Abstract
We aimed to determine the 10-year trend in the incidence of Severe fever with thrombocytopenia syndrome (SFTS) in Japan. This retrospective observational study used a publicly available national database. Trends in the incidence of SFTS with annual percent changes (APC) were examined using Joinpoint regression analysis with stratification by patient age, season, and region. The association between disease incidence and environmental factors was investigated using Spearman’s rank correlation. Between 2013 and 2022, there were 803 notified cases (397 males and 406 females) of SFTS, with 79.5% aged ≥ 65 years. The annual incidence rate increased continuously with an APC of 9.6%. The incidence peaked between May and June, with 80.8% of cases observed between May and October. The incidence was predominantly higher in western Japan, and the mean annual incidence rate was the highest in Miyazaki prefecture, with 0.89 per 100,000 people. Correlations between the SFTS incidence rates and environmental factors were observed in western Japan, with forest area (correlation coefficient, 0.80), followed by agricultural population rate (0.70). SFTS incidence is continuously increasing in Japan, especially among the elderly population. Environmental factors such as broader forest areas and increased agricultural population were possibly associated with the incidence.
Similar content being viewed by others
Introduction
Global warming has reportedly led to expanding habitat ranges and extended activity times for ticks, and accordingly, tick-borne infectious diseases have become a major concern worldwide1,2,3. Severe fever with thrombocytopenia syndrome (SFTS), caused by SFTS virus (SFTSV), or Huaiyangshan banyangvirus that is currently renamed as Dabie Bandavirus4, is an emerging tick-borne infectious disease in far eastern Asian countries5,6. The most updated systematic review and meta-analysis study demonstrated global upward trends in both notification and death rates, with the overall pooled notification rate at 18.93 (95% confidence interval [CI]: 17.02–21.05) and the overall pooled notification deaths rate at 3.49 (95% CI 2.97–4.10) per 10 million people7. While, the case fatality rate showed a significant decrease globally, with the overall pooled case fatality rate at 7.80% (95% CI: 7.01–8.69%). Comparing the three Eastern Asian countries, Japan has a substantially lower pooled notification rate at 2.46 cases per 10 million people without a statistically significant rising trend, while those in China and South Korea are 18.59 and 48.49, respectively7. However, since the first detection in Yamaguchi prefecture8, the number of SFTS cases has been increasing in Japan, mainly involving older people living in the western countryside9,10. The seasonal peak of SFTS reportedly occurs between April and September in eastern Asian countries7, with long daylight hours, shrubs, and forested areas considered risk factors for the disease10,11,12. However, other epidemiological features and temporal trends of SFTS in Japan remain unclear.
SFTS is transmitted not only by tick bites but also directly via the body fluids of infected companion animals such as cats6,13. The seroprevalence of SFTSV antibodies among Japanese veterinary staff is reported to be elevated at 4.2%13, suggesting occupational infections may occur through contact with domestic animals during care. In Japan, several prefectures have reported high positivity rates of anti-SFTSV antibodies in wild animals13,14,15,16,17,18. While anti-SFTSV antibodies were not detected in deer in Hokkaido, a northern island in Japan14, other prefectures in the western region showed high seroprevalences of anti-SFTSV antibodies across various animal species, such as deer (55–65%), boars (12–39%), and cat (33%)13,16,17. Notably, SFTSV can infect many other mammalian species7, indicating the potential threat of this zoonotic disease.
Until recently, no specific treatments have been available for patients with SFTS. In the absence of effective therapy, the mortality rate of SFTS remains very high, reaching 20–35%2,3. However, recent reports have suggested the clinical efficacy of favipiravir for treating SFTS19,20, and in May 2024, the Japanese government approved the drug as the first therapeutic agent for SFTS worldwide21. Due to insufficient clinical data, the real-world effectiveness of favipiravir has yet to be determined.
To further understand the clinical burden of SFTS, we analyzed the 10-year incidence trends of SFTS in Japan, with particular focus on vulnerable populations, geographic distribution, and associations with environmental factors.
