Abstract
To develop and validate a machine learning-based prediction model to predict axillary lymph node (ALN) metastasis in triple negative breast cancer (TNBC) patients using magnetic resonance imaging (MRI) and clinical characteristics. This retrospective study included TNBC patients from the First Affiliated Hospital of Soochow University and Jiangsu Province Hospital (2016–2023). We analyzed clinical characteristics and radiomic features from T2-weighted MRI. Using LASSO regression for feature selection, we applied Logistic Regression (LR), Random Forest (RF), and Support Vector Machine (SVM) to build prediction models. A total of 163 patients, with a median age of 53 years (range: 24–73), were divided into a training group (n = 115) and a validation group (n = 48). Among them, 54 (33.13%) had ALN metastasis, and 109 (66.87%) were non-metastasis. Nottingham grade (P = 0.005), tumor size (P = 0.016) were significant difference between non-metastasis cases and metastasis cases. In the validation set, the LR-based combined model achieved the highest AUC (0.828, 95%CI: 0.706–0.950) with excellent sensitivity (0.813) and accuracy (0.812). Although the RF-based model had the highest AUC in the training set and the highest specificity (0.906) in the validation set, its performance was less consistent compared to the LR model. MRI-T2WI radiomic features predict ALN metastasis in TNBC, with integration into clinical models enhancing preoperative predictions and personalizing management.
Similar content being viewed by others
Introduction
Triple-negative breast cancer (TNBC) is a highly aggressive subtype of breast cancer (BC) that accounts for approximately 10-20.8% of all BC cases1. This specific subtype is characterized by a lack of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER-2), exhibiting a higher risk of recurrence rates, rapid progression, and poor prognosis2.The presence of axillary lymph nodes (ALN) is the key factor in staging prognosis and treatment planning for TNBC patients. A timely and accurate detection of ALN can help to make an early therapeutic decision, reduce recurrence risk, and ultimately help patients’ long-term survival3,4. Although lymph node biopsy is the gold standard for determining the presence or absence of metastasis, due to its invasive procedure, it carries associated risks such as lymphedema, arm pain, and limited mobility, as well as frequently producing negative results that trigger unnecessary health issues5,6. This opens up a need for effective and non-invasive methods that can properly predict ALN metastasis without causing health effects to the patients.
Traditional imaging techniques like ultrasound, X-ray, and Computed Tomography Quantification (CTquan’c) have limited sensitivity and specificity in assessing the ALN status. While CT scans provide detailed morphological characteristics, their radiation exposure makes them unsuitable for repeated use7. Magnetic resonance imaging (MRI) is widely used in the evaluation of breast diseases due to its excellent soft tissue resolution without radiation exposure8. Radiomics is an emerging field that utilizes advanced imaging systems and data analysis to help generate quantitative features from medical images. Radiomics analysis has been employed in several breast cancer related studies such as identification of TNBC9, prediction of response to neoadjuvant chemotherapy in TNBC patients10,11), survival prediction in TNBC patients12 and so on. However, there are few reports on the analysis of MRI radiomics for preoperative lymph node metastasis in TNBC. This approach significantly addresses the diagnosis gaps by capturing fine details from MRI images beyond visual assessment, potentially providing a non-invasive predictive alternative.
Although MRI offers essential insights into breast cancer, there are limited studies available on MRI-based radiomic specifically for predicting axillary lymph node involvement TNBC, which indicates an image of the gap in the clinical practice. This study aims to develop and validate a prediction model for ALN metastasis in TNBC patients based on MRI and clinical characteristics.
Methods
Study design and population
In this retrospective study, patients with surgically or immunopathological confirmed TNBC were enrolled at the First Affiliated Hospital of Soochow University and Jiangsu Province Hospital of Traditional Chinese Medicine between May 2016 and May 2023. This study was approved by the institutional review board (Ethics No. LL202001) and written informed consent was waived. Patients were categorized into lymph node metastasis and non-metastasis groups based on axillary lymph node biopsy or surgical pathology results. Stratified randomization was used to ensure that the ratio of metastasis/non-metastasis cases in the training set (70%) and the validation set (30%) is consistent with that of the original dataset (1:2). This was specifically implemented through the following steps: First, the data were stratified based on metastasis status. Then, independent sampling was conducted within each stratum using a random seed method.
