Abstract
Hepatocellular carcinoma is a leading cause of cancer-related death, with unresectable HCC (uHCC) presenting considerable clinical challenges; treatment options are limited, warranting the combination of locoregional therapies. We compared the efficacy of transcatheter arterial embolization–hepatic artery infusion chemotherapy (TAE–HAIC) plus targeted therapy and immunotherapy to other therapeutic combinations for treating uHCC. We conducted a multicenter, retrospective cohort study, utilizing propensity score matching to compare treatment outcomes between patients receiving TAE–HAIC with targeted therapy and immunotherapy and those receiving other combination therapies. Data were collected from multiple centers, and survival outcomes, tumor control, and overall efficacy were evaluated between matched groups. TAE–HAIC plus targeted therapy and immunotherapy significantly improved overall survival and progression-free survival compared to other therapeutic combinations. The combination therapy demonstrated superior tumor control rates and a reduced risk of disease progression. Statistically significant improvements in survival metrics and clinical outcomes were observed in the TAE–HAIC group, underscoring the efficacy of this approach. This study supports the use of TAE–HAIC combined with targeted therapy and immunotherapy for patients with uHCC. These findings offer valuable insights into optimizing treatment strategies and highlight the potential for this combination to improve patient outcomes and survival.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is a common and aggressive malignancy, ranking sixth in incidence and third in cancer-related mortality in 20201. Simultaneously, surgical resection offers favorable outcomes in early-stage HCC; approximately 70% of patients present at advanced stages where curative treatments are not feasible2. Thus, developing effective therapeutic strategies for patients with unresectable HCC (uHCC) is critical.
Transcatheter arterial chemoembolization (TACE) remains the most widely used locoregional treatment for uHCC3. Recent studies have demonstrated the efficacy and safety of hepatic artery infusion chemotherapy (HAIC) in managing uHCC, providing an alternative to TACE4. Concurrently, the integration of targeted therapies with immune checkpoint inhibitors (ICIs)—demonstrated in landmark trials (IMbrave150, ORIENT-32, SHR1210)—significantly improves survival and objective response rates (ORRs) in HCC5,6. Building on this rationale, recent studies explore combining transarterial therapies (TACE/HAIC) with systemic agents (targeted therapy + ICIs), revealing synergistic benefits: TACE-based combinations enhance progression-free survival (PFS) and overall survival (OS) 7,8,9, and HAIC-based regimens similarly show favorable outcomes10,11. These findings position multi-modal therapy as a promising strategy for uHCC.
Although the clinical benefits of TACE combined with targeted therapy and ICIs, as well as HAIC combined with targeted therapy and ICIs, have been extensively studied, the comparative efficacy of different local treatments—TACE, HAIC, and transcatheter arterial embolization (TAE) combined with HAIC—when paired with targeted therapy and ICIs remain under explored. Furthermore, clinical evidence on quadruple therapy (TAE + HAIC + targeted therapy + ICIs) for uHCC is constrained by limited sample sizes and insufficient comparative analyses across regimens. As a real-world study with broader patient enrollment and direct therapeutic comparisons, our work addresses these gaps, providing clinically actionable data to optimize combination strategies for uHCC.
We therefore aimed to compare the safety and clinical efficacy of triple therapy (TACE or HAIC combined with targeted therapy and ICIs) to quadruple therapy (TAE + HAIC + targeted therapy + ICIs) for treating uHCC. To mitigate selection bias, propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) was used to ensure robust comparisons between the two treatment cohorts.
Methods
Study population
This multicenter, retrospective cohort study included patients with uHCC who were treated with transarterial therapy combined with targeted therapy and ICIs between August 2018 and November 2023 across the following four centers in China: (1) Zhujiang Hospital of Southern Medical University, (2) Shunde Hospital of Southern Medical University, (3) The Sixth Affiliated Hospital of South China University of Technology, and (4) Hunan Provincial People Hospital (The First Affiliated Hospital of Hunan Normal University). The study was conducted in compliance with the STROCSS guidelines12. Due to the retrospective nature of the study, the Ethics Committee of Zhujiang Hospital of Southern Medical University waived the need of obtaining informed consent (approval number: 2021-KY-081–01).
Inclusion criteria
-
(1)
Age ≥ 18 years.
