Abstract
The increasing global demand for cleaner energy solutions highlights the need to combine hydrogen with advanced biofuels for use in low-temperature combustion (LTC) engines. This study experimentally investigates the performance and emissions of an LTC engine fueled with six test fuels: Diesel, Citronella biofuel, three hydrogen-enriched blends (H20, H40, H60) and H40E (H40 + 10%R-EGR). Among the tested fuels, the H40 blend demonstrated the highest brake thermal efficiency (BTE), surpassing that of conventional diesel. Emission analysis showed notable reductions in hydrocarbons (HC) by 65%, carbon monoxide (CO) by 37%, carbon dioxide (CO₂) by 8%, NOx by 6%, and smoke opacity by 20.8% compared to diesel. Notably, H40E further lower NOx formation by 3% than H40 but HC and CO were higher by 2 to 5%. Combustion analysis revealed that hydrogen enhanced pre-mixed combustion, resulting in improved in-cylinder pressure and heat release rate. The experimental results were validated using Sankey diagram visualization, confirming improved energy distribution. The findings indicate that hydrogen-enriched biofuels, particularly the H40 blend offer a promising pathway for cleaner and more efficient engine operation, supporting the global transition away from fossil fuels.
Similar content being viewed by others
Introduction
The ever-increasing global energy demand, especially from the transportation sector, plays a significant role in national economic development, particularly in developing nations. Countries like India, Malaysia, Pakistan, and Bangladesh are heavily reliant on fossil fuel imports from Gulf countries, which has significant implications for both economic resilience and environmental sustainability1,2. The COVID-19 pandemic further highlighted the vulnerability of energy supply chains and brought renewed attention to the need for sustainable and independent energy solutions. Post-pandemic, emissions have risen steeply, urging governments to adopt stringent emission-reduction strategies and shift toward cleaner fuels3,4,5.
In the pursuit of low-emission alternatives, hydrogen has emerged as a frontrunner. It offers high energy density, zero-carbon emissions upon combustion, and compatibility with internal combustion (IC) engines. Several countries have now prioritized hydrogen in their national energy policies, investing substantially in hydrogen production and infrastructure development6,7. As a result, hydrogen-powered IC engines are gaining market traction. According to projections, the global hydrogen IC engine market will expand from USD 12 million in 2024 to USD 327 million by 2035, driven by government policies, technological innovations, and a global push for decarbonization8,9.
The Asia-Pacific region is expected to lead this shift, with Japan and South Korea unveiling comprehensive hydrogen roadmaps that include vehicle subsidies, tax incentives, and investment in hydrogen refueling infrastructure10,11. Liquid hydrogen, in particular, is poised for rapid growth due to advances in its production, storage, and transportation12,13. Simultaneously, India has launched its National Green Hydrogen Mission with multi-billion-dollar investments in green hydrogen production. Projects like NTPC’s hydrogen hub at Pudimadaka, Indian Railways’ hydrogen-powered locomotives, and private-sector ventures from Reliance, JSW Energy, and Adani are positioning India as a global leader in hydrogen energy14,15,16. Malaysia is also investing in large-scale clean hydrogen production, including the Sarawak hydrogen energy project and green methanol initiatives supported by Petros and Sarawak Petchem17,18,19.
Despite these advances, the use of hydrogen in diesel engines presents inherent technical challenges. Hydrogen’s high flame speed and elevated auto-ignition temperature make stable combustion difficult under conventional diesel engine conditions20,21. Research into overcoming these issues has largely focused on two strategies: fuel modification and engine modification. Fuel modification involves blending hydrogen with diesel or biofuels to improve combustion efficiency and reduce pollutant emissions22,23. For example, using hydrogen-enriched biofuels such as cashew nut oil or algae oil has shown to lower CO, CO2, and HC emissions while improving brake thermal efficiency (BTE) due to better air–fuel mixing and higher energy content24,25,26. However, a common drawback observed in most studies is the elevation of NOx emissions, which remains a major health and environmental concern27,28.
Engine modification strategies aim to address combustion challenges by redesigning parameters such as injection timing, fuel reactivity, and cylinder pressure29. Reactivity Controlled Compression Ignition (RCCI) engines have emerged as an effective solution, offering better control over combustion phasing and temperature. RCCI employs two fuels: a low-reactivity fuel (hydrogen, introduced through intake air) and a high-reactivity fuel (diesel or biofuel, directly injected)30,31. This setup facilitates controlled ignition and smoother combustion. However, even in RCCI mode, hydrogen-enriched fuels tend to produce high in-cylinder temperatures, leading to persistent NOx formation, albeit at lower levels than in conventional CI engines32,33,34.