Inclusion criteria adapted for the study were: (1) Patients underwent breast MRI T2-weighted imaging with Short Tau Inversion Recovery (T2WI-STIR) sequence scanning before surgery or biopsy; (2) Immunopathological results from tumor surgery or biopsy confirmed ER, PR, and HER-2 negativity; (3) Patients had axillary lymph node biopsy or surgical biopsy. Exclusion criteria for this study were: (1) Incomplete clinical or pathological data; (2) History of prior breast radiotherapy or chemotherapy; (3) Poor MRI image quality; (4) Pregnancy or lactation.
MRI examination
Breast MRI examinations were performed using Siemens Skyra 3.0 T and GE750W 3.0T MRI scanners with breast-specific coils. Patients were given instruction on relaxation and breathing techniques to minimize artifacts. The scanning position required the patient to lie prone with an elevated abdomen, allowing both breasts to rest naturally downward in the coil, with hands positioned comfortably. The scanning range began from the lower boundary of the breast, extending upwards to the axilla and covering both breasts. The scanning sequences included axial T1-weighted imaging, T2WI-STIR, diffusion imaging, and dynamic contrast-enhanced imaging. The T2 scanning parameters were Siemens Skyra 3.0 T MRI: T2WI-STIR (TR 4490 ms, TE 53 ms, TI 230 ms, slice thickness 4 mm, matrix 320 × 320). Tumor location (left or right breast) and lesion size (diameter in cm) were recorded.
Pathological examination
Following biopsy or surgery, all patients underwent immunopathological examination to access lymph node status, tumor Nottingham grade, Ki-67, E-cadherin, and CK5/6 expression level. Patients were divided into two groups: those with axillary lymph node metastasis and those without.
Tumor segmentation
T2WI-STIR images were saved in DICOM format. A radiologist with over ten years of experience in diagnostic imaging manually delineated regions of interest (ROI) using ITK-SNAP software (version 3.6, http://www.itksnap.org). Another radiologist with over ten years of experience reviewed and confirmed each segmentation, making necessary corrections and resolving disagreements through consultation. During delineation, diffusion and dynamic contrast-enhanced images were referenced to avoid including cystic or hemorrhagic necrotic areas within the tumor and ensure complete lesion coverage.
Radiomic feature extraction and selection
Radiomic features complying with the image biomarker standardization initiative13 were extracted from the segmented ROIs utilizing the Python-based Pyradiomics open-source package (Version 3.0.1, https://github.com/Radiomics/pyradiomics). The extracted features were categorized into six distinct groups: (1) First Order Feature, (2) Gray Level Co-occurrence Matrix Feature (GLCM), (3) Gray Level Dependence Matrix Feature (GLDM), (4) Gray Level Run Length Matrix Feature (GLRLM), (5) Gray Level Size Zone Matrix Feature (GLSZM), and (6) Shape-Based Features.
Feature selection and dimensionality reduction were performed using the R software packages “mlr3verse” and “glmnet” (version 4.2, http://www.Rproject.org). Prior to the dimensionality reduction process, all radiomics features were normalised to zero mean and unit variance to reduce the risk of overfitting due to excessive features. The optimal subset of radiomic features was identified through least absolute shrinkage and selection operator (LASSO) regression with 10-fold cross-validation. The selection of radiomic features was determined by adjusting the lambda (λ) parameter to one standard error above the minimum.
Model development
Univariate analysis was used to identify clinical and pathological significance features and subsequently used to build the clinical prediction model. The selected radiomic features were utilized to develop the radiomics model.
For our analysis, we constructed and validated five predictive models: a clinical model and a radiomics model built with Logistic Regression (LR), and three combined models integrating clinical and radiomic features using LR, Random Forest (RF), and Support Vector Machine (SVM) algorithms. The model selection strategy was designed to balance predictive performance and clinical interpretability. Logistic regression provides linear decision boundaries with explicit feature coefficients, random forest captures non-linear interactions through ensemble trees, and SVM handles high-dimensional data via kernel transformation. This tripartite framework covers the predominant modeling paradigms in radiomics studies while avoiding higher overfitting risks from excessive model complexity. In order to optimise the performance of the machine learning model, we employed a randomised search-based cross-validation method, which efficiently searches for the optimal hyper-parameter configurations by randomly sampling the parameter combinations in a predefined parameter space and combining them with a 10-fold cross-validation to evaluate the performance of each set of parameters. Ultimately, based on the results of random search and cross-validation, we determine the optimal hyperparameter combination for each model to achieve the best classification performance. Hyperparameter optimisation is strictly limited to the use of training cohorts, and the validation set is dedicated to targeting the models for final performance evaluation.
