Abstract
Reproductive seasonality in mammals is often influenced by top down and bottom up effect. While tropical and equatorial regions exhibit continual breeding due to stable resources, tropical India experiences pronounced seasonality in forage availability. This study investigates the breeding timing of chital (Axis axis) in the tropical deciduous forests of Central India at Kanha Tiger Reserve, where forage availability varies seasonally. Field observations focused on antler conditions in males and lactation signs in females to estimate breeding timing. Rutting peak, as determined by males with hard antlers, coincided with nutritional peak induced by the monsoon, which probably was also the cue for ovulation in females. Lactation, the most nutritionally demanding period, peaked with the post-fire sprouting of herbaceous vegetation in April and fawn weaning synchronized with ample food availability. This synchrony between reproductive cycles and forage availability emphasizes the adaptive strategies of chital to maximize offspring survival. Furthermore, the peak in tiger births coincided with the fawning peak of chital, making them vulnerable prey during a nutritionally demanding period for tigresses, who are restricted in their movements near vulnerable cubs. Understanding the links between trophic levels and their seasonality offers important insights for wildlife management and conservation strategies.
Similar content being viewed by others
Introduction
Synchrony or birthing peaks are seen as a response to either seasonal environments or as an anti-predatory adaptation1,2. While tropical and equatorial mammals exhibit a lack of seasonality in breeding due to uniform resource availability, tropical India is highly seasonal in resource availability due to the rainfall being primarily restricted to the monsoon season. However, reproductive seasonality in ungulates may also be influenced by factors beyond forage abundance, including predation risk, social structure, mate availability, and habitat configuration3, as synchronized birthing can reduce individual predation risk via predator swamping, while habitat structure and group sociality can influence access to mates and protection. Study on different African ungulates found that breeding synchronizes with seasons of abundant forage resources, an adaptation that enhances offspring survival by ensuring that young are born during favourable conditions4. Rainfall patterns also significantly influence ungulate reproductive behaviour as it affects food availability5. Increased rainfall can lead to lush vegetation, providing better nutrition for pregnant or lactating females and improving offspring survival rates. The reproductive patterns of ungulates can vary significantly based on environmental conditions. Ungulates are timed to coincide with their breeding season with periods of highest plant quality to support offspring nutrition6. This adaptation ensures that lactating females and weaned offspring obtain the required energy and protein from the forage5,7,8,9. These relationships underscore the importance of understanding ecological dynamics to better manage ungulate populations and their habitats.
As environmental conditions continue to change due to human impact, ongoing research will be required to understand how ungulates adapt to these challenges. Changes in forage quantity and quality significantly impact the body condition of ungulates, especially females during gestation and lactation, when their energy and protein requirements are heightened. Many studies emphasize the critical role that adequate nutrition plays in ensuring the health and reproductive success of these animals during these demanding periods4,10,11,12,13,14. The onset of oestrus in females is significantly affected by conditions required for a successful pregnancy and subsequent lactation, such as food availability or favourable weather conditions15,16, as increased time and energy expenditures for food searching can lead to higher mortality in reproducing adults11. We hypothesize that though the ultimate cue for timing reproduction is likely to be the monsoon rains and the associated growing season for all vegetation, we believe that it may be possible for ungulates to adjust seasonality of reproduction to local conditions. In the case of Kanha, it would be the management practice of cool burning of grasslands and the associated new flush of sprouts that are highly digestible with high protein and energy content.
In most of the cervids, antler cycles serve as external indicators of internal reproductive changes17. In most deer species, males undergo a yearly cycle characterized by the growth and subsequent shedding of antlers18,19,20. Antlers play a crucial role in male-male confrontations to establish dominance and secure mates, serving as an indication to females of male fitness and condition during the rutting season, which is a period of intense mating activity21,22,23,24. Testosterone levels peak seasonally during the rut season25, and when testosterone levels fall below threshold after the rutting season, antlers are cast off in most cervids25,26,27. Consequently, peak rutting activity often coincides with the period when the largest proportion of males exhibit burnished antlers, indicative of their reproductive readiness28,29,30. In deer, observing actual fawning is a rare event and is often studied by implanting radio transmitters in the vagina of pregnant females, which are expelled out at the time of parturition31,32. This allows for the study of timing and specific location of parturition. We indexed the timing of fawning by estimating the proportion of lactating females in the population5,33.