-
(2)
Diagnosis of uHCC and treated with combination therapy
-
(3)
Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2
-
(4)
At least one measurable target lesion, assessable using the modified Response Evaluation Criteria in Solid Tumors (mRECIST)13.
-
(5)
Child–Pugh class A-B
-
(6)
Normal organ and hematologic function
Exclusion criteria
-
(1)
Child–Pugh class C
-
(2)
Prior local or systemic anticancer therapies
-
(3)
Presence of other malignant tumors
-
(4)
Severe cardiovascular, pulmonary, or renal disease
-
(5)
Incomplete clinical data
-
(6)
Loss to follow-up
Patients were categorized into two cohorts based on treatment type: (1) triple therapy (TACE or HAIC + targeted therapy + ICIs) and (2) quadruple therapy (TAE + HAIC + targeted therapy + ICIs). Systemic therapies were administered within one month before and after the first transarterial therapy, based on patient preference after consultation with their physician. The treatment plans for all patients were determined by the attending physicians based on clinical experience and patient preferences. Moreover, each center adopted a combined treatment approach for the patients, and the treatment plans could only be altered if the patients were intolerant to the treatment or tumor progression occurred.
HCC diagnosis
HCC was diagnosed based on the radiologic criteria of the American Association for the Study of Liver Diseases. According to the Japan Liver Cancer Research Group, uHCC was defined as tumors not amenable to radical resection due to size, number, or invasion of major blood vessels. Conversion to resectable HCC was defined as achieving R0 resection with sufficient residual liver function, Child–Pugh class A, ECOG PS of 0–1, no extrahepatic lesions, no major vascular invasion, and no contraindications for hepatectomy.
Targeted therapy and ICI administration
The following targeted therapies were administered:
-
Lenvatinib (12 mg/day for body weight ≥ 60 kg or 8 mg/day for body weight < 60 kg)
-
Sorafenib (400 mg twice daily)
-
Donafenib (200 mg twice daily)
-
Apatinib (250–500 mg once daily)
-
Bevacizumab (7.5 mg/kg IV every 3 weeks)
Administered ICIs
-
Camrelizumab (200 mg)
-
Sintilimab (200 mg)
-
Toripalimab (240 mg)
-
Tislelizumab (200 mg)
-
Atezolizumab (1,200 mg)
-
Nivolumab (3 mg/kg)
ICIs were administered intravenously every 3–4 weeks until disease progression or treatment-related toxicity occurred. Dosages were adjusted based on tolerance and clinical response.
Transarterial therapy
Senior interventional radiologists with at least 10 years of clinical experience performed the transarterial therapies. For TACE, the femoral artery was accessed using the Seldinger technique, and catheters were inserted into the celiac trunk or superior mesenteric artery for arteriography. A microcatheter was placed into the tumor-feeding arteries, followed by an infusion of iodized oil (5–10 mL) for tumor embolization. Chemotherapeutic drugs were delivered through an arterial infusion pump or injected into the catheter during the procedure. The procedure was repeated if residual tumors were detected during postoperative imaging.
For HAIC, a catheter was placed into the tumor-feeding artery following the same technique as used for TACE. The FOLFOX regimen, which includes oxaliplatin (85–130 mg/m2), leucovorin (400 mg/m2), 5-fluorouracil (5-FU) bolus (400 mg/m2), and continuous 5-FU infusion (2,400 mg/m2 over 46 h), was administered. Continuous 5-FU (1,200 mg/m2 over 23 h) was used. HAIC was repeated every 3–4 weeks.
TAE was performed first for the TAE + HAIC group, followed by the administration of FOLFOX-based chemotherapy. The combination of TAE and HAIC was repeated every 3–4 weeks.
Data collection and follow-up
Baseline clinical data, including physical examinations, blood tests (liver function, hepatitis virus markers, alpha-fetoprotein [AFP]), and imaging evaluations (ultrasonography, CT, MRI), were collected. Follow-up was conducted until 1 March, 2024. Tumor responses were assessed using contrast-enhanced CT or MRI every 3–4 weeks. Patients who met the criteria for conversion to resectable HCC underwent surgical resection with postoperative adjuvant therapy, as determined by a multidisciplinary team.
Postoperative management
Radical resection was defined as R0 resection with sufficient residual liver function, which required discontinuation of targeted therapy for 1 week and ICIs for 1 month. Follow-up therapy was tailored based on the recommendation of the multidisciplinary team and patient preferences.