Several researchers have explored after-treatment systems and exhaust gas recirculation (EGR) techniques to mitigate NOx emissions. Cold EGR, in particular, has proven more effective than hot EGR in lowering combustion temperatures and curbing NOx levels35,36. Studies have shown that using hydrogen-enriched algae oil with 15% EGR can reduce NOx emissions by up to 30%, although excessive EGR can adversely impact combustion efficiency and engine power37. These findings underscore the need for a balanced strategy that integrates optimized fuel blends, combustion control, and effective NOx reduction techniques. The investigator checked the hydrogen-enriched biofuel along with EGR in a diesel engine38,39,40. In this study, the author compared hot EGR with cold EGR at 25 °C and confirmed that cold EGR further lowered NOx emissions compared to hot EGR. The researcher used pure algae oil enriched with hydrogen in a diesel engine35. They reported that with 15% exhaust gas recirculation (EGR), the hydrogen-enriched algae fuel reduced NOx formation by 30% compared to fuel without EGR. This reduction may be due to the higher oxygen dilution and specific heat capacity of the test fuel, which resulted in a lower peak in-cylinder temperature (ICT)36,41.
A critical review of the literature reveals notable research gaps: (i) limited integration of hydrogen with fifth-generation biofuels derived from sustainable sources like citronella, (ii) underutilization of R-EGR (Recirculated Exhaust Gas with optimized routing and temperature control) in hydrogen-biofuel dual fuel engines, and (iii) sparse experimental validation of such combinations in Low Temperature Combustion (LTC) regimes. In light of these gaps, the present work introduces a novel combination of hydrogen-enriched fifth-generation biofuel with R-EGR strategy in a Low Temperature Combustion (LTC) engine. This integrated approach seeks to reduce NOx emissions significantly while maintaining high thermal efficiency and combustion stability. Unlike previous studies that either focused on fuel modification or after-treatment separately, this research holistically combines advanced biofuel production, hydrogen enrichment, and advanced engine control through LTC and R-EGR.
Materials and methodology
In this research, a hydrogen gas cylinder was used to enrich the biofuel. The hydrogen was procured from a local scientific industry. The biofuel used was citronella grass oil, which was extracted through an energy-intensive process. The feedstock for the citronella oil was collected from a local water resource. The reference diesel was procured from a local Shell fuel station.
Background and extraction of Citronella grass oil
Citronella grass (Cymbopogon nardus), known for its resilience and versatility, has gained attention as a potential source of biofuel. This hardy plant thrives in diverse water conditions, including impure water, and reaches maturity quickly, typically within one to two weeks, and grows up to one meter in height. It is predominantly grown in tropical and subtropical regions, such as Southeast Asia, India, Sri Lanka, and Africa, where it enjoys warm and humid climates42. The essential oils derived from citronella grass, widely recognized for their insect-repellent properties, also have significant biofuel potential. Citronella oil is typically yellow to brown in color and has a fresh, lemony scent. The biomass of citronella grass can be converted into bioethanol, a renewable energy source, contributing to sustainable energy solutions43. In this work, citronella oil was extracted through an energy-intensive process, typically involving steam distillation, which requires substantial heat energy to separate the essential oils from the plant material. The detailed extraction concept was already present in our previous work (41). Additionally, the extracted oil was confirmed by Fourier Transform Infrared Spectroscopy (FT-IR) and Gas Chromatography-Mass Spectrometry (GC-MS), as presented in our previous work (41). This process ensures the efficient extraction of high-quality oil suitable for various applications. Its rapid growth, adaptability, and dual-purpose use make citronella grass a promising candidate in the biofuel industry.
Fuel property analysis
The physical and chemical properties of the test fuels were analyzed to assess their suitability for use in LTC engines. All properties were measured using standardized procedures in accordance with ASTM test methods to ensure accuracy and reproducibility. The results are presented in Table 1, which includes a direct comparison between citronella biofuel and conventional diesel. As seen in the table, citronella biofuel exhibits several advantageous properties over diesel. It has a significantly higher flash point, indicating enhanced safety during handling and storage. Its cetane number (~ 60) suggests superior ignition quality, which is beneficial in compression ignition engines. The lower sulfur content also promises reduced SOx emissions. However, citronella’s higher viscosity (5.8 cSt) may impact atomization during injection, possibly requiring engine or injector modifications. Additionally, the lower calorific value (~ 35 MJ/kg) compared to diesel implies a potential reduction in energy output per unit mass of fuel. Despite these drawbacks, the oxygen-rich nature of citronella biofuel can promote better combustion efficiency and reduced particulate matter emissions.