Statistical analysis
Statistical analyses were performed using SAS (version 9.4, SAS Institute Inc., USA) and R (version 4.2; available at http://www.R-project.org). Quantitative data that follow normal distribution were described as mean ± standard deviation (SD), and group comparisons were used in the t-test. Meanwhile, non-normally distributed quantitative data values were described as medians with interquartile ranges (IQR). Categorical data were summarized as counts (N) and percentages (frequencies), and chi-square or Fisher’s exact tests were used to analyze. Two-sided p-values < 0.05 were defined as statistically significant.
Spearman or Pearson correlation coefficients analysis was used to assess the correlation. The area under the curve (AUC) was used to measure the overall performance of the model. To determine the optimal probability threshold for classification, the Youden index was used. The threshold corresponding to the maximum value of this index was selected as the optimal threshold. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated based on this threshold to assess the effectiveness of each model. The p-value of the ROC curve was calculated using the pROC package in R software. Specifically, DeLong’s nonparametric algorithm was used to test the null hypothesis that the AUC is equal to 0.5 (i.e., no discrimination). Calibration curves and decision curve analysis (DCA) were employed to evaluate model fit and clinical benefit.
Results
Patient’s characteristics
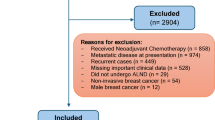
A total of 163 patients were included in this study after excluding 31 cases based on the selection criteria. The exclusions were due to: 13 cases with inconsistent scanning parameters, 7 cases with incomplete clinical or pathological data, 6 cases with poor image quality, and 5 cases with a history of neoadjuvant chemotherapy prior to the examination. (Fig. 1). ALN metastasis was observed in 54 (33.13%) patients, while 109 (66.87%) patients were classified as ALN non-metastatic. The patients were categorized into two groups: a training group (n = 115; 70.55%), which included 38 ALN metastatic patients and 77 non-metastatic patients, and a validation group (n = 48; 29.45%), consisting of 16 patients with ALN metastatic disease and 32 patients with ALN non-metastatic disease.
Nottingham grade, and tumor size, expression were significantly different between non-metastatic and metastatic patients (p < 0.05). In contrast, lesion location, patient age, Ki-67 levels, E-cadherin expression, CK5/6 and P63 expression showed no significant differences between the two groups (Table 1).
Radiomic feature selection
1,132 quantitative radiomic features were extraced. LASSO regression analysis was then performed to identify the most predictive features, resulting in four optimal features: one first-order gray-level feature and three wavelet texture features (Fig. 2). Among these, the wavelet_HHH_glcm_Imc1 feature demonstrated the highest predictive performance, achieving an AUC of 0.743 (95% CI: 0.649–0.837, p < 0.001). At a cutoff value of -0.110, this feature exhibited a sensitivity of 0.737 and a specificity of 0.688. On the other hand, the wavelet_LLH_glcm_Correlation feature showed the lowest predictive accuracy, with an AUC of 0.636 (95% CI: 0.526–0.746, p = 0.015), yielding a sensitivity of 0.763 and a specificity of 0.571 at a cutoff value of 0.426. The remaining two features, log_sigma_5_0_mm_3D_glcm_JointEntropy and wavelet_HHH_glcm_Imc2, had AUC values of 0.723 and 0.730, respectively (Table 2).
The selection of optimal feature subset using least absolute shrinkage and selection operator (LASSO) regression with ten-fold cross-validation. (A) The vertical axis represents the AUC value, the upper horizontal axis represents the number of radiomic features in the model, and the lower horizontal axis represents the Log(λ) value. When the tuning parameter λ and Log(λ) reach − 2.4, the AUC value of the model is maximized, selecting four radiomic features accordingly. (B) The vertical axis represents the coefficient values, the upper horizontal axis represents the number of radiomic features in the model, and the lower horizontal axis represents the Log(λ) value. As the Log(λ) value increases, the coefficients of the radiomic features gradually compress to 0.
