Abstract
The spread of invasive aquatic species in canals and wetlands poses significant challenges, including reduced water availability, disruption of native biodiversity, and obstruction of irrigation infrastructure. This study examines the distribution and environmental associations of two prominent invasive species Pontederia crassipes and Pistia stratiotes within the Lake Burullus wetland in Egypt. Field surveys were conducted to assess plant morphology and abundance, alongside measurements of water quality parameters including dissolved oxygen (DO), phosphate (PO4–P), ammonium (NH4–N), nitrite (NO2–N), nitrate (NO3–N), total nitrogen (TN), total phosphorus (TP), turbidity, and oxidizable organic matter (OOM). Remote sensing data, particularly the Normalized Difference Vegetation Index (NDVI), were used to monitor the spatial and seasonal dynamics of Pontederia in the Elshaklouba drain. The findings indicated that plant abundance was associated with specific water quality variables; however causality could not be determined due to the observational design of the study. NDVI analysis confirmed increased Pontederia densities during the summer months, consistent with field observations. The study also documented local management practices, primarily mechanical removal and the use of physical barriers, and briefly compared these with biological and integrated control strategies reported in recent literature. Additionally, the potential application of these species in phytoremediation and bioenergy applications is discussed, underscoring their dual role as both ecological threats and potential resources.
Similar content being viewed by others
Introduction
Coastal wetlands play a vital role in supporting both human populations and biodiversity, offering numerous benefits such as flood control, storm protection, and climate regulation1. However, these ecosystems face increasing pressure from invasive alien plant species (IAPS), which disrupt ecological processes and degrade habitat quality. This issue is particularly evident in the Burullus wetland complex in the Egypt’s Nile Delta, where aquatic invasive species like Pontederia crassipes and Pistia stratiotes threaten the sustainability of these wetland ecosystems. According to the National Invasive Species Council2, the IAPSare defined as, “an alien species whose introduction does or is likely to cause economic or environmental harm or harm to human health.” These species can significantly impact local biodiversity, impair ecosystem services, and reduce environmental quality. They disrupt natural water cycles, lower water availability, and deteriorate water quality by increasing soil erosion, altering nutrient dynamics, and contributing to excessive nutrient accumulation3,4,5,6. Despite these considerable impacts, there is a lack of detailed researches on the specific effects of invasive species on water quality, particularly studies that incorporate advanced monitoring tools and techniques.
Invasive species can worsen the impacts of water pollution by altering the physical and chemical properties of the ecosystem they invade. For instance, some invasive aquatic plants can change sediment composition and water quality, impeding the growth of native plants and disrupting local food webs7. Moreover, some invasive species may introduce new parasites, stressing native populations already weakened by pollution7. This dynamic not only threatens biodiversity but also compromises the health of ecosystems that provide essential services, such as water filtration and habitat stability. The interplay between invasive species and pollution underscores the need for integrated management strategies that address both issues simultaneously.
Invasive species can negatively impact the environment, economy and human health, so the early monitoring and detection are very significant. Traditional field survey methods may be replaced or integrated with more efficient and cheaper methods such as remote sensing methods8. Many authors used Landsat data for detection water hyacinth in aquatic systems using vegetation spectral indices in the detection process9,10. Invasive species not only impact human health but also disrupt natural physical processes. They can alter the amount and timing of runoff, increase erosion and sedimentation in addition to influence on water availability11. Invasive species can disrupt the nitrogen. The disruption significantly impacts essential algae and higher plants, altering the structure of the food web by changing nitrogen absorption12. Any alteration in nitrogen levels in water body affect negatively on ecosystem as degradation of water quality.
While the ecological impacts of Pontederia crassipes and Pistia stratiotes are well-documented globally, there remains a significant gap in understanding their spatial–temporal dynamics within the Lake Burullus wetland, a Ramsar site of international importance. Previous studies have largely focused on field-based assessments or coarse-resolution imagery, limiting the ability to monitor rapid vegetation changes. This study addresses this gap by integrating high-resolution Landsat OLI8 images with advanced vegetation indices to map and analyze the spread of invasive aquatic plants over time. To our knowledge, this is the first study to apply such a remote sensing-based approach in Lake Burullus, offering new insights into the potential of satellite data for wetland management and conservation.