Methods
Data source
This 10-year retrospective observational study was conducted on data collected between 2013 and 2022, obtained from the Infectious Diseases Weekly Report of Japan22. Since 2013, clinical data on patients with stipulated diseases have been accumulated at the National Institute of Infectious Diseases based on the Act on the Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases (the Infectious Diseases Control Law). SFTS is a notifiable disease classified as a category IV infectious disease that should be reported immediately after diagnosis23. Thus, when patients with the disease are diagnosed, medical practitioners are responsible for informing public health centers, and the reported data are then summarized by the National Epidemiologic Surveillance of Infectious Disease and made available to the public on an online website22. Clinically diagnosed cases of SFTS were not included because the law stipulates that only cases detected by laboratory tests, such as reverse transcription polymerase chain reaction testing, fluorescent antibody testing, and neutralizing antibody testing, are required for reporting. Details and definitions of these laboratory tests are clearly outlined in the Pathogen Detection Manual of the National Institute of Infectious Diseases24. In actual practice, since there is no commercially available test for SFTS in Japan, medical doctors submit patient samples to public health centers for the laboratory diagnosis as administrative testing, which helps prevent reporting bias in the diagnosed cases. Accordingly, SFTS cases have been reported throughout the country.
Data on the Japanese population and environmental factors, such as forest area, field area, agricultural population rate, temperature (average, maximum, and minimum), and daylight hours were obtained from the Vital Statistics provided by the Japanese Ministry of Health, Labor, and Welfare25. These environmental factors were selected because they have been reported as risk factors for SFTS in previous literature10,11,12. Data for the forest area, field area, and agricultural population are compiled every five years. The latest publicly available information for forest area was from 2019, field area was from 2021, and agricultural population was from 2020.
Categorization
Data for individual age was available in 5-yearly age groups. For those aged ≥ 70 years, the 5-yearly age group data was unavailable, with only compiled data accessible. For trend analysis purposes, we categorised the age groups into < 50 years, 50–64 years, and ≥ 65 years. To investigate the temporality of the disease onset, we divided the entire year into six bimonthly groups: January–February (1st to 8th week), March–April (9th to 17th week), May–June (18th to 26th week), July–August (27th to 35th week), September–October (36th to 44th week), and November–December (45th to 52nd or 53rd week). For geographical analysis, we categorized prefectures into seven regions (Hokkaido/Tohoku, Kanto, Hokuriku/Chubu, Kinki, Chugoku, Shikoku, and Kyushu).
Data processing and statistical analyses
The SFTS incidence per 100,000 population, temperature, and daylight hours were calculated as average values between 2018 and 2022. The agricultural population rate was calculated by dividing the agricultural population of each prefecture by the total population. Considering the uneven distribution of SFTS cases, we evaluated environmental factors by dividing the data into Japan as a whole (47 prefectures) and western Japan (24 prefectures).
To estimate the trends in SFTS, the Joinpoint regression model was applied by sex and age (Joinpoint Regression Program, version 4.5.2.0, June 2024, Statistical Research and Applications Branch, National Cancer Institute)26. The analysis identified the year in which significant trend changes occurred and estimated the magnitude of the increase or decrease. Each joinpoint showed a statistically significant change in trend, and the annual percent changes (APC) was calculated for each of these trends using generalized linear models assuming a Poisson distribution27. In addition, average APC (AAPC) over the entire study period was estimated. To compare the regional incidence and trend, we calculated the mean incidence rate per 100,000 people and APC for each prefecture. We included prefectures with ≥ 15 SFTS cases over a 10-year period because accurate trends cannot be assessed when the case number is low. To compare the bimonthly incidence, we applied the Kruskal-Wallis analysis using EZR software, a graphic user interface for R 3.5.2 software (The R Foundation for Statistical Computing, Vienna, Austria)28. For the association between the incidence of SFTS and environmental factors, Spearman’s rank correlation coefficients were calculated using the Spearman rank method in EZR software. All data collection and calculations were performed independently and in duplicate by two researchers (SF and HA) to ensure the accuracy of the analysis. Statistical significance was set at p < 0.05.