Outcomes and assessments
Primary endpoints included PFS and OS. Secondary endpoints included the objective response rate (ORR), conversion rate, and incidence of adverse events (AEs). PFS was defined as the time from the initiation of combination therapy to disease progression or death, while OS was defined as the time from initiation to death from any cause. Tumor responses were evaluated using mRECIST, with responses classified as progressive disease (PD), stable disease (SD), partial response (PR), or complete response (CR). The ORR was defined as the sum of PR and CR, while the disease control rate (DCR) was defined as the sum of PR, SD, and CR. The tumor change, calculated as [(post-treatment tumor diameter—pre-treatment tumor diameter)/pre-treatment tumor diameter] × 100%, partially reflects the ORR and thus validates the therapeutic efficacy. AEs were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
Statistical analysis
Baseline characteristics were summarized as percentages or medians (interquartile ranges). Chi-square and Fisher’s exact tests were used for categorical variables. Propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) were used to address group imbalances. These techniques can be effectively applied using the MatchIt package in R. The variables included in the propensity score model were age, hepatitis virus status, cirrhosis, number of tumors, and ECOG score. The 1:3 nearest neighbor matching method was applied with a 0.2 standard deviation caliper. For IPTW, inverse propensity scores were calculated for weighting. Survival data were analyzed using the Kaplan–Meier method, and survival curves were compared using the log-rank test. Univariate and multivariate Cox regression analyses were performed to identify prognostic factors. All tests were two-tailed, and a P < 0.05 was considered statistically significant. Statistical analysis was performed using R software (version 4.2.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical characteristics
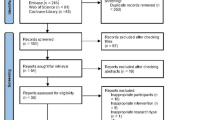
Overall, 235 patients with uHCC who received transarterial therapy combined with targeted therapy and ICIs were initially considered for this study. After excluding 35 ineligible patients, 200 patients were enrolled, with 155 in the Triple therapy group and 45 in the Quadruple therapy group (Fig. 1). The Quadruple group had a significantly higher proportion of younger patients, those with a single tumor, and those with an ECOG score of 0 than the Triple therapy group. PSM generated 43 patients in the Quadruple group and 100 patients in the Triple group. IPTW simulated 186 patients in the Quadruple group and 201 patients in the Triple group. Following PSM, no significant differences were observed between the groups (P > 0.05). Additionally, IPTW matched patients for variables including sex, age, hepatitis virus status, liver cirrhosis, and combination therapy. Table 1 summarizes the baseline characteristics before and after matching.
Among the enrolled patients, 112 received targeted therapy, ICIs, and TACE (T-I-T group), while 43 received targeted therapy, ICIs, and HAIC (T-I-H group). Table S1 details the specific number of patients treated with different targeted and ICI therapies. No significant differences in baseline clinical characteristics were observed between these two groups (Table S2).
Moreover, no significant differences were observed in PFS, OS, or ORR between the two groups (P > 0.05) (Figure S1a, b; Table S3). Given that there were no significant differences in baseline characteristics or survival prognosis between the two groups, we combined the two treatment modalities into a triad group for statistical analysis.
Efficacy outcomes
Before PSM, tumor progression occurred in 158 patients, and 93 patients died. Kaplan–Meier survival analysis of PFS and OS before PSM is shown in Figure S2a, b. The median PFS in the Quadruple group was 11.03 months, which was significantly better than the median PFS of 7.67 months in the Triple group (HR = 0.64, 95% CI: 0.44–0.93, P < 0.05). Similarly, the median OS in the Quadruple group was 24 months, significantly longer than the median OS of 20 months in the Triple group (HR = 0.55, 95% CI: 0.33–0.92, P < 0.05). The Quadruple group also had a significantly higher ORR than the Triple group (P < 0.05), although no significant difference in DCR was observed (P = 0.118; Table 2).
After PSM, 121 patients with tumor progression were identified: 39 in the Quadruple group and 82 in the Triple group. Patients in the Quadruple group exhibited significantly better PFS and OS both after PSM (PFS: HR = 0.64, 95% CI: 0.44–0.95, P < 0.05; OS: HR = 0.55, 95% CI: 0.31–0.95, P < 0.05; Fig. 2a, b) and after IPTW (PFS: HR = 0.60, 95% CI: 0.42–0.84, P < 0.05; OS: HR = 0.47, 95% CI: 0.27–0.80, P < 0.05; Fig. 3a, b). The Quadruple group demonstrated a significantly reduction in tumor change on the waterfall plot compared with the Triple group, along with a markedly improved ORR (P < 0.001) (Fig. 4a, b, Table 2).