The main objectives of this study were to assess the impact of invasive aquatic plants, specifically Pontederia crassipes and Pistia stratiotes, on the ecological dynamics of the Burullus wetland complex under varying salinity and nutrient conditions. A particular focus was placed on examining the behavior and distribution of invasive species, especially Pontederiawithin the study area and its relationship to key water quality parameters and distribution, using Landsat satellite data. The Elshaklouba drain served as a case study for applying remote sensing data. Furthermore, the study aimed to integrate remote sensing with environmental indicators to monitor and assess the spatial distribution and density of invasive species, particularly Pontederia, across Burullus Lake. By analyzing spatial and environmental data, this study sought to identify the main factors driving the spread of these species, evaluate their ecological impacts, and provide insights for effective management strategies to mitigate their effects on the wetland ecosystem.
Materials and methods
Study area
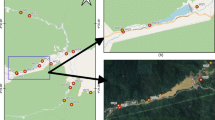
The study area is located in the central part of the Nile Delta and encompasses the Burullus wetland complex. It lies between longitudes 30°30′ and 31°10′E and latitudes 31°19′ and 31°36′N. It is known as a brackish water wetland complex with a mean depth of 115 cm13. This wetland receives drainage waters from eight main drains, listed from east to west: East Elburullus, Elkashaa, Tirra, Drain 7, Damru, Elshaklouba, and Elhoks. A freshwater canal, known as the Brinbal canal, is located at the western part of the lake. Another main drain, the Kitchener drain, does not discharge its water directly into the wetland but rather through lateral drains, with water reaching the lake via the Elkashaa drain14. The Burullus wetland complex is characterized by its variability in formations and biodiversity15,16. Sampling sites were distributed across the Burullus wetland complex, as illustrated in Fig. 1. Field photographs illustrating invasive alien aquatic plant species within the study area are presented in Fig. 2.
Onsite field images showing the distribution of invasive alien plants along the Burullus wetland. (A) Tirra drain, (B) Drain 7 indicating presence of P. stratioes beside E. crassipes, (C) Dense presence of Pontederia, (D) Dense presence front of Elkobry elaloy at Elshaklouba, (E) Elhoks station, (F) Digging practice in front of Brinbal outlet, (G) Halophytes species nearby Elboughaz and (H) beside Cornish Baltim city. All field images were captured during the field trip.
Collection of water samples
Water samples were collected on September 3, 2024, from the Burullus wetland complex, focusing on the drainage network, to monitor the floating invasive aquatic species Pontederia crassipes and Pistia stratiotes. At the time of sampling, the plants were widespread throughout the lake, especially in the southern areas. The collected water samples were transported to the laboratory at Baltim station of the National Institute of Oceanography and Fisheries, NIOF, Egypt for further analysis. The samples were filtered using 0.45 µm membrane filters for nutrient analysis. Ammonium ions in the samples were fixed in the field17.
Analyses of water parameters
The methodology employed in this study is summarized in Table 1. Several water parameters were analyzed to assess the environmental conditions influencing the presence and distribution of invasive plants, including temperature (T°C), pH, salinity, nutrients, chlorophyll a, oxidizable organic matter (OOM), and dissolved oxygen (DO).
Environmental characteristics and impacts of two invasive aquatic plants
Pontederia crassipes(commonly known as Water hyacinth) is a major macrophyte that disrupts water resources in many countries, potentially causing significant economic losses due to its rapid spread21. It is listed among the world’s 100 worst invasive alien species22. The morphological characteristics of P. crassipes vary with growth conditions. Petioles are elongated with circular leaves in dense stands and short with kidney-shaped leaves in less dense mats23. It belongs to the family Pontederiaceae and is an effective phytoremediator for metal ions such as cadmium (Cd) and chromium (Cr)24.
Pistia stratiotes belongs to the Araceae family, is a perennial monocotyledon with thick, soft leaves. This floating macrophyte is found on the water surface. The roots extend directly beneath the base of the floating leaves and emit a distinct musty odor25,26. The morphological characteristics of Pistia stratiotes are influenced by salinity, which can reduce leaf width. Conversely, increases in organic carbon and nutrient ions enhance plant biomass27. It thrives in slow-moving water habitats such as canals, ponds, and lakes28.
Measurements of plant parts
Specimens of Pontederia crassipes and Pistia stratiotes were collected in the field. The tested species were selected from the most abundant mats. The length of the petioles and stolons, as well as the height and width of the leaves, was measured. Measurements were taken from the minimum and maximum dimensions of Pontederia plants29.