Many studies on breeding seasonality have been conducted on North American and African ungulates, but in the Indian subcontinent only a few ungulate species have been studied for their reproductive patterns28,34. However, these studies have primarily focused on observing the seasonality of the antler cycle. Management decisions like prey augmentation35, where animals from high-density areas were captured and moved to low-density areas to increase the carrying capacity of large carnivores, also need information about rutting and fawning. Translocation during prey augmentation in the fawning season can cause stress due to possible mother-fawn separation, and aggressive behaviour of males that bear hard antlers during the rutting season can increase mortality, making it essential to understand the breeding season of the source chital population beforehand. In this study, we record the reproductive condition of chital throughout the year from representative samples to decipher seasonality and its likely drivers in the dry deciduous forest system of Kanha National Park.
Results
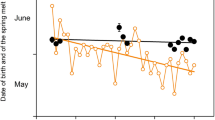
Fawning occurred throughout the year as lactation was recorded in all months. However, the proportion of lactating females in the population peaked in March-April. April has the highest proportion of lactating females (78 ± 0.8%), followed by March (73.5 ± 1.1%), while November recorded the lowest proportion (6.93 ± 0.37%) (Fig. 1, Table S1). Seasonally, summer had the highest proportion of lactating females (72.26 ± 0.24%), followed by monsoon (48.9 ± 0.46%), and winter (30.79 ± 0.34%) (Fig. 1, Table S1). None of the 200 sampled sub-adult females were observed to be lactating.
Seasonality of breeding and fawning in chital (Axis axis) as indexed by proportion of different antler stages of males and lactating females in relation to resource availability indexed by NDVI in Kanha Tiger Reserve. The bars show the proportion of males in different antler stages in the population. The blue line indicates the NDVI value for each month, and the red line represents the proportion of lactating females in the population. The dotted lines indicate the standard errors for the NDVI and the proportion of lactating females.
In July, nearly all adult males have hard antlers (97.05 ± 0.15%). Between May and November, over 60% of the male population displayed hard antlers, while more than 50% of the adult male population sprouted velvet-covered antlers between December and April (Fig. 1, Table S2). The proportion of shed antler male observation is low due to the short interval between antler shedding and the emergence of new antlers.
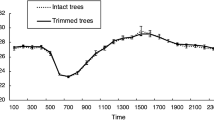
In Kanha Tiger Reserve, grassland growth peaks during the monsoon and post-monsoon periods but declines during the dry winter season with minimal rainfall. The NDVI value during the post-fire season (April) shows an upward trend indicating the emergence of freshly grown grass shoots. Lactating peaked at the time of the new grass flush when the NDVI values start increasing (Fig. 1, Table S4) after a lowest in March. Births of tiger litters were observed throughout the year with a peak in December. Their weaning started after 3–4 months which coincided with the time of fawning (Fig. 2).
Percent tiger litter weaned (n = 31) depending on their birth month data obtained from Kumar, 2019 vs. proportion of lactating chital females in chital population in Kanha tiger reserve every month. Blue bars show the number of tiger litter and red line show the percentage of lactating chital females in population.
Discussion
There seems to be a debate regarding the presence of distinct breeding seasonality in chital, especially in tropical regions where food and water are relatively stable throughout the year (Table S4). Though Krishnan (1972)36 reported chital lack any birth peak throughout peninsular India, many studies have found distinct seasonal peaks in chital rutting behaviour in India (Table S4). Studies were done in Hawaii37, Texas38, Australia39, Nepal30,40 also found a distinct peak in rutting behaviour of chital depending on resource availability. We also found similar result where fawning is influenced by resource availability. The Central Indian landscape has distinct seasonal variation, which affects the availability and quality of forage during different times of the year. Mixed foragers like Chital shift their diet towards browsing during summer when grass nutrient quality declines41,42, emphasizing the importance of dietary quality. The dry grasses become non-nutritious and are filled with silica and lignin content43. Due to the burning of grasslands during February to reduce the fuel load of grasses, preventing uncontrolled fire during the summer season, fresh biomass appears post-fire season, which is high in crude protein44,45,46,47 and other minerals like calcium and magnesium48. NDVI is a measure of green biomass, though this peaks after the monsoon along with the vegetation bulk, it is the sprouting of young grasses that provides the much needed digestible energy and proteins to support lactation and weaning of fawns that is important. Moreover, chital feed on most new flush of vegetation, irrespective of species and only become selective once the vegetation matures and produces secondary metabolites as plant defences against herbivory49. Therefore, the upward change in trend of NDVI is also important factor for chital.