Conversion rate
Overall, 31 patients met the criteria for radical HCC resection (14 in the Triple group and 17 in the Quadruple group). The conversion rate was higher in the Quadruple group both before (P < 0.05) and after PSM (P < 0.05) (Table 3). Patients who underwent conversion to resectable HCC exhibited significantly better PFS and OS than those who did not undergo conversion (PFS: HR = 0.41, 95% CI: 0.26–0.66, P < 0.05; OS: HR = 0.22, 95% CI: 0.1–0.49, P < 0.05; Figure S3a, b).
Safety and tolerability
Common treatment-related adverse events (AEs) occurred in 155 (77%) patients, with grade ≥ 3 AEs observed in 40 (20%) patients (Table 4). The incidence and severity of AEs were similar between the two groups (P = 0.1 before PSM; P = 0.081 after PSM). No cases of hepatic failure or treatment-related deaths were reported.
Prognostic factor analysis
Before PSM, multivariate analysis identified age, local treatment, AFP levels, and conversion surgery as independent prognostic factors for PFS, while local treatment, ECOG score, AFP levels, and conversion surgery were independent prognostic factors for OS (Table S4). After PSM, multivariate analysis revealed that local treatment AFP, and conversion surgery were independent prognostic factors for PFS. Similarly, local treatment, AFP, and conversion surgery were independent prognostic factors for OS (Table 5).
Discussion
This study demonstrates that patients with uHCC receiving quadruple therapy (TAE + HAIC + targeted therapy + ICIs) had significantly better survival outcomes than those receiving triple therapy (TACE or HAIC + targeted therapy + ICIs), as evidenced by improved PFS, OS, ORR, and conversion rates. Notably, this was achieved without an increase in AE incidence. These findings suggest that quadruple therapy may be an effective and safe treatment option for patients with uHCC.
Notably, 12 patients (27.9%) in the Quadruple group underwent hepatectomy, indicating that quadruple therapy may make some patients with uHCC eligible for surgical resection, leading to potential disease-free survival. Patients who underwent conversion to resectable HCC showed significantly better survival outcomes (median PFS: 14.93 vs. 7.47 months). Although the CR rate was lower in the Quadruple group than in the Triple group, it was likely because many patients in the Quadruple group achieved PR before surgical resection, with the best tumor remission measured before surgery. After PSM, we identified local treatment, AFP levels, and conversion surgery as independent prognostic factors for PFS, and local treatment, ECOG score, and conversion surgery as independent prognostic factors for OS. Clinical trials have shown that higher AFP levels are associated with poorer survival in patients with HCC14,15, also observed in our cohort. Similarly, a poor ECOG score is associated with worse survival outcomes, particularly in patients treated with immunotherapy for melanoma and non-small cell lung cancer16,17; this was also observed in our cohort. Additionally, conversion surgery was a protective factor for both PFS and OS.
Although TACE and HAIC are distinct transarterial therapies for uHCC, this study primarily compared the prognostic outcomes of different treatment modalities. Since there were no significant baseline differences between the T-I-T and T-I-H groups, we combined these groups to form the Triple therapy cohort. This study used different types of targeted drugs and ICIs, all of which are commonly used in HCC treatment. Given that the effectiveness of these drugs can vary, we conducted a baseline comparison and found that the Quadruple and Triple groups were well-balanced. The result confirm that, although drug heterogeneity may influence efficacy via tumor biology, it was not a significant confounder in this study. By following the guidelines to minimize variations in interventional procedures across centers, multi – center data can better represent real – world universality18,19.
