hippocampal cells transfected with ANGPTL4 OE were cultured in 1% of FBS and 1 g/L glucose in medium and cultured in a 1% O2 cell culture incubator, and hippocampal cells were stimulated using additionally SP600125 (10 µM, 1 h);
JC-1 mitochondrial membrane potential assay
Mitochondrial membrane potential of hippocampal cells was detected using JC-1 dye (C2006; Beyotime Biotechnology, Shanghai, China). Cells were inoculated into six-well plates, and approximately 5 × 10^5 cells were cultured per well. After treatment, cells were collected and washed twice with PBS. Cells were incubated with JC-1 staining working solution according to the manufacturer’s instructions. After warming at 37 °C for 20 min, the cells were again washed twice with PBS. Cells were observed using a fluorescence microscope (Nikon, Japan), and the intensity of red fluorescence (JC-1 aggregates, representing high membrane potential) and green fluorescence (JC-1 monomers, representing low membrane potential) was recorded. Changes in mitochondrial membrane potential were quantified by the red/green fluorescence ratio.
Statistical analysis
Data are presented as mean ± standard error of the mean. Statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software, USA). For statistical comparisons, one-way ANOVA with Bonferroni post hoc test was used to compare multiple experimental groups, and Student’s t-test was used if comparing two groups. p < 0.05 was considered statistically significant.
Results
Pathway analysis of VaD-related differentially expressed genes and ANGPTL4 upregulation in the GSE122063 dataset
In dataset GSE122063, the differentially expressed genes between VaD-FrontalCortex and healthy control samples included 152 significantly up-regulated genes and 251 significantly down-regulated genes; the differentially expressed genes between VaD-TemporalCortex and healthy control samples included 128 significantly up-regulated genes and 269 significantly down-regulated genes, of which ANGPTL4 was a significantly up-regulated gene. Noticeably, ANGPTL4, SYK and JNK (MAPK8) showed significant up-regulation in both brain regions, and these genes may be involved in the pathological process of VaD by modulating inflammatory responses and MAPK signalling pathways. Wayne diagram analysis revealed 221 intersecting genes. GO analysis showed that these differentially expressed genes were enriched in collagen-containing extracellular matrix, integrin complex, and collagen trimer in cellular component (CC); and in molecular function (MF); in molecular function (MF), they were enriched in extracellular matrix structural constituent, extracellular matrix structural constituent conferring tensile strength and integrin binding; The KEGG pathway analysis revealed that the differential genes were significantly enriched in PI3K-Akt signaling pathway, VEGF signaling pathway and ErbB signaling pathway. correlation analysis showed that ROS could promote SYK expression, SYK further promoted JNK (MAPK8) expression, and JNK (MAPK8) promoted BNIP3 expression. Correlation analysis revealed a positive correlation between ROS and SYK, a positive correlation between SYK and JNK (MAPK8), a positive correlation between JNK (MAPK8) and BNIP3, and a positive correlation between ANGPTL4 and SYK and JNK (MAPK8) (Fig. 1).
Bioinformatics analysis. A, Differential gene volcano plots for VaD-FrontalCortex and healthy control samples; B, Differential gene volcano plots for VaD-TemporalCortex and healthy control samples; C, Wayne plots; D, GO enrichment analyses histograms; E, KEGG enrichment analyses bubble plots; F, ROS and SYK correlation scatter plots; G, SYK and JNK correlation scatterplot; H, JNK and BNIP3 correlation scatterplot; I, ANGPTL4 and SYK correlation scatterplot; J, ANGPTL4 and JNK (MAPK8) correlation scatterplot.
ANGPTL4 overexpression exacerbates cognitive deficits in 2-VO rats
In the Morris water maze (MWM) experiment, ANGPTL4 overexpression (OE) significantly reduced the frequency of crossing the original platform (F = 44.80, P < 0.0001) in 2-VO rats, indicating that ANGPTL4 overexpression exacerbated spatial memory deficits in rats. Conversely, ANGPTL4 knockdown (KD) treatment significantly improved the cognitive function of 2-VO rats (F = 21.64, P < 0.0001), as evidenced by the comparison of the frequency of crossing the original platform with that of the VaD group (Fig. 2).
ANGPTL4 aggravates hippocampal tissue damage in 2VO rats via p-Syk
As observed by hematoxylin-eosin (HE) staining, neurons in the CA1 area of the hippocampus of rats in the VaD group showed significant morphological changes, including a reduction in the number of neurons and structural abnormalities.ANGPTL4 OE treatment further exacerbated these injuries, whereas ANGPTL4 KD treatment significantly improved the morphology of neurons in the CA1 area of the hippocampus (Fig. 3A).