We found that the parturition peak of chital is synchronous with the appearance of fresh grass forage biomass, i.e., the months of March and April. Moreover, fawns born at this time were weaned off during the monsoon period, when there is ample forage available for them to feed on. The birth peak of ungulates has been observed to coincide with seasonal rainfall and the appearance of green foliage in tropical and subtropical regions50. The influence of food availability on ungulate breeding has also been reported in Red deer51 and chital deer of Bandipur and Mudumalai52.
In Kanha, most rutting occurs in late summer and monsoon season, although some hard antler males are present in the population throughout the year. Deer females conceive only when they have good body condition53,54 and Kelly et al. (2022) found that this is true for chital also. In Kanha, chital has better body condition when ample food is available41. Similarly, fawning starts in January. Since fawning is represented by lactating females and lactation occurs up to 4 months in chital, it is difficult to determine the exact fawning peak.
Peak in tiger cub birth occurs when more than half of the female chital were lactating. When weaning of tiger cubs starts after a few months, it is the same time when the number of lactating females is also highest. When weaning starts, the need for prey also increases, as the mother needs to provide food for weaned cubs. During this period, chital are most vulnerable to predation, with males being in hard antlers and preoccupied with the rut, females caring for fawns and weak due to lactation, and ample availability of fawns. Tigers in the Kanha landscape showed a functional response by selective predation on the abundant chital fawns and rutting males55, resulting in numerical response by timing their birth peak at this time. Similar pattern has also been reported in mountain lions (Puma concolor)56. The mountain lion birth peak is synchronized with the birth of North American ungulates in May-June.
There are certain cues like resource abundance that track ecological rhythms that influence reproductive behaviour. The intricate relationship between seasonal forage dynamics, reproductive patterns, nutritional ecology, and predation underscores the importance of understanding these dynamics for effective wildlife management and conservation planning. Birthing and rutting peaks of chital in Kanha, which is a deciduous forest-grassland system, were determined by resource availability and were unlikely to be in response to predation. From Schaller’s (1967)57 study it can be inferred that chital fawning occurred during December-February and the peak in tiger cubs reaching weaning stage was during June. These timings differ significantly from those observed by us. We believe that chital and subsequently tigers have adapted to the cool burning of grasses a management practice that was initiated since the creation of the Protected Area. This is an interesting finding since it suggests that nutritional factors and not photoperiod play an important role in determining reproduction timing in tropical ungulates58,59,60. This result is open to further verification by more robust experimental studies on tropical ungulates especially chital.
This study underscores the complexity of chital reproductive strategies in tropical systems. Controlled burning enhances forage quality and providing essential nutrition for lactating females. The alignment of chital reproductive cycles with forage availability, and of tiger births with vulnerable chital demographics, emphasizes the importance of ecological synchrony in predator-prey systems.
The interplay between seasonal forage dynamics, reproductive patterns, nutritional ecology, and predation is vital for effective wildlife management and conservation. Understanding these dynamics in the deciduous forest-grassland system of Kanha reveals that chital birthing and rutting peaks are driven more by resource availability than predation pressures. Effective conservation strategies must consider these intricate ecological relationships to support the sustainable management of chital populations and their habitats.