Remote sensing data collection
Landsat OLI8 images were obtained from the Earth Explorer website and pre-processed using radiometric calibration in ENVI 5.330. The selected images acquisition dates were: 26 November 2023, 01 March 2024, 02 April 2024, 13 June 2024, 08 August 2024, 10 September 2024 and 03 October 2024. The spatial resolution of image bands was 30 m. These images were used assess the density of vegetation cover along the drain, particularly the invasive alien water hyacinth, which is highly distributed in the drain water. NDVI density detection was performed using ArcGIS 10.5 to calculate the area covered by vegetation. The NDVI classes were categorized into four levels based on vegetation density31 (Table 2). This analysis was based on the methodology of Mucheye et al.10 for water hyacinth detection, using the following equation:
According to Mucheye et al.10, water hyacinth is associated with NDVI values ranged between 0.6 and 0.95.
Statistical analysis
To assess the relationship between water quality and invasive plants in the study area, Principal Component Analysis (PCA) was employed as an optimization technique to highlight correlations and extract key features. Additionally, a linear regression model, based on correlation analysis, was used to estimate vegetation density as an indicator of invasive plant presence.
Results and discussion
Human activities and water quality roles in distribution of invasive plants
In the study area, various human activities surrounding and within the vicinity of Lake Burullus were identified including agricultural, industrial, fish farms, and urban areas32. These activities effect badly on water quality. Peters et al.33 mentioned many water quality issues which were related to anthropogenic activities namely; organic matters, metal ions, nutrients especially nitrogen and phosphorus, pesticides, herbicides, exotic and invasive species and others. Understanding these activities is essential to evaluating its impacts. Most of studied drains were characterized by agricultural wastes with the exception for Elhox drain (site 6) which receives industrial wastes. In addition, no invasive aquatic plants were recoreded at Elboughaz area (site 9). Similarly, the site of Baltim (site 10) lacked the presence of these two plant species32,34. A sentinel 2 10m land use/cover map was implemented to indicate different activities around the sampling sites as illustrated in Fig. 3.
Based on field observations, invasive aquatic plant species such as Pontederia and Pistia were absent in areas with elevated salinity levels. This pattern is consistent with previous researches; for instance, Haller et al.35 reported that Pistia exhibits low salinity tolerance and does not survive in water with salinity exceeding 2.5 ppt. In our study, Pistia was observed only in Drain 7 (Table 3), where salinity was relatively low (1 ppt; Table 4). Similarly, Pontederia crassipes (water hyacinth) is known to be sensitive to salinity, with higher levels inhibiting its growth and potentially leading to mortality36. While our findings align with these reports, it is important to note that the absence of these species in salt-impacted areas may be influenced by multiple interacting factors, and salinity alone cannot be confirmed as the sole determinant.
Water hyacinth has been associated with reduced phytoplankton productivity, likely due to its dense mats which can deplete dissolved oxygen and limit light penetration37,38. In our study, Pontederia distribution appeared to coincide with specific physicochemical conditions, including moderate nutrient levels, pH, and turbidity. For example, the highest Pontederia abundance was recorded at Elshaklouba, where turbidity was low and both water temperature and pH fell within the species’ reported optimal ranges39,40,41. While these associations suggest favorable conditions for Pontederia, we caution against inferring direct causality, as other unmeasured environmental or biological factors may also contribute.
Previous studies have emphasized the potential of Pontederia and Pistia in phytoremediation42. In our study area, sites with higher plant abundance also exhibited lower levels of oxidizable organic matter (OOM) and nutrients, which may reflect the plants’ capacity to absorb or retain pollutants. However, these patterns are correlative, and further experimental or longitudinal studies are needed to confirm the extent of their remediation effects. Conversely, dense Pistia mats may reduce wind-induced mixing, potentially leading to thermal stratification, lower oxygen levels, and increased nutrient concentrations43,44,45. These findings underscore the complex and context-dependent roles of invasive aquatic plants in shaping water quality.