TACE combined with HAIC and TKIs and PD-1 inhibitors is superior to TACE alone in patients with HCC and portal vein tumor thrombosis, with a reported ORR of 53.7% versus 7.8%20. Our study is superior to the aforementioned research, likely due to the inclusion of patients with BCLC A or B, which nonetheless corroborates the efficacy of combination therapy. Hu et al. demonstrated that lenvatinib plus PD-1 inhibitors showed certain therapeutic effects in patients with uHCC, with a conversion success rate of 55.4% in the combination therapy group, an ORR of 62.5%, and a PFS of 10.1 months21. These findings attest to the effectiveness of combination therapy in conversion treatment, further validating the rationality of our study. Moreover, the incorporation of local therapy in our research enhanced therapeutic efficacy. Chen et al. revealed that the triple therapy of TACE combined with lenvatinib and camrelizumab exhibited significant conversion effects in patients with uHCC, with an ORR reaching 76.4%, like our study’s results22. Yuan et al. demonstrated comparable efficacy of TACE-HAIC combined with targeted and immunotherapy in HCC with portal vein tumor thrombosis, reporting an ORR of 42.1% and median PFS of 14.8 months, which aligns with our outcomes23. In contrast, a recent study applying TACE-HAIC plus TKIs and PD-1 inhibitors for uHCC showed comparatively inferior efficacy relative to our findings (median PFS 9.2 vs. 11.03 months; OS 18.2 vs. 24.0 months)24; our study enrolled a substantially larger cohort and directly compared different therapeutic regimens. All these research outcomes confirm the rationality of our study and further substantiate the significant advantages of our combination therapy in terms of therapeutic efficacy. We hope that this study provides a safe and effective treatment option for patients with uHCC, improving ORR and long-term survival prognosis.
This study’s most common treatment-related Aes were elevated ALT and AST levels, thrombocytopenia, fever, and fatigue. While Aes were common, only 20% of patients (40/200) experienced grade ≥ 3 Aes, and no treatment-related deaths occurred. The incidence and severity of Aes were similar between the Quadruple and Triple groups. However, the incidence of abdominal pain was higher in the Quadruple group, which may be attributable to the addition of embolization therapy.
Our study possesses several notable strengths. To our knowledge, this represents the first direct comparison of quadruple therapy versus triple therapy for uHCC. We minimized potential biases by applying both PSM and IPTW, strengthening the reliability of our results. Furthermore, Cox regression analyses were used to identify independent prognostic factors, further enhancing the robustness of our conclusions. Notably, the quadruple therapy group achieved a higher ORR (79.1%) than historical reports of triple therapy (42–74%)25,26,27,28, potentially attributable to synergistic mechanisms or the inclusion of selected BCLC A/B stage patients.
This enhanced efficacy may be explained by complementary biological mechanisms. Transarterial chemoembolization and hepatic arterial infusion chemotherapy induce tumor necrosis and antigen release, which may prime antitumor immunity29,30,31,32. ICIs subsequently restore T-cell cytotoxic function by blocking inhibitory pathways33. Many tumor cells rely on specific tyrosine kinase signaling pathways for proliferation and survival. Concurrently, targeted drugs suppress tumor angiogenesis and modulate the immunosuppressive microenvironment, thereby potentiating immunotherapy effects34,35,36. The rapid tumor cell death induced by targeted therapy may further release neoantigens, amplifying systemic immune responses37,38.
Despite these strengths, it is not without limitations. First, although we used PSM to balance baseline characteristics, the study’s retrospective nature inherently introduces potential biases. For example, the types of drugs used were not standardized across patients, though we analyzed baseline characteristics related to drug choices and found no significant differences. Second, protocol-permitted adjustments of therapy duration and dosage based on tumor response or patient tolerance may influence outcome. Third, the relatively small sample size may have limited the accuracy and generalizability of the results. Finally, real-world implementation faces challenges including potential selection biases from socioeconomic or geographic factors, along with different hospitals and doctors in treatment habits and professional levels. Consequently, large-scale multicenter prospective trials are essential to validate these findings, address methodological limitations, and optimize clinical translation. We anticipate this work will offer new treatment options for uHCC.
Currently, a variety of biomarkers and clinical characteristics, such as Programmed Death-Ligand 1 (PD-L1), tumor mutation burden (TMB), interferon-γ (IFN-γ), pathological features, and baseline clinical characteristics, have demonstrated promising potential in predicting treatment responses in oncology39,40,41,42. We anticipate that future research will build upon these findings to develop predictive models based on similar features, enabling the effective prediction of treatment responses to different combination therapies.
Conclusions
Quadruple therapy consisting of targeted therapy, ICIs, and TAE plus HAIC offers superior efficacy to triple therapy, providing a promising treatment modality with manageable toxicity for patients with uHCC.