Immunohistochemical results showed that the expression of p-Syk was significantly increased in the hippocampal CA1 region of rats in the VaD group compared with the Sham group (P < 0.0001, t = 17.12). ANGPTL4 OE treatment further upregulated the expression of p-Syk proteins (P < 0.0001, t = 11.91), while ANGPTL4 KD treatment significantly suppressed the expression of tp-Syk proteins (P < 0.0001, t = 10.24) (Fig. 3B).
ANGPTL4 affects mitochondrial autophagy in 2VO rat hippocampal cells by regulating p-Syk, p-JNK, and BNIP3 protein expression
In the CA1 region of the hippocampus of VaD rats, Western blot analysis showed that the expression of p-Syk (P < 0.0001, t = 10.17), Syk (P < 0.0001,t = 12.00), p-JNK (P < 0.0001,t = 7.238) and JNK (P < 0.0001,t = 17.69)pr oteins was significantly increased in the ANGPTL4 OE group compared with the VaD group. In addition, the expression of BNIP3, a key protein in mitochondrial autophagy, was also significantly upregulated after ANGPTL4 OE treatment (P < 0.0001,t = 7.830). These changes suggest that overexpression of ANGPTL4 may have led to increased mitochondrial autophagy and exacerbated apoptosis through activation of the p-Syk and p-JNK signalling pathways as well as increased expression of BNIP3, thereby affecting cognitive function in 2VO rats. In contrast, ANGPTL4 knockdown (KD) treatment significantly suppressed the expression of these proteins (p-Syk: P < 0.0001, t = 10.60; Syk: P < 0.0001, t = 10.52; p-JNK: P < 0.0001, t = 8.920; JNK: P < 0.0001, t = 14.47; BNIP3: P < 0.0001, t = 12.34), suggesting that inhibition of ANGPTL4 expression attenuates the degree of activation of these signalling pathways, which may help to attenuate the onset of mitochondrial stress and apoptosis (Fig. 4).
ANGPTL4 regulates mitochondrial autophagy through syk/jnk signalling pathway
In in vitro experiments, we further verified the effect of ANGPTL4 expression on Syk and JNK signalling pathways. By transfecting ANGPTL4 overexpression plasmid into hippocampal cells, the results showed that the expression of ANGPTL4 (P = 0.005, t = 5.610), integrin (P < 0.01, t = 6.152), p-Syk (P < 0.01, t = 5.272), and p-JNK (P < 0.01, t = 4.839) proteins were significantly up-regulated. integrin, as a cell-surface receptor protein, interacts with ANGPTL4 and may be a key step in the activation of the Syk signalling pathway. Subsequently, we treated ANGPTL4 overexpressing hippocampal cells with a Syk inhibitor (R788 disodium) and found that the expression of both p-Syk (P < 0.001, t = 11.81) and p-JNK (P < 0.01, t = 8.129) proteins was significantly decreased, and similarly, the use of a JNK inhibitor significantly suppressed the expression of p-JNK (P < 0.001, t = 10.99) (Fig. 5A).
Mitochondrial autophagy-related proteins BNIP3, PINK1, and Parkin by Western blot revealed that ANGPTL4 overexpression (OE) treatment exacerbated mitochondrial stress in hippocampal cells (BNIP3: P < 0.001, t = 13.23; PINK1: P < 0.001, t = 5.576; Parkin: P < 0.01, t = 6.84). However, when hippocampal cells with ANGPTL4 OE were treated with Syk inhibitor (R788 disodium) and JNK inhibitor(SP600125), it was found that these inhibitors significantly suppressed the regulatory effects of ANGPTL4 OE on the above signalling pathways and mitochondrial autophagy. R788 disodium (BNIP3:P < 0.0001,t = 15.58; PINK1:P < 0.01, t = 8.258; Parkin: P < 0.001,t = 11.11), SP600125 (BNIP3:P < 0.001,t = 17.24; PINK1:P < 0.001, t = 9.422; Parkin: P < 0.01,t = 9.525) This suggests that ANGPTL4 regulates mitochondrial autophagy through the Syk/JNK signalling pathway (Fig. 5B).