Although this research provides important information regarding chital reproductive seasonality with respect to forage availability and its impact on predator-prey dynamics, it is limited by the lack of direct observation of mating and fawning events, but instead used indirect measures like antler condition and lactation. Future studies might aim for more precise and direct observations of mating behaviour and parturition, perhaps with the aid of radio telemetry based tracking techniques to validate the timing and determinants of seasonality in chital breeding. Further investigation into the impact of climate change on resource availability and timing would be a key follow-up to determine the potential effects these changes have on reproductive cycles in the future.
Materials and methods
Study area
Kanha Tiger Reserve is located in the Satpura Maikal hill ranges of the Central Indian highlands61. It was among the first nine Tiger Reserves gazetted in 1973. It is located between 80°26’10’’ E to 81°04’40.00’’E and 22°01’5.0” N to 27°27’48.00” N in Balghat and Mandla districts of Madhya Pradesh. The area of the Core Zone is 917.43 km2, and that of the Buffer Zone is 1134.39 km2, adding up to 2051.82 km2 as the total area of Kanha Tiger Reserve. The Tiger Reserve has flat hilltops, varying degrees of slopes, and meadows in the valleys, which offer unique, diverse types of wildlife habitat, forming ideal niches for various species of plants and animals.
There are three distinct seasons in Kanha Tiger Reserve. These are monsoon, summer, and winter. Summer extends from mid-February to June end, monsoon is from June end week to mid-October, and winter is from mid-October to mid-February. Due to a great variation in temperature, humidity, wind velocity, and precipitation in different seasons, these factors serve as regulators of vegetation and the habits of wild animals in the reserve. Kanha Tiger Reserve lies in a dry deciduous zone, and the availability of water is a very important factor for the survival of animals and plants. Major forest types of the area can be divided into four categories: sal forest, miscellaneous forest, bamboo mixed forest, and grasslands. Controlled burning practices during February are carried out in the grasslands of Kanha each year for habitat improvement and management purposes.
Kanha hosts high densities of both prey and predators. Chital, which is a major prey species of all the carnivores present in Kanha, has one of the highest densities in India62.
Field method
Estimating proportion of population in breeding condition
Observation of actual mating events and fawning in wild populations is rare, especially in tropical forest systems. We therefore indexed breeding condition in males by assessing their antler condition17,29 and fawning in females by assessing their lactation state5,63,64,65.
For obtaining an unbiased representation of the population in samples, we conducted intensive surveys along fixed vehicle routes to observe the breeding status of male and female chital groups. Each route was visited once a week for a whole year during 2022. An average of 21 Km road covered per day by vehicle for chital group observation which cover different habitat types of the study area. Initially, the population was categorized into adults, sub-adults, and juveniles where juvenile were (≤ 1 year), sub-adult were (< 2 years but > 1 year) and adult (> 2 years)57 (Figure S3). For assessing breeding signs, only adult individuals were considered. To assess the involvement of sub-adult females in breeding, a total of 200 sub-adult females were observed for lactation signs during the peak lactation period. We subdivided adult males into hard antler, velvet, and shed antler male groups as per their antler stages at different times of the year (Figure S1). We expected that the peak breeding season will coincide with the time when the largest proportion of bucks exhibit hard antlers.
To determine the fawning season, lactation signs were recorded whenever an adult female was observed, as the presence of lactating mothers indicated the birth of a fawn5,63,64,65. In chital udders and teats become visible only a few days before parturition and remain prominent up to four months after parturition66,67 (Figure S2).
Analytical method
Mating and fawning season
Data on various stages of male antlers and lactating females were pooled month-wise for exploratory analysis. Then the data were categorized into three distinct seasons: winter, summer, and monsoon, and reanalysed. Since the same route was followed weekly for observations, the computation was done using multiple sampling with replacement approach68 which accounts for the possible repeated sampling of the same individual.