Data availability
All data generated or analysed during this study are included in the manuscript or Supplementary Information. Further inquiries can be directed to the corresponding author.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71, 209–249 (2021).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019).
Lencioni, R., de Baere, T., Soulen, M. C., Rilling, W. S. & Geschwind, J. F. H. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 64, 106–116 (2016).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Ren, Z. et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 22, 977–990 (2021).
Xie, L. et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: A single-arm, open-label, phase 2 trial. J. Immunother. Cancer. 8, e000798 (2020).
Cai, M. et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: A retrospective cohort study. Front. Immunol. 13, 848387 (2022).
Xin, Y. et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol. Int. 17, 753–764 (2023).
Wang, W.-J. et al. Efficacy and safety of TACE combined with lenvatinib and PD-1 inhibitors for unresectable recurrent HCC: A multicenter, retrospective study. Cancer Med. 12, 11513–11524 (2023).
Liu, B.-J. et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy 13, 1395–1405 (2021).
Lin, L.-W., Ke, K., Yan, L.-Y., Chen, R. & Huang, J.-Y. Efficacy and safety of hepatic artery infusion chemotherapy combined with tyrosine kinase inhibitors plus programmed death-1 inhibitors for hepatocellular carcinoma refractory to transarterial chemoembolization. Front Oncol. 13, 1178428 (2023).
Rashid, R. et al. The STROCSS 2024 guideline: Strengthening the reporting of cohort, cross-sectional, and case-control studies in surgery. Int J Surg. 110, 3151–3165 (2024).
Llovet, J. M. & Lencioni, R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 72, 288–306 (2020).
Xie, D. et al. Development and validation of a nomogram for patients undergoing transarterial chemoembolization for recurrent hepatocellular carcinoma after hepatectomy. J. Hepatocell. Carcinoma. 11, 693–705 (2024).
Ohama, H. et al. Clinical usefulness of newly developed prognostic predictive score for atezolizumab plus bevacizumab for hepatocellular carcinoma. Cancer Rep. (Hoboken). 7, e2042 (2024).
Yao, J. et al. Efficacy and safety of combined immunotherapy and antiangiogenic therapy for advanced non-small cell lung cancer: A two-center retrospective study. Int. Immunopharmacol. 89, 107033 (2020).
Reschke, R. & Ziemer, M. Rechallenge with checkpoint inhibitors in metastatic melanoma. J. Dtsch. Dermatol. Ges. 18, 429–436 (2020).
Vogel, A. et al. Hepatocellular carcinoma: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 36, 491–506 (2025).
Kudo, M. et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 Update. Liver Cancer. 10, 181–223 (2021).
Sun, C. et al. Concurrent clinical and pathological response predicts favorable prognosis of patients with gastric cancer after neoadjuvant therapy: A real-world study. BMC Cancer 23, 996 (2023).
Zhang, W. et al. Lenvatinib plus anti-PD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: a single-arm, phase II trial. J. Immunother. Cancer. 11, e007366 (2023).
Wu, X. K. et al. Transcatheter arterial chemoembolisation combined with lenvatinib plus camrelizumab as conversion therapy for unresectable hepatocellular carcinoma: a single-arm, multicentre, prospective study. EClinicalMedicine. 67, 102367 (2024).
Yuan, Y. et al. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int. J. Surg. 109, 1222–1230 (2023).
Pang, B. et al. Real-world efficacy and safety of TACE-HAIC combined with TKIs and PD-1 inhibitors in initially unresectable hepatocellular carcinoma. Int. Immunopharmacol. 137, 112492 (2024).
Liu, B.-J. et al. Sorafenib combined with embolization plus hepatic arterial infusion chemotherapy for inoperable hepatocellular carcinoma. World J. Gastrointest. Oncol. 12, 663–676 (2020).
He, M.-K. et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther. Adv. Med. Oncol. 13, 17588359211002720 (2021).
Wu, J.-Y. et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A multicenter retrospective study. J. Hepatocell. Carcinoma. 8, 1233–1240 (2021).
Yang, F. et al. Transarterial chemoembolization combined with immune checkpoint inhibitors and tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: Efficacy and systemic immune response. Front. Immunol. 13, 847601 (2022).