To explore the mitophagy/apoptosis crosstalk, we simultaneously detected apoptosis-related protein Bax, Caspase-3, and Caspase-8 protein expression (Fig. 5C). The results revealed that Bax (P < 0.01, t = 4.750), Caspase-3 (P < 0.01, t = 5.116), and Caspase-8 (P < 0.01, t = 5.059) proteins were likewise significantly increased by the treatment of ANGPTL4 OE, and that the treatment of R788 disodium and SP600125 resulted in a significant increase in the expression of Bax (R788 disodium: P < 0.0001,t = 18.16; SP600125: P < 0.0001,t = 20.85), Caspase-3 (R788 disodium: P < 0.0001,t = 19.98; SP600125: P < 0.0001,t = 18.07), Caspase-8 ( R788 disodium: P < 0.0001,t = 14.73; SP600125:P < 0.001,t = 13.80) expression was significantly reduced.
ANGPTL4 regulates apoptosis through syk/jnk signalling pathway
Based on the experimental results of 2.5, we further investigated the mechanism of ANGPTL4 regulating apoptosis through the Syk/JNK signalling pathway. Using red fluorescence and green fluorescence to label apoptotic and surviving cells respectively, the results showed that red fluorescence was significantly enhanced and green fluorescence was significantly weakened in ANGPTL4 overexpression treatment group, which indicated that hippocampal cell apoptosis was increased(P < 0.01, t = 6.103) and the surviving cells were decreased(P < 0.01, t = 5.298) (Fig. 6A).
Upon treatment of ANGPTL4 overexpressing hippocampal cells with Syk inhibitor and JNK inhibitor, red fluorescence was significantly attenuated and green fluorescence was significantly enhanced, indicating a decrease in apoptosis and an increase in surviving cells.R788 disodium(P < 0.01, t = 6.525;P < 0.05, t = 4.366), SP600125(P < 0.01, t = 6.671;P < 0.05, t = 4.425).This further validated the role of ANGPTL4 in regulating apoptosis through the Syk/JNK signalling pathway (Fig. 6B). These results suggest that ANGPTL4 regulates mitochondrial autophagy and apoptosis through the Syk/JNK signalling pathway during the pathological process of vascular dementia and affects the survival status of hippocampal cells.
Discussion
Vascular dementia (VD) is one of the most common cognitive dysfunctions after Alzheimer’s disease, which is mainly caused by cerebrovascular lesions, and has a complex pathological mechanism involving multiple factors, including impaired blood-brain barrier, neuroinflammatory response, oxidative stress and mitochondrial dysfunction6,19,20,21. During these processes, mitochondrial autophagy, as an important intracellular clearance mechanism, is able to remove damaged mitochondria and maintain cellular homeostasis and balance22. However, excessive mitochondrial autophagy may lead to apoptosis and further aggravate brain damage. Fibroblast growth factor (ANGPTL4), as a multifunctional regulatory protein, exhibits significant roles in several pathological processes. The aim of this study was to investigate the role of mitochondrial autophagy and its triggered apoptosis mediated by ANGPTL4 through the integrin/p-Syk signalling pathway in VD and to gain insight into the relationship between these signalling pathways.
The role of ANGPTL4 in VD through activation of the p-Syk signalling pathway has attracted our attention. p-Syk is an important tyrosine kinase with multiple functions in cell signalling. p-Syk activation can regulate cellular inflammatory responses and apoptotic processes through a variety of downstream effector molecules23,24. p-Syk expression is upregulated by ANGPTL4 that may exacerbate neuroinflammation by promoting the release of inflammatory factors, thereby exacerbating nerve injury25. In further studies, we found that ANGPTL4 was able to regulate the expression of p-JNK, a member of the c-Jun N-terminal kinase family, which has important roles in stress response, inflammation, and apoptosis26,27,28.ANGPTL4, by up-regulating the activity of p-JNK, may promote the enhancement of the intracellular stress response, thereby exacerbating mitochondrial autophagy29. Notably, p-JNK not only plays a role in the autophagy process, but also plays a key role in the regulation of apoptosis. p-JNK activation affects cell survival and death by modulating a series of downstream effector molecules, such as Bcl-2 family proteins28.
This study also revealed the mechanism by which ANGPTL4 affects mitochondrial autophagy through BNIP3 protein, a pro-apoptotic member of the Bcl-2 family, whose expression is significantly increased under hypoxic conditions and promotes mitochondrial autophagy by binding to LC3 protein on the outer membrane of mitochondria30.The up-regulation of ANGPTL4 might lead to mitochondrial autophagy enhancement. During the pathological process of VD, a moderate increase in mitochondrial autophagy contributes to the removal of damaged mitochondria and the maintenance of cellular function. However, excessive mitochondrial autophagy leads to a large loss of mitochondria, affecting cellular energy metabolism and ultimately triggering apoptosis31.