Nutrient levels are a key factor influencing the invasion of water hyacinth46. Previous studies noted that water hyacinth is highly productive with increased nitrogen levels, although it can grow at low levels. This aligns with our findings, as all sites with high nitrogen levels exhibited Pontederia presence and expansion, except those with high salinity. Pontederia varies in height from a few centimeters to nearly a meter, with leaves typically 15–20 cm in length and width47. Plant size and morphology are influenced by nutrient concentrations, particularly phosphorus (P) and nitrogen (N)48. In this study, Pontederia morphological characteristics varied across locations, potentially due to nutrient availability. The maximum leaf height and width were recorded at Elshaklouba, ranging from 13–14 cm, with a maximum petiole length of 56 cm. The lowest maximum leaf height and width were observed at Elkashaa and Brinbal, with values of 6.5–6.5 cm and 6–6.5 cm, respectively (Table 3). Ismail et al.49 reported that floating macrophytes thrive on the water surface under favorable water quality conditions, with phenotypic plasticity aiding their adaptation. Submerged species are more common in shallow water bodies. Floating aquatic macrophytes can be used to remove or reduce nutrient loads from wetland water, mitigating the spread of other invasive aquatic weeds. Species like Pontederia can adsorb significant amounts of nutrients50.
Management processes
In the study area, two primary management practices were identified for controlling invasive aquatic plants. The first involves mechanical removal typically using excavators to clear drains, lagoons, and streams from unwanted plants51. The removed plant biomass is typically deposited on canal banks to dry (Wade, 1990). The second practice includes establishing of nets across drainage channels to prevent the spread of invasive plants into lake waters. While these mechanical approaches offer immediate results, they are often labor-intensive, costly, and require repeated application. As Tang52 and Khan et al.53,54 have noted, more sustainable and cost-effective strategies are needed for long-term control.
In contrast, biological control methods such as the introduction of host-specific insects or pathogens have shown promise in reducing invasive plant populations over time with minimal environmental disruption. However, they may take longer to establish and require careful ecological assessment. Recent studies advocate for integrated management approaches that combine mechanical removal with biological agents, offering a more balanced and effective solution by leveraging the strengths of each method. Despite these advancements, many regions, including South Africa, Lake Mutirikwi in Zimbabwe, and sites around Lake Victoria in Ethiopia, continue to rely primarily on mechanical or physical methods54, underscoring the need for broader adoption of integrated strategies.
The Elshaklouba drain site was selected for monitoring Pontederia invasive species using Landsat OLI8 satellite images, as illustrated in Fig. 4. It is located in the southwestern part of the lake. According to the NDVI categorization, there were four classes: no vegetation, low-density vegetation, moderate-density vegetation, and high-density vegetation. Based on Mucheye et al.10, water hyacinth spread was concentrated within NDVI values of 0.6–0.95. High-density vegetation along the drain was observed during the summer season. This result aligns with Wirngo et al.55, who found that water hyacinth was present in the Wouri River throughout the year and was more prevalent during the dry season than the rainy season. NDVI density area values are shown in Table 5 and Fig. 5.

Source Landsat image from https://earthexplorer.usgs.gov/ and shape file produces using ArcGIS 10.5.
Case study of Elshaklouba drain to observe distribution of Pontederia Crassipes.
Reclassification was applied as follows: C1 (< 0.01) represented non-vegetative surfaces such as water, soil, urban areas, and bare lands; C2 (0.01–0.3) indicated low vegetation cover; C3 (0.31–0.60) represented moderate-density vegetation; and C4 (> 0.61) indicated high-density vegetation. Class 4 is most indicative of water hyacinth presence. It is probable that class 4 represents high-density Pontederia mats, while sparse Pontederia may fall within class 3. Another interpretation is that older, denser Pontederia may not reflect as strongly in the green bands as younger, more nutrient-rich plants. One limitation of this study is the reliance of multispectral imagery to detect and observe invasive plant species along the lakes. Additionally, interference from other plant reflectance may impact the results. Validation was conducted using random sampling along the drain and field observations. Pixels where Pontederia mats were densely distributed corresponded to values equal to or greater than 0.7, while moderate-density mats had values lower than 0.6 (Fig. 6).
Statistical analysis
PCA analysis
Based on Principal Component Analysis (PCA) (as shown in Fig. 7), salinity and dissolved oxygen (DO) are the most influential factors explaining the environmental variation across the study area. Invasive floating plants, such as Pontederia crassipes, reduce light and oxygen, leading to fish kills and negatively impacting the aquatic ecosystem38. PCA is widely recognized as an effective multivariate tool for identifying sources of pollutants along the study area. It simplifies complex datasets by converting them into a set of new components, where each component represents a cluster of interrelated variables56. Data showed that the first two components; PCA1 and PCA2 explain about 66% of the total variance in the dataset. Site 10 (Baltim) is associated with higher nutrient levels and turbidity which indicating poor quality; these may be attributed to high agricultural wastes along this site. El-Mezayen and Abd El-Hamid57 mentioned that high nutrients levels are attributed to an increase quantity of fertilizers from agricultural wastes. Despite of recent improvement of Buruulus Lake, it suffers from high content of nutrients that increase water pollution58.