Test matrix
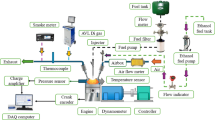
In the present study, a single-cylinder, four-stroke, water-cooled diesel engine (Kirloskar model) was modified to operate in Reactivity-Controlled Compression Ignition (RCCI) mode. The detailed schematic of the experimental setup is shown in Fig. 1. The engine was operated at a constant speed of 1500 rpm with a power output of 5.2 kW and a compression ratio of 6:1. The direct injection system operated at an injection pressure of 180 bar. Five different test fuels were evaluated, including pure diesel (D100), pure citronella biofuel (B100), and three blends of hydrogen-enriched citronella biofuel designated as H20, H40, and H60, where the number represents the percentage volume of hydrogen supplied. In RCCI mode, hydrogen was introduced with the intake air during the suction stroke as the low-reactivity fuel (LRF), while the high-reactivity fuel (HRF), either diesel or biofuel, was injected directly at the end of the compression stroke. The test fuel matrix is summarized in Table 2.
Each fuel was tested under steady-state conditions at fixed engine speed, and performance was evaluated based on brake power (BP). The instrumentation included a crank encoder for crank angle measurement, an AVL pressure sensor with a data acquisition system for in-cylinder pressure analysis, an AVL DiGas 444 five-gas analyzer for emission measurements, and an AVL 437 smoke meter for smoke opacity analysis. An exhaust gas recirculation (EGR) system with a manual flow regulator and temperature control was integrated into the engine, enabling precise flow regulation of both hot and cold R-EGR configurations. Each experimental point was repeated three times to ensure repeatability, and average values were used for analysis.
Regulated - Exhaust gas recirculation set up
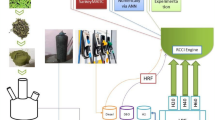
The R-EGR system is a crucial advancement for reducing nitrogen oxide (NOx) emissions in internal combustion engines, particularly diesel engines. In this study, we’ve modified a Kirloskar diesel engine to incorporate an R-EGR setup that precisely controls the inflow of reformed exhaust gases. The system is streamlined with two key components: the Reforming Reactor and the R-EGR valve. The Reforming Reactor converts the exhaust gases into a hydrogen-rich synthetic gas (syngas), comprising mainly hydrogen (H₂) and carbon monoxide (CO). Introducing this syngas back into the combustion chamber enhances the combustion process, leading to more efficient fuel burn and a significant reduction in NOx emissions. The presence of hydrogen promotes faster flame propagation and more complete combustion, which not only reduces emissions but can also improve engine efficiency. The R-EGR valve is electronically controlled, allowing for real-time adjustments based on engine operating conditions such as load, speed, and temperature. By modulating the amount of syngas reintroduced into the combustion chamber, the valve ensures optimal combustion conditions are maintained, effectively reducing NOx formation while avoiding any adverse effects on engine performance. The detailed R-EGR process flow is illustrated in Fig. 2, showing how exhaust gases are diverted from the exhaust manifold into the reforming reactor. After reforming, the hydrogen-rich gases pass through the R-EGR valve before mixing with the incoming air in the intake manifold. This seamless integration allows for continuous and precise adjustment of the gas composition entering the combustion chamber.
Test procedure and safety regulation
Before commencing the experimental investigations, a thorough inspection of all essential systems was carried out, including switches, oil tank, fuel tank, lubrication system, and exhaust system. Following confirmation that all systems were functioning correctly, the test was initiated using diesel fuel until the engine reached steady-state operating conditions. Afterward, the diesel fuel was drained, and various test fuels were introduced sequentially. Each fuel was tested three times to ensure consistency and repeatability. The average of the three trials was used for data analysis, and corresponding graphs were plotted based on these averaged values. All tests were conducted under controlled and repeatable conditions, following guidelines established by ASTM D613 for fuel testing and ISO 15,550 for engine performance testing. Instrument calibration was performed prior to each experiment, and the engine was operated exclusively under steady-state conditions to minimize transient effects. The low variability in repeated measurements confirmed the reliability and reproducibility of the experimental methodology. To ensure accurate results, compliance with recognized testing protocols and the ASTM and ISO standards was maintained throughout the study.