ANGPTL4 forms a complex signalling network through the co-regulation of Syk and JNK signalling pathways. In this network, Syk and JNK interact with each other to co-regulate mitochondrial autophagy and apoptosis. activation of Syk can promote the activation of JNK through upstream signalling, whereas the activation of JNK further amplifies the signal strength of mitochondrial autophagy and apoptosis. Notably, Syk and JNK may exhibit different modes of regulation in different cellular environments and pathological conditions, and this complexity offers diverse possibilities for therapeutic strategies in VD.
Through the present study, we can see that a complex regulatory network is formed among ANGPTL4, Syk, JNK and BNIP3. ANGPTL4 directly or indirectly affects mitochondrial autophagy and apoptosis by regulating the activities of Syk and JNK. BNIP3, as a key regulator of mitochondrial autophagy, plays an important role in connecting mitochondrial function and apoptosis in this network. The complexity of this regulatory network suggests that therapeutic strategies for VD should comprehensively consider the interactions of multiple signalling pathways with a view to achieving optimal therapeutic effects.
In this study, we revealed the mechanism by which ANGPTL4 mediates mitochondrial autophagy and triggers apoptosis through the integrin/p-Syk signalling pathway during VD pathology. We found that ANGPTL4 forms a complex signalling network that affects mitochondrial function and cell survival through the regulation of the Syk/JNK signalling pathway. Our findings not only enriched the understanding of the pathogenesis of VD, but also suggested that ANGPTL4 and its related signalling pathway may be a new target for VD therapy. Future studies should further explore the potential therapeutic effects of inhibiting ANGPTL4 and its downstream signalling pathways, with a view to providing new strategies for the prevention and treatment of VD.
Through this study, we can see that a complex regulatory network is formed among ANGPTL4, Syk, JNK and BNIP3. ANGPTL4 directly or indirectly affects mitochondrial autophagy and apoptosis by regulating the activities of Syk and JNK. BNIP3, as a key regulator of mitochondrial autophagy, plays an important role in connecting mitochondrial function and apoptosis in this network. The complexity of this regulatory network suggests that therapeutic strategies for VD should comprehensively consider the interactions of multiple signalling pathways with a view to achieving optimal therapeutic effects.
In this study, we revealed the mechanism by which ANGPTL4 mediates mitochondrial autophagy and triggers apoptosis through the integrin/p-Syk signalling pathway during VD pathology. We found that ANGPTL4 forms a complex signalling network that affects mitochondrial function and cell survival through the regulation of the Syk/JNK signalling pathway. Our findings not only enriched the understanding of the pathogenesis of VD, but also suggested that ANGPTL4 and its related signalling pathway may be a new target for VD therapy. Future studies should further explore the potential therapeutic effects of inhibiting ANGPTL4 and its downstream signalling pathways, with a view to providing new strategies for the prevention and treatment of VD.
Data availability
The authors confirm that the data supporting the findings of this study are available within supplementary materials.
References
Korczyn, A. D., Vakhapova, V. & Grinberg, L. T. Vascular dementia. J. Neurol. Sci. 322 (1–2), 2–10 (2012).
Zheng, J. et al. Association of regular glucosamine use with incident dementia: evidence from a longitudinal cohort and Mendelian randomization study. BMC Med. 21 (1), 114 (2023).
Romay, M. C., Toro, C. & Iruela-Arispe, M. L. Emerging molecular mechanisms of vascular dementia. Curr. Opin. Hematol. 26 (3), 199–206 (2019).
Greenan, C. et al. A randomised controlled trial of calcium channel Blockade (CCB) with amlodipine for the treatment oF subcortical ischaemic vascular dementia (AFFECT): study protocol. Trials 17 (1), 324 (2016).
Bir, S. C. et al. Emerging concepts in vascular dementia: a review. J. Stroke Cerebrovasc. Dis. 30 (8), 105864 (2021).
Han, B. et al. Upregulation of neuronal PGC-1α ameliorates cognitive impairment induced by chronic cerebral hypoperfusion. Theranostics 10 (6), 2832–2848 (2020).
Li, J. et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res. Rev. 84, 101817 (2023).