NDVI change
Chital are the primary grazers at Kanha Tiger Reserve as more than 50% of their diet consists of grasses41. So change in the condition of grass affects the body condition of chital. To see the monthly productivity in the grasslands of Kanha, we analysed the Normalized Difference Vegetation Index (NDVI) of the grasslands of Kanha for the past 10 years from satellite imagery of LANDSAT 8 (USGS) in Google Earth Engine69. A harmonic model for monthly change in NDVI was fitted to the data to see monthly changes in the grasslands of Kanha (Figure S4). NDVI does not provide information about palatability of grass species or identification of specific grass species. We were interested in detecting the early flush of green biomass which is indicated by the increasing trend in NDVI values. When sprouting begins in grasslands, the NDVI value starts increasing from a low as the grasses turn green replacing the dry grass or bare land left after burning. Then monthly average values of NDVI were plotted to evaluate the synchrony between grass sprouting season and chital breeding in Kanha.
Birth of tiger Cubs
We used information from Kumar (2019)70 on the number of litters born each month for tigers in Kanha where the birth month of tiger litters were determined by aging the cubs. The birth records and the month of parturition were obtained through continuous sightings by experienced forest staff, researchers, camera trap pictures, and handheld photographs taken by tourists who shared the date and location information. Information on 31 litter births was recorded with reasonable certainty. Once the month of birth was ascertained, the relationship between chital fawning and tiger cub was explored by looking at the time of birth of offspring of both prey and predator.
Data availability
All data generated and analysed during this study are included in this published article and its supplementary information files.
References
Roeder, D. V., Husak, M. S., Murphy, M. T. & Patten, M. A. Combined roles for breeding synchrony, habitat and scale as predictors of extrapair paternity. Anim. Behav. 194, 139–150 (2022).
Hayward, M. W., de Tores, P. J., Dillon, M. J. & Fox, B. J. Local population structure of a naturally occurring metapopulation of the Quokka (Setonix brachyurus macropodidae: Marsupialia). Biol. Conserv. 110, 343–355 (2003).
Bowyer, R. T., McCullough, D. R., Rachlow, J. L., Ciuti, S. & Whiting, J. C. Evolution of ungulate mating systems: integrating social and environmental factors. Ecol. Evol. 10, 5160–5178 (2020).
Sinclair, A. R. E., Mduma, S. A. R. & Arcese, P. What determines phenology and synchrony of ungulate breeding in the serengeti?? Ecology 81, 2100–2111 (2000).
Priyadarshini, K. V. R. Interactions between Forage Recruitment and Activity Pattern of the Indian Blackbuck (Saurashtra University, Rajkot, 2005).
Mduma, S. A., Sinclair, A. R. E. & Hilborn, R. Food regulates the Serengeti wildebeest: a 40-year record. J. Anim. Ecol. 68, 1101–1122 (1999).
Western, D. Size, life history and ecology in mammals. Afr. J. Ecol. 17, 185–204 (1979).
Bronson, F. H. Mammalian Reproductive Biology (University of Chicago Press, 1989).
Owen-Smith, N., Mason, D. R. & Ogutu, J. O. Correlates of survival rates for 10 African ungulate populations: density, rainfall and predation. J. Anim. Ecol. 74, 774–788 (2005).
Oftedal, O. T. Pregnancy and lactation. In Bioenergetics of Wild Herbivores (eds Hudson, R. J. & White, R. G.) 215–238 (CRC, 1985).
Robbins, C. T. Wildlife Feeding and Nutrition (Elsevier Science, 1993).
Parker, K. L., Gillingham, M. P., Hanley, T. A. & Robbins, C. T. Energy and protein balance of free-ranging black-tailed deer in a natural forest environment. Wildl. Monogr. 143, 1–48 (1999).
Gaillard, J. M., Festa-Bianchet, M., Delorme, D. & Jorgenson, J. Body mass and individual fitness in female ungulates: bigger is not always better. Proc. R. Soc. Lond. B Biol. Sci. 267, 471–477 (2000).
Cook, R. C., Murray, D. L., Cook, J. G., Zager, P. & Monfort, S. L. Nutritional influences on breeding dynamics in elk. Can. J. Zool. 79, 845–853 (2001).
McGinnes, B. S. & Downing, R. L. Factors affecting the peak of white-tailed deer fawning in Virginia. J. Wildl. Manag. 41, 715–719 (1977).
Asher, G. W. Reproductive cycles of deer. Anim. Reprod. Sci. 124, 170–175 (2011).