Results
The incidence of SFTS
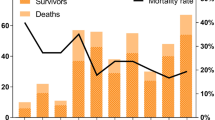
The total number of SFTS cases in Japan during the study period was 803 (397 males, 406 females). The annual incidence increased 2.5-fold from 2013 to 2022; 48 to 118 cases (Fig. 1a). Males accounted for 49.4% of the cases with no sex predilection. Patients aged ≥ 65 years accounted for 79.5% of the cases (Fig. 1b). Overall, the annual incidence increased at a rate of 9.6% (95% CI: 6.4–13.6%) from 2013 to 2022, with a higher APC in men (10.4%, 95% CI: 7.8–13.1%). By age, a consistent and significant increasing trend was observed in patients aged ≥ 65 years for both males and females, but not in the younger age groups (Table 1).
Temporal distribution of incidence of SFTS
The distribution of the incidence of SFTS from the 1st to the 52nd (or 53rd) weeks in 2013–2022 is shown in Fig. 2a. The highest number of cases was observed in the 23rd and 26th weeks, with 38 cases each. In the bimonthly seasons, the incidence of SFTS peaked in May–June (Fig. 2b). The Kruskal-Wallis analysis was used to calculate the p-value of < 0.001 among the bimonthly groups. During the epidemic months from May to October, 649 cases (80.8%) were notified. AAPCs of each bimonthly season showed significant increases in January–February (25.4%), March–April (9.9%), May–June (10.1%), and September–October (10.1%) (Table 2). In contrast, the incidence in November–December period showed a decreasing trend (AAPC, −4.2%, 95% CI: −17.3–9.1%).
Geographical features
The mean incidence rate of SFTS was distributed predominantly in western Japan, with the highest rates in Miyazaki Prefecture (0.89 cases per 100,000 people) and Kochi Prefecture (0.86 cases per 100,000 people) (Fig. 3a). The number of prefectures notifying SFTS cases increased from 13 to 26 of the 47 prefectures between 2013 and 2022. By region, AAPCs in western regions increased, except in the Shikoku region: 28.5% in Hokuriku/Chubu, 18.5% in Kinki, 15.5% in Chugoku, and 8.8% in Kyushu regions (Supplemental Table 1). Each of the Hokkaido/Tohoku and Kanto regions reported zero and two cases, respectively. By prefecture, AAPCs showed significantly increasing trends in Mie (26.9%), Shimane (26.4%), Okayama (19.1%), Nagasaki (13.4%), and Kumamoto (15.1%) prefectures (Fig. 3b). Detailed data on the annual and mean incidence rates by prefecture is available in Supplemental Table 2.
Regional distribution of the SFTS annual incidence rates and results of the Joinpoint trend analysis by prefecture. (a) SFTS annual incidence per 100,000 people (mean rates for the 10-year period). (b) Average APC by prefecture. For Figure b, the analysis was eligible for prefectures with ≥ 15 SFTS cases over a 10-year period. SFTS, severe fever with thrombocytopenia syndrome; APC, annual percentage change. This figure was created using Microsoft Excel and includes map data from Bing (GeoNames, Microsoft, Zenrin).
Environmental factors
Associations between the incidence of SFTS and environmental factors are shown in Fig. 4. Across the country, rises in the average (coefficient, 0.63), maximum (coefficient, 0.38), and minimum (coefficient, 0.56) temperatures were significantly associated with the SFTS incidence. However, no relationships were observed with the forest area, field area, agricultural population rate, or daylight hours. In contrast, in western regions, greater forest area (coefficient, 0.80) and higher agricultural population rate (coefficient, 0.70) were associated with the SFTS incidence, despite no correlation with temperature parameters or daylight hours.