Wu, S. et al. Gastrodin and Gastrodigenin improve energy metabolism disorders and mitochondrial dysfunction to antagonize vascular dementia. Molecules 28, 6 (2023).
Xu, S. et al. STAT2-induced linc02231 promotes tumorigenesis and angiogenesis through modulation of hnRNPA1/ANGPTL4 in colorectal cancer. J. Gene Med. 25 (8), e3506 (2023).
Avalle, L. et al. STAT3 induces breast cancer growth via ANGPTL4, MMP13 and STC1 secretion by cancer associated fibroblasts. Oncogene 41 (10), 1456–1467 (2022).
Zuo, Y. et al. Dual role of ANGPTL4 in inflammation. Inflamm. Res. 72 (6), 1303–1313 (2023).
Aryal, B. et al. ANGPTL4 in metabolic and cardiovascular disease. Trends Mol. Med. 25 (8), 723–734 (2019).
Yang, R. et al. Egr-1 is a key regulator of the blood-brain barrier damage induced by meningitic Escherichia coli. Cell. Commun. Signal. 22 (1), 44 (2024).
Bakthavatsalam, D., Craft, J. W. & Kazansky, J. R. Identification of inhibitors of integrin cytoplasmic domain interactions with Syk. Front. Immunol. 11, 575085 (2020).
Ennerfelt, H., Frost, E. L. & Shapiro, D. A. et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell 185 (22), 4135–52e22 (2022).
Ennerfelt, H. & Lukens, J. R. Microglia rely on SYK signalling to Mount neuroprotective responses in VaDs of alzheimer’s disease and multiple sclerosis. Clin. Transl Med. 13 (1), e1178 (2023).
Farkas, E. et al. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 108 (1), 57–64 (2004).
Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing Spatial and related forms of learning and memory. Nat. Protoc. 1 (2), 848–858 (2006).
O’brien, J. T. & Thomas, A. Vascular dementia. Lancet (Lond. Engl.) 386 (10004), 1698–1706 (2015).
Zhao, Y. et al. NAD(+) improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion VaDs through Sirt1/PGC-1α pathway. J. Neuroinflamm. 18 (1), 207 (2021).
Zhu, T. et al. Puerarin alleviates vascular cognitive impairment in vascular dementia rats. Front. Behav. Neurosci. 15, 717008 (2021).
Doblado, L. et al. Mitophagy in human diseases. Int. J. Mol. Sci. 22, 8 (2021).
Yi, Y. S. et al. Syk-MyD88 axis is a critical determinant of inflammatory-response in activated macrophages. Front. Immunol. 12, 767366 (2021).
Shi, W., Sun, Y. & Wang, J. et al. Trem1 mediates neuronal apoptosis via interaction with SYK after spinal cord ischemia-reperfusion injury. Am. J. Transl. Res. 13 (6), 6117–6125 (2021).
Ye, X. C. et al. Dectin-1/Syk signaling triggers neuroinflammation after ischemic stroke in mice. J. Neuroinflamm. 17 (1), 17 (2020).
Graczyk, P. P. JNK inhibitors as anti-inflammatory and neuroprotective agents. Future Med. Chem. 5 (5), 539–551 (2013).
Goberdhan, D. C. & Wilson, C. JNK, cytoskeletal regulator and stress response kinase? A Drosophila perspective. BioEssays 20 (12), 1009–1019 (1998).
Nadel, G., Maik-Rachline, G. & Seger, R. JNK cascade-induced apoptosis-a unique role in GqPCR signaling. Int. J. Mol. Sci. 24, 17 (2023).
Jin, Q. et al. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 14, 576–587 (2018).
Poole, L. P. & Macleod, K. F. Mitophagy in tumorigenesis and metastasis. Cell. Mol. Life Sci. 78, 3817–3851 (2021).
Zhang, J. & Ney, P. A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 16 (7), 939–946 (2009).
Author information
Authors and Affiliations
Contributions
Designing the experiment: Chunhong Yao, Jinfeng Wang. Performing the experiments: Chunhong Yao, Jinfeng Wang. Analyzing the data: Chunhong Yao. Preparing the figures and/or tables: Chunhong Yao. Drafting the work or revising it critically for important content: Chunhong Yao, Jinfeng Wang.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yao, C., Wang, J. ANGPTL4 promoted the cognitive impairment in vascular dementia via increasing integrin/p-Syk signalings induced mitochondrial autophagy and apoptosis in the hippocampus. Sci Rep 15, 25312 (2025). https://doi.org/10.1038/s41598-025-07811-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07811-y