Bubenik, G. A. et al. Effect of antiandrogen cyproterone acetate on the development of the antler cycle in Southern pudu (Pudu puda). J. Exp. Zool. 292, 393–401 (2002).
Bubenik, G. A., Schams, D. & Coenen, G. The effect of artificial photoperiodicity and antiandrogen treatment on the antler growth and plasma levels of LH, FSH, testosterone, prolactin and alkaline phosphatase in the male white-tailed deer. Comp. Biochem. Physiol. Part. A: Physiol. 87, 551–559 (1987).
Tomás, W. M. Seasonality of the antler cycle of Pampas deer (Ozotoceros bezoarticus leucogaster) from the Pantanal wetland, Brazil. Stud. Neotropical Fauna Environ. 30, 221–227 (1995).
Ungerfeld, R., González-Sierra, U. T. & Bielli, A. Seasonal antler cycle in a herd of Pampas deer (Ozotoceros bezoarticus) in Uruguay. Mammalian Biology. 73, 388–391 (2008).
Bubenik, G. A., Brown, R. D. & Schams, D. Antler cycle and endocrine parameters in male axis deer (Axis axis): seasonal levels of LH, FSH, testosterone, and prolactin and results of GnRH and ACTH challenge tests. Comp. Biochem. Physiol. Part. A: Physiol. 99, 645–650 (1991).
Clements, M., Clutton-Brock, T., Albon, S., Pemberton, J. & Kruuk, L. Getting the timing right: antler growth phenology and sexual selection in a wild red deer population. Oecologia 164, 357–368 (2010).
Vanpé, C. et al. Assessing the intensity of sexual selection on male body mass and antler length in roe deer Capreolus capreolus: is bigger better in a weakly dimorphic species? Oikos 119, 1484–1492 (2010).
Heckeberg, N. Origination of antlerogenesis. J. Morphol. 278, 182–202 (2017).
Bubenik, G. A. Neuroendocrine regulation of the antler cycle. In Horns, Pronghorns, and Antlers (eds Bubenik, G. A. & Bubenik, A. B.) 265–297 (Springer New York, 1990). https://doi.org/10.1007/978-1-4613-8966-8_8.
Putman, R. J. The Natural History of Deer (Cornell University Press, 1988).
Bartoš, L. Social status and antler development in red deer. In Horns, Pronghorns, and Antlers (eds Bubenik, G. A. & Bubenik, A. B.) 442–459 (Springer New York, 1990). https://doi.org/10.1007/978-1-4613-8966-8_17.
Raman, T. S. Antler cycles and breeding seasonality of the Chital Axis axis Erxleben in Southern India. JOURNAL-BOMBAY Nat. HISTORY Soc. 95, 377–391 (1998).
Ramesh, T., Kalle, R., Sankar, K., Qureshi, Q. & Downs, C. T. Aspects of breeding biology of Chital (Axis axis) and Sambar (Rusa unicolor) in the Western Ghats. Acta Ethologica. 16, 147–155 (2013).
Bhusal, A. et al. Breeding seasonality of Chital (Axis axis) in the Hetauda Valley of Nepal. Forestry: J. Inst. Fores Nep. 17, 174–183 (2020).
Bowman, J. L. & Jacobson, H. A. An improved vaginal-implant transmitter for locating white-tailed deer birth sites and fawns. Wildlife Soc. Bulletin. 26, 295–298 (1998).
Conant, E. R., Conway, W. C., Wallace, M. C. & Tatman, N. M. Vaginal implant transmitters as a tool for pronghorn Fawn capture. Wildlife Soc. Bulletin. 44, 156–162 (2020).
Jhala, Y. V. & Isvaran, K. Behavioural ecology of a grassland antelope, the Blackbuck Antilope cervicapra: linking habitat, ecology and behaviour. In The Ecology of Large Herbivores in South and Southeast Asia. Ecological Studies Vol. 225 (eds Ahrestani, F. & Sankaran, M.) 151–176 (Springer, 2016).
Rice, C. G. Reproductive biology of Nilgiri tahr, Hemitragus hylocrius (Mammalia: Bovidae. J. Zool. 214, 269–284 (1988).