Correlation between SFTS annual incidence rates and each environmental factor. SFTS, severe fever with thrombocytopenia syndrome. Spearman’s rank correlation coefficient analysis was performed for the statistical analysis. *Factors with a significant correlation with SFTS incidence rate. Y-axis: average SFTS annual incidence per 100,000 people (mean rates for the 5-year period) between 2018 and 2022. (a) X-axis: Average temperature (°C) in each prefecture between 2018 and 2022. (b) X-axis: Maximum temperature (°C) in each prefecture between 2018 and 2022. (c) X-axis: Minimum temperature (°C) in each prefecture between 2018 and 2022. (d) Forest area. X-axis: Square measure (ha) of forest area in each prefecture in 2019. (e) Field area. X-axis: Square measure (m2) of field area in each prefecture in 2021. (f) Agricultural population rate. X-axis: Agricultural population rata (agricultural population/total population in each prefecture) in 2020. (g) X-axis: Daylight hours (hr) between in each prefecture 2018 and 2022. This figure was created using Microsoft Excel and includes map data from Bing (GeoNames, Microsoft, Zenrin).
Discussion
We utilized a publicly available national database to clarify the trends in the incidence of SFTS in Japan over a 10-year period. In particular, we examined the temporal variation and regionality of the disease incidence across the country and its association with environmental factors, aspects that have not been fully explored in the preceding literature. A consistent increase was observed in people aged 65 years and older, with approximately 80% of the total cases being notified between May and October. The mean incidence rate was highest in Miyazaki (0.89 per 100,000 people), and significant increases in APCs were observed in five other prefectures located in the western regions. In terms of association with environmental factors, greater forest area and higher agricultural population rate were significantly associated with the SFTS incidence in western Japan.
The global incidence of SFTS has been predominantly reported in east and southeast Asia, particularly China, Japan, and South Korea3,7,12,29. The incidence is increasing in these countries, notably among older individuals7. In China, 93.3% of patients diagnosed with SFTS were 40–84 years12, and the median ages in Japanese and South Korean cases were greater than 70 years3,12,29. Our data uncovered that 79.5% of patients were older than 65 years, with no difference between sexes. Age-stratified trend analysis revealed a significant increase in APC of 8.7% (95% CI: 4.9–13.2%) in the older population, which has also been observed in China and South Korea3,12,29. The establishment of SFTS surveillance and increased awareness of the disease may contribute to the upward trend in SFTS incidence29. However, the actual disease prevalence is likely increasing in the context of global warming. Furthermore, the higher incidence of SFTS among the elderly is reportedly due to the increased opportunities for tick exposure through agricultural activities11. Indeed, the majority of patients presenting to hospitals with tick bites are elderly individuals aged over 70 years, which corresponds to the demographic most susceptible to SFTS occurrence30,31. Younger populations are less likely to engage in agricultural activities and have less exposure to ticks due to differences in residential environment, which may explain the lower incidence of SFTS in this age group. Another contributing factor is that elderly individuals exhibit compromised immune function, which may attenuate their immune response to SFTSV. However, it remains unclear whether immunosenescence influences the age-specific epidemiological distribution of the disease11. Further research is warranted to elucidate the relationship between age- associated immune function and SFTS susceptibility.
Most SFTS cases occurred between April–May and October in China and South Korea, with a peak in May and June12,29. Our study showed an epidemiological similarity. Understandably, this endemic peak coincides with the peak of tick-bite events30,31; most patients bitten by ticks visit hospitals from April to August. Notably, 63.9% of Japanese patients with SFTS had a history of entering mountains or agricultural fields30,31, and 84.6% of Chinese patients were farmers, with 72.4% having a fieldwork history32. Public health initiatives should focus on raising awareness regarding the risks of tick bites, especially for the population engaged in agricultural activities during these epidemic periods.
Tick bites are the primary mode of SFTS transmission, making preventive efforts critical. Among various tick species, Amblyomma testudinarium, Haemaphysalis longicornis, Ixodes nipponensis, and Rhipicephalus microplus are common vectors of SFTSV2. Geographically, Ixodes persulcatus and Ixodes ovatus are predominant in northern and central Japan, while A. testudinarium and H. longicornis are commonly present in western Japan30. Risk factors for tick bites include older age and longer outdoor activities, particularly engaging in yardwork or gardening33. Older farm workers would have a greater chance of tick exposure because they are less likely to use insect repellent or check their bodies and clothes for ticks after possible exposures12,33. Of note, the highest risk factor for tick-borne diseases was associated with exposure in the neighbourhood (odds ratio: 4.08, 95% CI; 2.49−6.68), rather than exposure elsewhere34. These facts suggest that avoiding tick exposure in daily life is an important measure to protect oneself from SFTS.