On the other hand, sites 7 and 9 are associated with high levels of DO. Finally, PCA is an effective tool to distinguish among different sites along the study area. PCA data showed that sites; Drain 7 and West Elburullus have the same properties with high total nitrogen, nitrate, nitrite and turbidity. These may be attributed to discharge of different wastes. Elkashaa (site 1) is associated to chl_a, pH and salinity as shown from PCA analysis. These are related to high effluent to this drain from mixed different pollutants sources. These finding reflect the impact of human activities such as agricultural runoff, indusial dishrags and urban wastes on the water quality of Burullus Lake.
Correlation analysis
Data indicated that NDVI has a strong positive correlation with bands B5, B6, and B7, with R2 values of 0.82, 0.73, and 0.65, respectively, as shown in Fig. 8. Invasive plants can be identified using NDVI and other spectral indices. Evaluating the performance of multiple indices beyond the commonly used ones is crucial for mapping and modeling invasive species distribution59. Iqbal et al.60 stated that invasive plants exhibit a significant spectral signature in the shortwave infrared (SWIR) band due to their influence on soil and vegetation moisture. Furthermore, numerous studies have demonstrated a strong relationship between NDVI and the near-infrared (NIR) band.
Regression analysis for invasive species coverage
Bands as B5, B6, and B7 were selected as indication to invasive plants due to their strong correlation with NDVI. A regression model for invasive plants as an NDVI indicator was applied, yielding an R2 value of 0.71, as shown in Fig. 9. The resulting equation can be used to identify or map the distribution of invasive plants using NDVI and their reflectance in the near-infrared (NIR) and shortwave infrared (SWIR) bands.
Conclusions
This research highlighted that Pistia cannot tolerate salinity levels above 2.5 ppt, resulting in its absence in saline areas. Conversely, Pontederia thrived in low turbidity conditions, with higher abundance at sites like Elshaklouba. Both species effectively reduced total organic matter and nutrient levels, thereby improving water quality. Additionally, morphological variations in Pontederia were linked to nutrient concentrations, showcasing their adaptability. Overall, these findings suggest the potential for using these species in phytoremediation efforts in affected ecosystems. The distribution of invasive aquatic plants along the Burullus wetland is influenced by multiple factors, including nutrient concentrations, salinity levels, and human activities.
The integration of remote sensing data with field observations proved to be an effective approach for tracking the seasonal spread of invasive aquatic species species. In particular, Pontederia crassipes and Pistia stratiotes demonstrated a strong capacity to absorb pollutants such as phosphate, nitrogen compounds, and oxidizable organic matter, indicating their potential use in phytoremediation. Conversely, their removal can enhance water quality by increasing dissolved oxygen levels and improving light penetration, which benefits aquatic ecosystems. However, the study faced limitations; while NDVI provided useful insights, the use of spectral signatures would have offered greater accuracy in detecting plant distribution patterns.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Costanza, R. et al. Changes in the global value of ecosystem services. Glob. Environ. Change 26, 152–158 (2014).
ISAC. Invasive Species Definition Clarification and Guidance White Papers. ISAC Rep. 1–7 https://www.doi.gov/sites/doi.gov/files/uploads/isac_definitions_white_paper_rev.pdf (2006).
Bartz, R. & Kowarik, I. Assessing the environmental impacts of invasive alien plants: A review of assessment approaches. NeoBiota 43, 69–99. https://doi.org/10.3897/NEOBIOTA.43.30122 (2019).
Jones, B. A. & McDermott, S. M. Health impacts of invasive species through an altered natural environment: Assessing air pollution sinks as a causal pathway. Environ. Resour. Econ. 71, 23–43. https://doi.org/10.1007/s10640-017-0135-6 (2018).
Kueffer, C. Plant invasions in the Anthropocene. Science 358, 724–725. https://doi.org/10.1126/science.aao6371 (2017).
Pejchar, L. & Mooney, H. A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 24, 497–504. https://doi.org/10.1016/j.tree.2009.03.016 (2009).
Alidoost Salimi, P. et al. A review of the diversity and impact of invasive non-native species in tropical marine ecosystems. Mar. Biodivers. Rec. 14, 11. https://doi.org/10.1186/s41200-021-00206-8 (2021).