Phumanee, W. et al. Coexistence of large carnivore species in relation to their major prey in Thailand. Global Ecol. Conserv. 32, e01930 (2021).
Krishnan, M. An ecological survey of larger mammals of Peninsular India. J. Bombay Nat. Hist. Soc. 69, 469–501 (1972).
Graff, W. & Nichols, L. The Axis deer in Hawaii. J. Bombay Nat. History Soc. 63, 629–734 (1966).
Ables, E. D. The Axis deer in Texas. The Caeser Kleberg Research Programme. The Texas Agricultural Experiment Station. A & M University System. Texas 44–49 (1974).
Kelly, C. L. et al. Dancing to a different tune: changing reproductive seasonality in an introduced Chital deer population. Oecologia 200, 285–294 (2022).
Mishra, H. R. The Ecology and Behaviour of Chital (Axis axis) in the Royal Chitwan National Park, Nepal (University of Edinburg, 1982).
Awasthi, N. Resource Partitioning among Sympatric Ungulates in Kanha Tiger Reserve, Madhya Pradesh, India (Saurashtra University, Rajkot, 2020).
Watter, K., Baxter, G., Brennan, M., Pople, T. & Murray, P. Seasonal diet preferences of Chital deer in the Northern Queensland dry tropics, Australia. Rangel. J. 42, 211 (2020).
Kayongo-Male, H., Thomas, J. W., Ullrey, D. E., Deans, R. J. & Arroyo-Aguilú, J. A. Chemical composition and digestibility of tropical grasses. J. Agric. Univ. Puerto Rico. 60, 186–200 (1976).
White, L. M. Seasonal changes in yield, digestibility, and crude protein of vegetative and floral tillers of two grasses. J. Range Manag. 36, 402–404 (1983).
Akbarinia, A. & Koocheki, A. Investigation on effects of different harvesting stages on growth, productivity and quality of some barley’s varieties. J. Pejouhesh Sazandegi. 15, 40–43 (1992).
Arzani, H., Nikkhah, A. & Arzani, Z. Forage Quality in Three Provinces of Semnan, Markazi and Lorestan Rangelands, Final Report, Determining Economic Property Size Project (College of Natural Resources, University of Tehran, 1998).
Arzani, H. et al. Phenological effects on forage quality of five grass species. Rangel. Ecol. Manage. 57, 624–629 (2004).
Devi, A., Hussain, S. A., Sharma, M., Gopi, G. V. & Badola, R. Seasonal pattern of food habits of large herbivores in riverine alluvial grasslands of Brahmaputra floodplains. Assam Sci. Rep. 12, 482 (2022).
Owen-Smith, R. N. Adaptive Herbivore Ecology: from Resources To Populations in Variable Environments (Cambridge University Press, 2002).
Ogutu, J. O., Piepho, H. P., Dublin, H. T., Bhola, N. & Reid, R. S. Rainfall extremes explain interannual shifts in timing and synchrony of calving in Topi and warthog. Popul. Ecol. 52, 89–102 (2010).
Clutton-Brock, T. H., Albon, S. D. & Guinness, F. E. Fitness costs of gestation and lactation in wild mammals. Nature 337, 260–262 (1989).
Ahrestani, F. S., Van Langevelde, F., Heitkönig, I. M. A. & Prins, H. H. T. Contrasting timing of parturition of Chital Axis axis and Gaur Bos Gaurus in tropical South India – the role of body mass and seasonal forage quality. Oikos 121, 1300–1310 (2012).
Flajšman, K., Jerina, K. & Pokorny, B. Age-related effects of body mass on fertility and litter size in roe deer. PLoS ONE. 12, e0175579 (2017).
Paoli, A., Weladji, R. B., Holand, Ø. & Kumpula, J. Winter and spring Climatic conditions influence timing and synchrony of calving in reindeer. PLoS ONE. 13, e0195603 (2018).
Jhala, Y. V. et al. Spatial and Population Ecology of Tiger Co-Predator and Their Prey in Kanha Tiger Reserve. 104 (2014).