In recent years, concerns have been raised regarding the growing prevalence of host animals and the expansion of tick distribution and activity periods due to climate change1. The frequency of tick-bite events has been increasing over time in Japan31, and this trend potentially results in the rise in SFTS incidence. The increasing trends of SFTS incidence observed from January to June, as well as September to October, might be a consequence of the increased tick-bite events resulting from global warming, which can extend the activity periods of ticks. The case number of Japanese spotted fever, another tick-borne disease in Japan, is also growing among the elderly, and the five prefectures where we reported the increasing trends - Mie, Shimane, Okayama, Nagasaki, and Kumamoto - have also been reported as endemic areas35. People residing in these western rural areas need to be aware of the risk of tick bites that can cause these potentially fatal diseases.
SFTS develops as a zoonotic disease in both animals and humans. Anti-SFTSV antibodies have been found in wild and domestic animals across various SFTS-endemic regions in Japan, including Ehime (18.7% of wild and domestic animals)15, Yamaguchi (64.6% of wild deer and 38.5% of wild boars)16, Oita (55% of wild deer, 12% of wild boars, and 27% of wild raccoons)17, Nagasaki (33.1% of cats)13, and Miyazaki (68.3% of Japanese badgers and 22.6% of Japanese raccoon dogs)18. The prevalence of SFTS serology in humans is yet to be determined in Japan; however, the seropositivity rate in the most endemic area (Miyazaki) is 0.9% of the general population36. A particularly high SFTS seroprevalence (4.2%: 3 of 71) was observed among veterinarians and nurses working at animal hospitals in Nagasaki13. Transmission of SFTSV from infected companion animals to humans is possible through direct contact with the body fluids of infected animals6. The progressive spread of SFTSV in various animals is of major concern in endemic countries6,7,36. Among the five prefectures showing increasing trends of SFTS incidence in our study, Nagasaki and Miyazaki have already reported spread among wild animals13,18. A national survey revealed that wild boar and deer populations have been declining since reaching peak levels in 201437. However, the habitat area of both species continued to expand into human living areas through 202037. Despite the population decline, seroprevalence remains elevated in endemic regions, suggesting enhanced wildlife exposure to SFTSV. Serological surveys would be useful in tracing the potential spread of SFTS in both animals and humans across Japan.
The importance of the association between the incidence of SFTS and environmental factors should also be addressed. We clearly demonstrated that the incidence of SFTS was correlated with broader forest area and increased agricultural populations in western Japan, similar to previous studies10,11,12. These environmental factors suggest a greater likelihood of tick exposure1,12, and provide reasonable explanations for the rise in SFTS incidence. On the contrary, no correlation between the disease incidence and temperature parameters was observed. A previous study indicated an association with SFTS incidence and prolonged daylight hours10, which was, however, not observed in the present study. Still, our data is insufficient to establish causality, warranting further epidemiological analysis with detailed environmental data.
The strength of this study is the use of a national database covering a 10-year period, with the inclusion of statistical trend analysis, highlighting an increasing trend in incidence, seasonal temporality, geographical features, and environmental factors. In addition to the geographical spread of ticks contaminated with SFTSV, the aging population and the heightened awareness of this disease may have contributed to the observed increase in the number of cases. This study had several limitations as well. First, underreporting of the disease may have resulted in the underestimation of the disease. Diagnosis of such a rare, emerging disease is challenging and requires an administrative laboratory testing that is not commercially available. Thus, a number of patients might have been clinically misdiagnosed. Second, due to the small number of cases, we could not calculate the age-adjusted incidence rates. Third, owing to the unavailability of clinical data, a detailed analysis of the clinical features was impossible and the prognosis of patients is unknown. Fourth, the environmental factors analysed differed depending on the year of data collection, leaving an inconsistency. Despite these limitations, this study provides important insights for comprehending the prevalence and characteristics of patients with SFTS in Japan.