Sladonja, B. & Damijanic, D. Remote sensing in invasive species detection and monitoring. Int. J. Environ. Sci. Nat. Res. 29, 556255. https://doi.org/10.19080/IJESNR.2021.29.556255 (2021).
Bubenheim, D., Genovese, V., Madsen, J. D. & Hard, E. Remote sensing and mapping of floating aquatic vegetation in the Sacramento-San Joaquin River Delta. J. Aquat. Plant Manag. 59, 46–54 (2021).
Mucheye, T., Haro, S., Papaspyrou, S. & Caballero, I. Water quality and water hyacinth monitoring with the Sentinel-2A/B satellites in Lake Tana (Ethiopia). Remote Sens. 14, 4921. https://doi.org/10.3390/rs14194921 (2022).
Wiesenborn, W. D. Salt cedar impacts on salinity, water, fire frequency, and flooding. Proc. Saltcedar Manag. Workshop 9–12 (1996).
Durand, P., Breuer, L., Johnes, P., Billen, G., Butturini, A., Pinay, G., Grinsven, H., Garnier, J., Rivett, M., Reay, D., Curtis, C., Siemens, J., Maberly, S., Kaste, Ø., Humborg, C., Loeb, R., De Klein, J., Hejzlar, J., Skoulikidis, N. & Wright, R. Nitrogen processes in aquatic ecosystems. Eur. Nitrogen Assess. 126–146 (2011).
El Bahrawy, A. N., Donia, N. S., Farouk, M. A. & Sayed, N. S. Analysis of Burullus Wetland ecosystem using DPSIR framework. J. Environ. Sci. 40, 101–124 (2017).
Elsayed, F. A., Okbah, M. A., El-Syed, S. M., Eissa, M. A. & Goher, M. E. Nutrient salts and eutrophication assessment in Northern Delta Lakes: Case study Burullus Lake Egypt. Egypt. J. Aquat. Biol. Fish. 23, 145–163 (2019).
EEAA. Lake Burullus, Burullus protected area. Nat. Biodivers. Unit Publ. 13 (2005).
Sheta, B. M., Abdelhalim, A. A., Elgyar, E. E., Khalil, M. A. & Ageba, M. F. Wetland habitat suitability and diversity for migratory and resident birds in the Ramsar Site Lake Burullus Egypt. Egypt. J. Aquat. Biol. Fish. 27, 253–274 (2023).
Grasshoff, K., Kremling, K. & Ehrhardt, M. Methods of seawater analysis. Methods Seawater Anal. 600 (1999).
APHA. Standard methods for the examination of water and wastewater. Stand. Methods (1999).
FAO. Permanganate value of organic matter in natural waters. Fish. Tech. Pap. 137, 169–171 (1975).
Strickland, J. D. H. & Parsons, T. R. A practical handbook of seawater analysis. Fish. Res. Board Can. Bull. 167 (1972).
Abdulrazzak, I. A. The effects of Eichhornia crassipes on the water resources. Int. J. Eng. Sci. 6, 51–55 (2017).
GISD. 100 of the World’s Worst Invasive Alien Species. Glob. Invasive Species Database. http://www.iucngisd.org/gisd/100_worst.php (2018).
Singh, P. & Kumari, B. Monographic study of Eichhornia crassipes (Mart.) Solms. Int. J. Bot. Stud. 2, 196–199 (2017).
Faisal, M. & Hasnain, S. Synergistic removal of Cr (VI) by Eichhornia crassipes in conjunction with bacterial strains. Pak. J. Biol. Sci. 6, 264–268. https://doi.org/10.3923/pjbs.2003.264.268 (2003).
Haines, H. H. The Botany of Bihar and Orissa. Bot. Bihar Orissa 330 (1978).
Anonymous. The Ayurvedic Pharmacopoeia of India. Ayurvedic Pharmacopoeia India 96 (1989).
El Sherbeny, G. A., El-Shehaby, O. A. & Mohsin,. Ecological study and morphological variation of Pistia stratiotes in the northeastern section of the Nile Delta Egypt. J. Environ. Sci. 44, 31–45 (2015).
Hussner, A. Long-term macrophyte mapping documents a continuously shift from native to non-native aquatic plant dominance in the thermally abnormal River Erft (North Rhine-Westphalia, Germany). Limnologica 48, 39–45 (2014).
Wu, C., Wang, M., Dong, Y., Cheng, Z. & Meng, H. Growth, bolting and yield of garlic (Allium sativum L.) in response to clove chilling treatment. Sci. Hortic. 194, 43–52 (2015).