Jansen, B. D. & Jenks, J. A. Birth timing for mountain lions (Puma concolor); testing the prey availability hypothesis. PLoS ONE. 7, e44625 (2012).
Schaller, G. B. The Deer and the Tiger a Study of Wildlife in India (University of Chicago Press, 1967).
McNaughton, S. J. Grazing as an optimization process: Grass-Ungulate relationships in the Serengeti. Am. Nat. 113, 691–703 (1979).
Owen-Smith, N. & Ogutu, J. O. Controls over reproductive phenology among ungulates: allometry and tropical-temperate contrasts. Ecography 36, 256–263 (2013).
Parker, K. L., Barboza, P. S. & Gillingham M. P. Nutrition integrates environmental responses of ungulates. Funct. Ecol. 23, 57–69 (2009).
Rodgers, W. & Panwar, H. Planning a wildlife protected area network in India. (1988).
Jhala, Y. V., Qureshi, Q., Gopal, R. & Nayak, A. K. Status of Tigers, Co-Predators, and Prey in India, 2018.National Tiger Conservation Authority, Govt. Of India, New Delhi, and Wildlife Institute of India (2020).
Fryxell, J. M. Food limitation and demography of a migratory antelope, the white-eared Kob. Oecologia 72, 83–91 (1987).
Eberhardt, L. E., Eberhardt, L. L., Tiller, B. L. & Cadwell, L. L. Growth of an isolated elk population. The J. Wildl. Management . 78, 369–373 (1996).
Laurian, C., Ouellet, J. P., Courtois, R., Breton, L. & St-Onge, S. Effects of intensive harvesting on moose reproduction. J. Appl. Ecol. 37, 515–531 (2000).
Weigl, R. Longevity of Mammals in Captivity: from the Living Collections of the World; a List of Mammalian Longevity in Captivity (Schweizerbart, 2005).
Myhrvold, N. P. et al. An amniote life-history database to perform comparative analyses with birds, mammals, and reptiles: ecological archives E096‐269. Ecology 96, 3109–3109 (2015).
Skalski, J. R., Ryding, K. E. & Millspaugh, J. J. Wildlife Demography: Analysis of Sex, Age, and Count Data (Elsevier Academic, 2010).
Gorelick, N. et al. Google Earth engine: Planetary-scale Geospatial analysis for everyone. Remote Sens. Environ. 202, 18–27 (2017).
Kumar, U. Tiger and Leopard: Population Ecology and Resource Partitioning of Sympatric Carnivores in Kanha Tiger Reserve M.P (Saurashtra University, 2019).
Acknowledgements
We thank Chief Wildlife Warden of Madhya Pradesh and management of Kanha Tiger Reserve for permissions and logistics for the study. We thank our field assistants Nirottam and Kanhaiya and the team of forest guards for their help in field data collection. We thank field director Kanha, S.K. Singh for logistic support. We thank Omkar Nar and Ninad Mungi for their help in preparing graphs. This study was funded by National Tiger Conservation Authority, Ministry of Environment, Forest and Climate change, Government of India.
Author information
Authors and Affiliations
Contributions
YVJ & QQ conceived, supervised, procured the resources for the study, SG did the field data collection, SG & YVJ did the data analysis, SG, UK and YVJ wrote the MS, and all the authors approved the MS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement and ARRIVE guidelines compliance
This study was based solely on non-invasive observational methods. All observations were conducted in the animals’ natural habitat without any physical contact, handling, or interference with the animals. No experimental procedures were performed on live animals. Hence, ARRIVE guidelines, was not applicable to this study. The study complies with the ethical standards for wildlife research as approved by the Chief Wildlife Warden, under Permit No. Serial No./D.M.II/Research-213/9027.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material. The online version contains supplementary material available at https://doi.org/10.1038/s41598-025-08093-0
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Goswami, S., Kumar, U., Qureshi, Q. et al. Ecological correlates of chital (Axis axis) reproductive seasonality in Kanha Tiger Reserve. Sci Rep 15, 22976 (2025). https://doi.org/10.1038/s41598-025-08093-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08093-0