Chang, X., Lu, X., Guo, J. & Teng, G.-J. Interventional therapy combined with immune checkpoint inhibitors: Emerging opportunities for cancer treatment in the era of immunotherapy. Cancer Treat. Rev. 74, 49–60 (2019).
Tischfield, D. J. et al. Transarterial embolization modulates the immune response within target and nontarget hepatocellular carcinomas in a rat model. Radiology 303, 215–225 (2022).
Li, S. et al. Hepatic artery infusion chemotherapy using fluorouracil, leucovorin, and oxaliplatin versus transarterial chemoembolization as initial treatment for locally advanced hepatocellular carcinoma: A propensity score-matching analysis. J. Vasc. Interv. Radiol. 32, 1267-1276.e1 (2021).
Lyu, N. et al. FOXAI: A phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut 67, 395–396 (2018).
Llovet, J. M. et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 293–313 (2021).
Matsuki, M. et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 7, 2641–2653 (2018).
Xia, Y. et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: A single-arm, open label, phase II clinical trial. J. Immunother. Cancer. 10, e004656 (2022).
Wang, Y. et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed. Pharmacother. 132, 110797 (2020).
Fu, Z. et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): A retrospective controlled study. Hepatol. Int. 15, 663–675 (2021).
Huinen, Z. R., Huijbers, E. J., van Beijnum, J. R., Nowak-Sliwinska, P. & Griffioen, A. W. Anti-angiogenic agents - Overcoming tumor endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 18, 527–540 (2021).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 378, 2078–2092 (2018).
Kockx, M. M., McCleland, M. & Koeppen, H. Microenvironmental regulation of tumour immunity and response to immunotherapy. J. Pathol. 254, 374–383 (2021).
Lei, X. et al. Augmenting antitumor efficacy of Th17-derived Th1 cells through IFN-γ-induced type I interferon response network via IRF7. Proc. Natl. Acad. Sci. USA. 121, e2412120121 (2024).
Chang, T. G. et al. LORIS robustly predicts patient outcomes with immune checkpoint blockade therapy using common clinical, pathologic and genomic features. Nat. Cancer. 5, 1158–1175 (2024).
Acknowledgments
We sincerely thank all individuals who supported us throughout the preparation of this manuscript. First and foremost, we express our deep gratitude to Mr. Pan Mingxin, whose insightful suggestions and encouragement significantly contributed to our understanding of translation studies. It was a privilege to study under his guidance and supervision. His dedication and exemplary work ethic were a true inspiration. We are also immensely grateful to our friends and colleagues for their invaluable assistance and camaraderie during the preparation of this paper. In addition, we would like to extend our heartfelt thanks to our families for their unwavering support. Lastly, we appreciate all those who took the time to review this manuscript and offer constructive feedback, which will be instrumental in guiding our future research.
Funding
This work was supported by the Science and Technology Projects in Guangzhou (grant number 2023B03J1247), the Guangdong Basic and Applied Basic Research Foundation (grant number 2021B1515230011), and Guangzhou Key Research and Development Program (grant number 2024B03J1381).
Author information
Authors and Affiliations
Contributions
Zhang C, Xu YY, Cai L and Zhou SY conceived and designed the study; Wang WD, Liang WC, Fu SJ, Pan MX, Yang J and Liu SL provided administrative support; Zhang C, Zhou SY, Feng JN, Li C and Guo WX provided study materials or patients; Zhang C, Cai L, He GL, Tan MH and Tao HS collected and assembled the data; Xu YY and Zhang C analyzed and interpreted the data; All authors contributed to manuscript writing; All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The authors affirm responsibility for the integrity of the study and ensure that any questions regarding the accuracy or integrity of any part of the work have been adequately addressed. The study adhered to the ethical principles outlined in the Declaration of Helsinki (2013 revision). Due to the retrospective nature of the study, the Ethics Committee of Zhujiang Hospital of Southern Medical University waived the need of obtaining informed consent (approval number: 2021-KY-081-01). Registry and the Registration No. of the study/trial: Chinese Clinical Trial Registry (No. ChiCTR2300073527). Informed consent for this retrospective analysis was waived.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, C., Xu, Y., Cai, L. et al. Efficacy of combined TAE, HAIC, targeted, and immunotherapy in unresectable HCC: a multicenter propensity score matching study. Sci Rep 15, 21184 (2025). https://doi.org/10.1038/s41598-025-08170-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08170-4