El-Zeiny, A. M. & El-Kafrawy, S. B. Assessment of water pollution induced by human activities in Burullus Lake using Landsat 8 operational land imager and GIS. Egypt. J. Remote Sens. Space Sci. 20, S49–S60. https://doi.org/10.1016/j.ejrs.2016.11.003 (2017).
Laksono, A., Saputri, A. A., Pratiwi, C. I., Arkan, M. Z. & Putri, R. F. Vegetation covers change and its impact on Barchan Dune morphology in Parangtritis Coast Indonesia. E3S Web Conf. 200, 02026. https://doi.org/10.1051/e3sconf/202020002026 (2020).
El-Alfy, M. A., Darwish, D. H., Basiony, A. I. & Elnaggar, A. A. GIS-based study on the environmental sensitivity to pollution and susceptibility to eutrophication in Burullus Lake Egypt. Mar. Geod. 44, 554–572. https://doi.org/10.1080/01490419.2021.1954111 (2021).
Peters, N. E., Meybeck, M. & Chapman, D. V. Effects of human activities on water quality, Part 8: Water quality and biogeochemistry. Encycl. Hydrol. Sci. https://doi.org/10.1002/0470848944.hsa208 (2006).
El-Alfy, M. A., Serag, M. S., Basiony, A. I., Fathi, M. & Darwish, D. H. Influence of land cover indices and surface temperature on the metals bioaccumulation by three Macrophytes in Lake Burullus Egypt. J. Coast. Conserv. 27, 5. https://doi.org/10.1007/s11852-023-00934-2 (2023).
Haller, W. T., Sutton, D. L. & Barlowe, W. C. Effects of salinity on growth of several aquatic macrophytes. Ecology 55, 891–894 (1974).
Bick, E. et al. Effects of salinity and nutrients on water hyacinth and its biological control agent, Neochetina bruchi. Hydrobiologia 847, 3213–3224. https://doi.org/10.1007/s10750-020-04314-x (2020).
Mironga, J. M., Mathooko, J. M. & Onywere, S. M. The effect of water hyacinth (Eichhornia crassipes) infestation on the physicochemical characteristics of Lake Naivasha Kenya. Int. J. Humanit. Soc. Sci. 2, 103–113 (2012).
Villamagna, A. M. & Murphy, B. R. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshw. Biol. 55, 282–298. https://doi.org/10.1111/j.1365-2427.2009.02294.x (2010).
Widyanto, L. S. Ekologi Eceng Gondok (Compilation of Paper and Report periode 1974–1979). SEAMEO BIOTROP Rep. (1981).
Krismono, Nastiti, A. S., Suryandari, A. & Haryadi, J. Eichhornia crassipes aquatic plant management technology for water resources enhancement. IOP Conf. Ser. Earth Environ. Sci. 521, 012011. https://doi.org/10.1088/1755-1315/521/1/012011 (2020).
Nguyen, T. H. T. et al. Habitat suitability of the invasive water hyacinth and its relation to water quality and macroinvertebrate diversity in a tropical reservoir. Limnologica 52, 67–74. https://doi.org/10.1016/j.limno.2015.04.002 (2015).
Ratnani, R. D., Hartati, I. & Kurniasari, L. Pemanfaatan eceng gondok (Eichornia crassipes) untuk menurunkan kandungan COD (Chemical Oxygen Demand), pH, bau, dan warna pada limbah cair tahu. Momentum 7, 41–47 (2011).
Attionu, R. H. Some effects of water lettuce (Pistia stratiotes L.) on its habitat. Hydrobiologia 50, 245–254 (1976).
EPPO. Pistia stratiotes. EPPO Datasheets Pests. https://gd.eppo.int/RESOURCES/special_projects/datasheets (2024).
Neuenschwander, P., Julien, M. H., Center, T. D. & Hill, M. P. Pistia stratiotes L. (Araceae). Biol. Control Trop. Weeds Using Arthropods 332–352 (2009).
Gao, L., Li, B. & Jin, L. Can water hyacinth (Eichhornia crassipes) be controlled by reducing nitrogen and phosphorus pollution of water bodies. Appl. Ecol. Environ. Res. 14, 77–91. https://doi.org/10.15666/aeer/1403_077091 (2016).
Jafari, N. Ecological and socio-economic utilization of water hyacinth (Eichhornia crassipes Mart Solms). J. Appl. Sci. Environ. Manag. 14, 43–49. https://doi.org/10.4314/jasem.v14i2.57834 (2010).