Predictive model construction and validation
In the training set, the AUC value (0.841, 95% CI: 0.758 ~ 0.925) of the combined model based on SVM is the highest, and its sensitivity, specificity, and accuracy (0.842, 0.805, and 0.817, respectively) are also superior to those of the other predictive models. In the validation set, LR-based combined model achieved the highest AUC (0.828, 95% CI: 0.706 ~ 0.950) and maintained excellent sensitivity (0.813) and accuracy (0.812). The RF-based model achieved the highest AUC in the training set with the highest specificity (0.906) in the validation set, the RF-based model showed less consistent performance overall compared to the LR model (Table 3; Fig. 3).
Nomogram development and model evaluation
The nomogram incorporates seven key predictive factors: Nottingham grade, tumor size (in cm), and four radiomic features (log_sigma_5_0_mm_3D_glcm_JointEntropy, wavelet_LLH_glcm_Correlation, wavelet_HHH_glcm_Imc1, and wavelet_HHH_glcm_Imc2). Each predictive factor in the model corresponds to a score range of 0–100, with higher-weighted factors assigned higher scores. By summing the scores of the seven predictive factors, the total score corresponds to the probability of lymph node metastasis in TNBC (Fig. 4).
Nomogram for predicting axillary lymph node metastasis in triple-negative breast cancer. Predictive factors in the nomogram include Nottingham grade, Size (tumor diameter in cm), JointEntropy -log_sigma_5_0_mm_3D_glcm_JointEntropy (radiomic feature, represented as x388 in the figure), Correlation-wavelet_LLH_glcm_Correlation (radiomic feature, represented as x469 in the figure), Imc1-wavelet_HHH_glcm_Imc1 (radiomic feature, represented as x991 in the figure), and Imc2-wavelet_HHH_glcm_Imc2 (radiomic feature, represented as x992 in the figure).
For example, a patient with a Nottingham grade of 3, tumor size of 8 cm, log_sigma_5_0_mm_3D_glcm_JointEntropy of 7.5, wavelet_LLH_glcm_Correlation of 0.3, wavelet_HHH_glcm_Imc1 of -0.5, and wavelet_HHH_glcm_Imc2 of 0.9 would have a total score of approximately 264. According to the nomogram, this corresponds to a predicted probability of lymph node metastasis of roughly 30–35%.
The calibration curve (Fig. 5A) demonstrates a good fit between the predicted and observed probabilities of ALN metastasis, as indicated by the close alignment of the ideal line, the apparent prediction line, and the bias-corrected calibration curve. Furthermore, decision curve analysis (Fig. 5B) shows a higher net benefit when using the nomogram for predicting axillary lymph node metastasis compared to not using a predictive model, as evidenced by the red curve representing the nomogram’s efficacy lying above the lines representing all or no patients having lymph node metastasis.
Calibration curve and decision curve. (A) Calibration curve. The horizontal axis represents the probability of lymph node metastasis predicted by the nomogram model, and the vertical axis represents the actual clinical observations. The Ideal diagonal dashed line represents the ideal line; the Apparent is the model’s prediction line; the Bias-corrected solid line is the calibration curve obtained after repeated bootstrapping. The alignment of these lines indicates good calibration. (B) Decision curve analysis. The horizontal axis represents threshold probability (indicating the proportion of patients with lymph node metastasis), and the vertical axis represents net benefit. The red curve represents the efficacy of lymph node metastasis prediction using the nomogram model. The yellow arc represents all patients having lymph node metastasis, and the green horizontal line represents no lymph node metastasis in all patients.
Discussion
This study evaluated the predictive factors for ALN metastasis in patients with TNBC by analyzing clinical characteristics and radiomic features derived from MRI scans. The integrated clinical-radiomic model has the potential to enhance preoperative prediction of ALN metastasis in TNBC patients, which could reduce the need for invasive biopsies and facilitate more personalized management strategies for these patients.