In conclusion, we investigated the epidemiological trends and environmental factors associated with SFTS incidence in Japan between 2013 and 2022, hopefully contributing to increased public awareness of the importance of tick prevention to reduce the incidence of tick-borne diseases. We clearly uncovered an increasing trend of SFTS among the Japanese older population. This zoonotic disease is predominant in western Japan from May to October, with outdoor or agricultural activities as risk factors. Our study underscores the importance of public health initiatives, particularly for older people, such as avoiding tick-infested fields and using insect repellent during months with a high risk of tick bites.
Data availability
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
References
Bouchard, C. et al. N increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 45, 83–89 (2019).
Seo, J-W., Kim, D., Yun, N. & Kim, D-M. Clinical update of severe fever with thrombocytopenia syndrome. Viruses 13. (2021).
Yokomizo, K., Tomozane, M., Sano, C. & Ohta, R. Clinical presentation and mortality of severe fever with thrombocytopenia syndrome in japan: A systematic review of case reports. Int. J. Environ. Res. Public. Health 19. (2022).
Kim, E-H. & Park, S-J. Emerging Tick-Borne Dabie bandavirus: virology, epidemiology, and prevention. Microorganisms 11 (2023).
Yu, X-J. et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl. J. Med. 364, 1523–1532 (2011).
Kobayashi, Y. et al. Severe fever with thrombocytopenia syndrome, japan, 2013–2017. Emerg. Infect. Dis. 26, 692–699 (2020).
Cui, H. et al. Global epidemiology of severe fever with thrombocytopenia syndrome virus in human and animals: a systematic review and meta-analysis. Lancet Reg. Health West. Pac. 48, 101133 (2024).
Takahashi, T. et al. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J. Infect. Dis. 209, 816–827 (2014).
Yamaji, K., Aonuma, H. & Kanuka, H. Distribution of tick-borne diseases in japan: past patterns and implications for the future. J. Infect. Chemother. 24, 499–504 (2018).
Ogawa, T. et al. Analysis of differences in characteristics of High-Risk endemic areas for contracting Japanese spotted fever, Tsutsugamushi disease, and severe fever with thrombocytopenia syndrome. Open. Forum Infect. Dis. 11, ofae025 (2024).
Liu, K. et al. Epidemiologic features and environmental risk factors of severe fever with thrombocytopenia syndrome, xinyang, China. PLoS Negl. Trop. Dis. 8, e2820 (2014).
Huang, X., Li, J., Li, A., Wang, S. & Li, D. Epidemiological characteristics of severe fever with thrombocytopenia syndrome from 2010 to 2019 in Mainland China. Int. J. Environ. Res. Public. Health 18. (2021).
Ando, T. et al. Severe fever with thrombocytopenia syndrome in cats and its prevalence among veterinarian staff members in Nagasaki. Japan Viruses 13. (2021).
Uchida, L. et al. Survey of tick-borne zoonotic viruses in wild deer in hokkaido, Japan. J. Vet. Med. Sci. 80, 985–988 (2018).
Kimura, T. et al. Seroprevalence of severe fever with thrombocytopenia syndrome (SFTS) virus antibodies in humans and animals in Ehime prefecture, japan, an endemic region of SFTS. J. Infect. Chemother. 24, 802–806 (2018).
Tatemoto, K. et al. Risk assessment of infection with severe fever with thrombocytopenia syndrome virus based on a 10-year serosurveillance in Yamaguchi Prefecture. J. Vet. Med. Sci. 84, 1142–1145 (2022).
Hashimoto, T. et al. Distribution of severe fever with thrombocytopenia syndrome virus and antiviral antibodies in wild and domestic animals in Oita prefecture, Japan. Am. J. Trop. Med. Hyg. 106, 1547–1551 (2022).
Kaneko, C. et al. Seroprevalence of severe fever with thrombocytopenia syndrome virus in medium-sized wild mammals in miyazaki, Japan. Ticks Tick. Borne Dis. 14, 102115 (2023).