Nwamo, R. D., Ajonina, G. N., Ngwasiri, P. N., Besack, F. & Moudingo, E.J.-H. Problems of invasive species of water hyacinth (Eichhornia crassipes [Mart.] Solms) in Cameroon with special reference to its eradication and valorization: A bibliographical review. Energy Environ. Res. 12, 56–69 (2022).
Ismail, S. N., Subehi, L., Mansour, L. A. & Mashhor, M. Invasive aquatic plant species of Chenderoh Reservoir, Malaysia and Jatiluhur Reservoir, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 380, 012004. https://doi.org/10.1088/1755-1315/380/1/012004 (2019).
Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 56, 15–39. https://doi.org/10.1146/annurev.arplant.56.032604.144214 (2005).
Wade, P. M. Physical control of aquatic weeds. Aquat. Weeds 93–135 (1990).
Tung, K. S. The effectiveness of mechanical control of water hyacinth (Eichhornia crassipes). J. Aquat. Plant Manag. 42, 28–32 (2004).
Khan, H., Uslu, O. & Gul, B. Management and utilization of water hyacinth (Eichhornia crassipes Mart.). Recent Adv. Biol. Ecol. Agric. Pract. 73–96 (2022).
Yigermal, H., Nakachew, K. & Assefa, F. Distribution, threats and management options for water hyacinth (Eichhornia crassipes) in Ethiopia: A review. J. Res. Weed Sci. 3, 9–23. https://doi.org/10.26655/JRWEEDSCI.2020.1.2 (2020).
Wirngo, H. Y., Bamgboye, A. I. & Ngwabie, N. M. Spatio-temporal distribution of water hyacinth (Eichhornia crassipes) in the River Wouri Littoral Region in Cameroon. TWIST 19, 175–184. https://doi.org/10.53555/twist.v19i4.3608 (2024).
Anh, H. Q. et al. Polychlorinated biphenyls in settled dusts from an end-of-life vehicle processing area and normal house dusts in northern Vietnam: Occurrence, potential sources, and risk assessment. Sci. Total Environ. 728, 138823. https://doi.org/10.1016/j.scitotenv.2020.138823 (2020).
El-Mezayen, M. M. & Abd El-Hamid, H. T. Assessment of water quality and modeling trophic level of Lake Manzala Egypt using remotely sensed observations after recent enhancement project. J. Indian Soc. Remote Sens. 51, 197–211. https://doi.org/10.1007/s12524-022-01635-3 (2023).
Abdel-Hamid, H. T., Kaloop, M. R. & Elbeltagi, E. Impact assessment of the land use dynamics and water pollution on ecosystem service value of the Nile Delta coastal lakes Egypt. J. Indian Soc. Remote Sens. 51, 963–981. https://doi.org/10.1007/s12524-022-01663-z (2023).
West, A. M. et al. Using multi-date satellite imagery to monitor invasive grass species distribution in post-wildfire landscapes: An iterative, adaptable approach that employs open-source data and software. Int. J. Appl. Earth Obs. Geoinf. 59, 135–146. https://doi.org/10.1016/j.jag.2017.03.009 (2017).
Iqbal, I. M., Balzter, H. & Shabbir, A. Identifying the spectral signatures of invasive and native plant species in two protected areas of Pakistan through field spectroscopy. Remote Sens. 13, 4009. https://doi.org/10.3390/rs13194009 (2021).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M. El-Alfy: Conceptualization, collection of samples, samples analyses, mappingand writing the original and final draft and revising the draft, H. Abd El-Hamid: Collection of samples, samples analyses, statistical analysis, mappingand writing the original and final draft, H. Kacem: writing the original draft andA. Keshta: Conceptualization, supporting field survey, writing original manuscriptand revising the draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This is an environmental study that does not include any animal or human, or biological tissues and ethical approval is not required.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The participant has consented to the submission of this article to the journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Alfy, M.A., Abd El-Hamid, H.T., Kacem, H.A. et al. Assessment of invasive aquatic plant dynamics in the Lake Burullus wetland complex integrating remote sensing techniques. Sci Rep 15, 23701 (2025). https://doi.org/10.1038/s41598-025-07892-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07892-9
Keywords
This article is cited by
-
Remote sensing and multivariate analysis to select suitable habitats for migratory and resident birds in BurullusLake, Egypt
Euro-Mediterranean Journal for Environmental Integration (2026)