Univariate analysis revealed that Nottingham grade, and tumor size were significantly associated with ALN metastasis (p < 0.05), aligning with previous studies that showed a significant correlation between higher histological grades and larger tumor size and increased lymph node metastasis rates14,15,16. Although previous studies found a positive correlation between CK5/6 expression and lymph node metastasis17,18), the difference in CK5/6 expression between our two groups was close to being statistically different, which may be related to the small sample size of our cohort or the natural characteristics of the patients. In addition, patient age, Ki-67, E-cadherin and P63 expression were not significantly associated with ALN status, which may indicate that these markers may not be as important in predicting TNBC lymph node metastasis.
MRI is routinely employed for the diagnosis and staging of breast cancer, offering superior soft tissue contrast without the risks associated with radiation exposure. Our study utilized T2WI radiomic features, which prior research suggests may be more reliable for assessing tumor characteristics compared to T1-weighted or contrast-enhanced images19. By leveraging these T2WI-derived radiomic features, our model provided a high-throughput, objective analysis that may assist clinicians in non-invasive lymph node assessment in TNBC20,21).
In our analysis of radiomic features, we initially identified 1,132 quantitative features from MRI scans. Through LASSO regression, four optimal features were selected, including one first-order gray-level feature and three wavelet texture features. The wavelet_HHH_glcm_Imc1 feature exhibited the strongest predictive performance with an AUC of 0.743 (95%CI: 0.649 ~ 0.837), indicating its potential utility in clinical practice for predicting ALN metastasis in TNBC patients. This finding supports the studies that showed the importance of radiomic features in breast cancer22,23). The other features demonstrating intermediate predictive performance establish the complexity of tumor characteristics captured through radiomics.
The development of predictive models incorporating both clinical and radiomic features revealed that combined models consistently outperformed individual models in both training and validation sets. The logistic regression (LR)-based combined model achieved the highest AUC of 0.828 (95%CI: 0.706 ~ 0.950) in the validation set, demonstrating excellent sensitivity (0.813) and accuracy (0.812). This confirmed that integrating clinical and radiomic data enhances predictive capabilities, as supported by previous researchers24,25). Previous studies have shown a positive correlation between ultrasound parameters (e.g., higher Data System category) and breast cancer ALN metastasis26. Deep learning models based on ultrasound have an AUC of up to 0.89 in predicting ALN metastasis in breast cancer23. The overall accuracy of PET/CT in diagnosing ALN metastasis has also been reported to be 74.1%27. However, these studies included cases that were not limited to TNBC, and direct comparisons of efficacy could not be made due to differences in the study cohort and the equipment and methods used in this study.
Parmar et al.28 used 12 radiomics-based machine learning methods to predict survival in non-small cell lung cancer patients, with results indicating that the RF algorithm had the best performance. In this study, the RF model performed well in the training set, but despite its high specificity (0.906) in the validation set, its AUC value (0.587, 95%CI: 0.409 ~ 0.765) was relatively low. This may be due to the small sample size in the validation set, which weakens the stability of the model. In addition, the high complexity of the RF and SVM models themselves may be more sensitive to noise in some features. As well as potential distributional differences that may exist between the two datasets could also be the cause of overfitting. Further validation of our results using richer tuning methods on larger datasets is needed in the future. Although the support vector machine (SVM)-based model showed strong performance on the training set, its slight decline in the validation set suggests potential overfitting, a common challenge in machine learning applications.
A nomogram based on the LR model was developed to enhance clinical utility, including essential predictive factors such as the Nottingham grade, tumor size, and chosen radiomic features. This nomogram delivers personalized predictions of ALN metastasis, enabling clinicians to assess better individual cases29,30). The calibration curve demonstrated a good fit between predicted and observed probabilities, indicating the nomogram’s reliability across risk levels. Additionally, decision curve analysis highlighted the nomogram’s clinical utility, showing a higher net benefit compared to not using a predictive model, thus supporting its potential role in guiding treatment decisions for TNBC patients.
Despite the promising results, this study also has some limitations. Firstly, due to the low incidence of TNBC, the sample size in this study was relatively small. Secondly, although our study population was from two centers, the validation of the model was not carried out exclusively in an external cohort due to the limitation in the sample size. Future studies that include larger samples in more centers are necessary. Thirdly, the study employed manual image segmentation, which can introduce variability, which, while accurate, can introduce variability. Future research would benefit from implementing automated segmentation techniques to enhance reproducibility. Another limitation is the exclusive use of a single imaging modality (T2WI) for radiomic feature extraction; integrating features from multiple imaging sequences could further improve predictive accuracy. Finally, our study used only three classical machine learning models, and future multicentre studies may use larger cohorts to further explore deep learning architectures. Nevertheless, this study establishes a promising framework for applying radiomics in predicting lymph node metastasis.