Yuan, Y. et al. Clinical efficacy and safety evaluation of favipiravir in treating patients with severe fever with thrombocytopenia syndrome. EBioMedicine 72, 103591 (2021).
Song, R., Chen, Z. & Li, W. Severe fever with thrombocytopenia syndrome (SFTS) treated with a novel antiviral medication, favipiravir (T-705). Infection 48, 295–298 (2020).
Pharmaceuticals and Medical Devices Agency, Japan. Available at: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/6250054. Accessed March 22. (2025).
National Institute of Infectious Diseases, Japan. Available at: https://www.niid.go.jp/niid/ja/allarticles/surveillance/2270-idwr/nenpou.html. Accessed March 22. (2025).
Ministry of Justice Act on the Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases. Available at: https://www.japaneselawtranslation.go.jp/en/laws/view/2830/en. Accessed March 22. (2025).
Pathogen Detection Manual of Severe Fever with Thrombocytopenic Syndrome (SFTS). Available at: https://www.niid.go.jp/niid/images/lab-manual/SFTS20200812.pdf. Accessed March 22. (2025).
Japanese Ministry of Health, Labor, & Welfare Vital Statistics. Available at: https://www.e-stat.go.jp/. Accessed March 22 2025. (2024).
Kim, H. J., Fay, M. P., Feuer, E. J. & Midthune, D. N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 19, 335–351 (2000).
Cayuela, A., Rodríguez-Domínguez, S., López-Campos, J. L., Otero Candelera, R. & Rodríguez Matutes, C. Joinpoint regression analysis of lung cancer mortality, Andalusia 1975–2000. Ann. Oncol. 15, 793–796 (2004).
Kanda, Y. Investigation of the freely available easy-to-use software EZR for medical statistics. Bone Marrow Transpl. 48, 452–458 (2013).
Choi, S. J. et al. Severe fever with thrombocytopenia syndrome in South korea, 2013–2015. PLoS Negl. Trop. Dis. 10, e0005264 (2016).
Natsuaki, M. Tick bites in Japan. J. Dermatol. 48, 423–430 (2021).
Fukushima, S., Sumida, T., Kawamata, O., Hidani, Y. & Hagiya, H. Epidemiology and clinical features of patients with tick bites in the Japanese spotted fever-endemic zone. J. Infect. Chemother. 31, 102570 (2025).
Dong, Y., Lin, S-H., Jiang, L. & Liu, H. Clinical characteristics and risk factors of 267 patients having severe fever with thrombocytopenia syndrome-new epidemiological characteristics of fever with thrombocytopenia syndrome: epidemiological characteristics of SFTS. Medicine 101, e31947 (2022).
Wilson, N. et al. Tick bite risk factors and prevention measures in an area with emerging Powassan virus disease. Public. Health Chall. 2 (2023).
Fischhoff, I. R., Bowden, S. E., Keesing, F. & Ostfeld, R. S. Systematic review and meta-analysis of tick-borne disease risk factors in residential yards, neighborhoods, and beyond. BMC Infect. Dis. 19, 861 (2019).
Otsuka, Y. et al. Trends in the incidence of Japanese spotted fever in japan: A nationwide, Two-Decade observational study from 2001–2020. Am. J. Trop. Med. Hyg. 108, 701–704 (2023).
Hidaka, K. et al. Seroprevalence for severe fever with thrombocytopenia syndrome virus among the residents of miyazaki, japan: an epidemiological study. J. Infect. Chemother. 30, 481–487 (2024).
Ministry of the Environment, Government of Japan. Available at: https://www.env.go.jp/en/index.html. Accessed June 12. (2025).
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This research received no specific grants from any funding agency, commercial sector, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
Study concept: HH, and SF. Data Analysis: SF, HH, and HA. Drafting: SF and HH. Supervision: TK and HH.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fukushima, S., Akazawa, H., Koyama, T. et al. Trends in the incidence of severe fever with thrombocytopenia syndrome in Japan: an observational study from 2013 to 2022. Sci Rep 15, 20715 (2025). https://doi.org/10.1038/s41598-025-07955-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07955-x