Conclusion
This study successfully developed and validated a combined clinical-radiomics model to predict ALN metastasis in preoperative TNBC patients. By integrating MRI-derived radiomic features with clinical data, the model shows accuracy and predictive capabilities compared to clinical or radiomic models separately. This non-invasive approach will be a promising tool for patient assessment and reduce invasive biopsies. Further investigation is needed in a larger and multicenter approach to enhance the study’s reproducibility and clinical application.
Data availability
All data generated or analysed during this study are included in this published article.
References
Thike, A. A. et al. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod. Pathology: Official J. United States Can. Acad. Pathol. Inc. 23 (1), 123–133 (2010).
Yin, L., Duan, J. J., Bian, X. W. & Yu, S. C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast cancer Research: BCR. 22 (1), 61 (2020).
Nos, C. et al. Prediction of tumour involvement in remaining axillary lymph nodes when the Sentinel node in a woman with breast cancer contains metastases. Br. J. Surg. 90 (11), 1354–1360 (2003).
Chakraborty, A. et al. Determinants of lymph node status in women with breast cancer: A hospital based study from Eastern India. Indian J. Med. Res. 143 (Supplement), S45–s51 (2016).
Patani, N. R., Dwek, M. V. & Douek, M. Predictors of axillary lymph node metastasis in breast cancer: a systematic review. Eur. J. Surg. Oncology: J. Eur. Soc. Surg. Oncol. Br. Association Surg. Oncol. 33 (4), 409–419 (2007).
Viale, G. et al. Predicting the status of axillary Sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer 103 (3), 492–500 (2005).
Hongna, T., Minghui, W. & Jing, Z. et al. The value of breast X-ray radiomics in predicting axillary lymph node metastasis in breast cancer. Chin. J. Radiol. 54 (9), 859–863 (2020).
Bing, N., Xindang, S. & Xin, L. Imaging characteristics of breast cancer and their relationship with lymph node metastasis. Chin. J. CT MRI. 16 (5), 84–86 (2018).
Hu, Z., Qin, Y., Liu, F., Wang, Y. & Liu, S. Ultrasound radiomics features to identify patients with Triple-Negative breast cancer: A retrospective, Single‐Center study. J. Ultrasound Med. 43 (12), 2419–2419 (2024).
Lee, H. et al. Prediction of early clinical response to neoadjuvant chemotherapy in Triple-negative breast cancer: incorporating radiomics through breast MRI. Sci. Rep. 14 (1), 21691 (2024).
Chen, Y. et al. Intratumoral microbiota-aided fusion radiomics model for predicting tumor response to neoadjuvant chemoimmunotherapy in triple-negative breast cancer. J. Transl Med. 23 (1), 352 (2025).
Wenwen, Jiang, Z. et al. Integrating ultrasound radiomics and clinicopathological features for machine learning-based survival prediction in patients with nonmetastatic triple-negative breast cancer. BMC Cancer. 25 (1), 291 (2025).
Zwanenburg, A. et al. The image biomarker standardization initiative: standardized quantitative radiomics for highthroughput image-based phenotyping. Radiology 295 (2), 328–338 (2020).
Zhixin, L., Bing, Z. & Mei, G. al e. Clinicopathological characteristics, diagnosis, and treatment analysis of triple-negative breast cancer. Chin. J. Clin. Oncol. Rehab. 18 (4), 305–308. (2011).
Abdul Aziz, A. A., Md Salleh, M. S. & Ankathil, R. Clinicopathological and prognostic characteristics of Malaysian triple negative breast Cancer patients undergoing TAC chemotherapy regimen. Int. J. Breast cancer. 2020, 8424365 (2020).
Wang, J., Lu, X., Zheng, X., Xia, C. & Li, P. Clinical value of preoperative ultrasound signs in evaluating axillary lymph node status in Triple-Negative breast Cancer. J. Oncol. 2022, 2590647 (2022).
Krag, D. N. & Single, R. M. Breast cancer survival according to number of nodes removed. Ann. Surg. Oncol. 10 (10), 1152–1159 (2003).
Inanc, M. et al. Cytokeratin 5/6, c-Met expressions, and PTEN loss prognostic indicators in triple-negative breast cancer. Med. Oncol. 31 (1), 801. (2014).
Romeo, V. et al. AI-enhanced simultaneous multiparametric (18)F-FDG PET/MRI for accurate breast cancer diagnosis. Eur. J. Nucl. Med. Mol. Imaging. 49 (2), 596–608 (2022).
Nijiati, M. et al. MRI-Based radiomics for preoperative prediction of lymphovascular invasion in patients with invasive breast Cancer. Front. Oncol. 12, 876624 (2022).
Cui, X., Zhu, H. & Huang, J. Nomogram for predicting lymph node involvement in Triple-Negative breast Cancer. Front. Oncol. 10, 608334 (2020).
Gao, X., Luo, W., He, L. & Yang, L. Nomogram models for stratified prediction of axillary lymph node metastasis in breast cancer patients (cN0). Front. Endocrinol. 13, 967062 (2022).
Zhou, L. Q. et al. Lymph node metastasis prediction from primary breast Cancer US images using deep learning. Radiology 294 (1), 19–28 (2020).
Bae, S. H. et al. The role of fluorodeoxyglucose-PET/computed tomography as a predictor of breast cancer characteristics and prognosis. Nucl. Med. Commun. 43 (1), 108–113 (2022).
Wang, S. et al. Construction and validation of a nomogram prediction model for axillary lymph node metastasis of cT1 invasive breast cancer. Eur. J. cancer Prevention: Official J. Eur. Cancer Prev. Organisation (ECP). 33 (4), 309–320 (2024).
Yun, S. J., Sohn, Y. M. & Seo, M. Risk stratification for axillary lymph node metastases in breast cancer patients: what clinicopathological and radiological factors of primary breast cancer can predict preoperatively axillary lymph node metastases? Ultrasound Q. 33 (1), 15–22 (2017).
Kutluturk, K. et al. Factors affecting the accuracy of 18F-FDG PET/CT in evaluating axillary metastases in invasive breast cancer. Niger J. Clin. Pract. 22 (1), 63 (2019).
Parmar, C., Grossmann, P., Bussink, J., Lambin, P. & Aerts, H. Machine learning methods for quantitative radiomic biomarkers. Sci. Rep. 5, 13087 (2015).
Iasonos, A., Schrag, D., Raj, G. V. & Panageas, K. S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncology: Official J. Am. Soc. Clin. Oncol. 26 (8), 1364–1370 (2008).
Daimiel Naranjo, I. et al. Radiomics and machine learning with multiparametric breast MRI for improved diagnostic accuracy in breast Cancer diagnosis. Diagnostics (Basel Switzerland). 11 (6), 919 (2021).
Funding
This work was mainly supported by the key program of Jiangsu Commission of Health (K2023027), the medicine plus X project from Suzhou medical school of Soochow university (grant number ML12203423), a grant from Infectious and Inflammatory Radiology Committee of Jiangsu Research Hospital Association (grant number GY202301), Jiangsu Province Capability Improvement Project through Science, Technology and Education (Jiangsu Provincial Medical Key Discipline Cultivation Unit, JSDW202242), Suzhou Key Laboratory of Medical Imaging (SZS2024032), the project of science and technology for livelihood SuZhou city (grant number SYSD2020074).
Author information
Authors and Affiliations
Contributions
Yunyun Shen and Renjun Huang carried out the studies, participated in collecting data, and drafted the manuscript. Yinghui Zhang and Yonggang Li performed the statistical analysis and participated in its design. Jianguo Zhu and Yonggang Li helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by Ethics Committee of Xinghai Hospital, Suzhou Industrial Park (Ethics No. LL202001) and the requirement for informed consent was waived by the Institutional Review Board of Xinghai Hospital, Suzhou Industrial Park because of the retrospective nature of the study. I confirm that all methods were performed in accordance with the relevant guidelines. All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, Y., Huang, R., Zhang, Y. et al. Prediction of axillary lymph node metastasis in triple negative breast cancer using MRI radiomics and clinical features. Sci Rep 15, 21923 (2025). https://doi.org/10.1038/s41598-025-08001-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08001-6