Strict adherence to safety protocols was ensured, particularly when handling hydrogen gas. A flame arrester was installed in the hydrogen supply line to prevent potential backfire, in accordance with the National Fire Protection Association (NFPA) 2 Hydrogen Technologies Code. Personal protective equipment (PPE) including flame-resistant clothing, gloves, safety goggles, and face shields were worn at all times during the testing process. Hydrogen cylinders were stored upright and securely fastened to prevent tipping, in line with ISO 14687 for hydrogen fuel quality. A dedicated cylinder cart was used for safe transportation of the hydrogen cylinders. Gas leaks were regularly checked using suitable leak detection methods, and the testing area was well-ventilated to prevent hydrogen accumulation. Emergency procedures were clearly defined and accessible in case of hydrogen leakage or fire. A schematic summary of the work flow is illustrated in Fig. 3.
Uncertainty study
Every experimental investigation requires an uncertainty study to quantify and eliminate errors arising from various factors, including environmental, instrument, human errors, and fuel mixing inconsistencies. The total uncertainty estimation was performed by incorporating all potential sources of error, including the mixing error, which is critical in the context of fuel blending and combustion processes. Variations in fuel-air mixture homogeneity can influence combustion efficiency and emissions, and this factor can significantly magnify overall uncertainties in performance and emission parameters.
The total uncertainty was calculated using the following equation:
Based on the study, the error performance was estimated for all engine performance and emission parameters. The thermal efficiency had an error of 0.5%, fuel consumption had an error of 0.7%, hydrocarbon (HC) emissions had an error of 0.4%, carbon monoxide (CO) emissions had an error of 0.15%, oxides of nitrogen (NOx) emissions had an error of 0.7%, and smoke had an error of 0.7%. By including the mixing error, the total uncertainty estimation for the experiment was found to be ± 1.31%.
Result and discussion
Performance test
The performance test was conducted to measure the Brake Thermal Efficiency (BTE) and Brake Specific Energy Consumption (BSEC) versus brake power using six different test fuels: diesel, citronella, H20, H40, H60 and H40E. The detailed test fuel matrix is mentioned in the Table 2.
Result of BTE
Usually, Brake Thermal Efficiency (BTE) tests are conducted to analyze the capacity of converting useful energy from test fuels. Hence, the output of BTE largely depends on the energy content of the test fuel44. Commonly, higher heating value fuels exhibit higher BTE. The results Fig. 4 indicate that the H40 blend achieved a 0.44% higher BTE than diesel, attributed to its greater energy content from hydrogen. The output of BTE were 31.90% for diesel, 28.42% for Citronella, 32.04% for H40, 30.75 for H20, 29.91% for H60 and 31.15% for H40E respectively at top load. For all test fuels, BTE increased with rising load. Notably, higher BTE was observed at top load compared to middle load, likely due to enhanced combustion resulting from better air-fuel mixing and increased cylinder temperatures at higher loads. Interestingly, diesel exhibited higher BTE at low loads compared to the other fuels. This may be because, at high loads, oxygen deficiency in diesel engines leads to incomplete combustion, reducing efficiency. In contrast, at low loads, sufficient oxygen is available, allowing diesel to combust more completely. Citronella oil showed lower BTE across all loads due to its minimal energy content, as confirmed in Table 2. Despite containing 60% citronella oil, the H40 blend outperformed all other test fuels, including diesel by 0.5–11%. This superior performance is largely owing to the higher energy content from hydrogen and the efficient combustion process facilitated by the RCCI engine configuration. Hydrogen’s high reactivity and fast flame speed improve combustion kinetics, enhancing overall engine efficiency. R-EGR involves recirculating a portion of reformed exhaust gases enriched with hydrogen back into the combustion chamber. This process is intended to enhance combustion by improving ignition properties and accelerating reaction rates. However, when H40 was tested with 10% R-EGR, it exhibited a slight decrease in BTE compared to H40 without R-EGR by 2.86%. This suggests that while R-EGR has the potential to improve combustion efficiency, an excessive amount in this case, 10% may lead to charge dilution. Charge dilution reduces oxygen concentration and flame temperature, adversely affecting combustion efficiency and leading to incomplete combustion. Supplying the H60 blend resulted in lower BTE than diesel, H40, and H20. Excess hydrogen beyond 40% may cause quenching effects and incomplete combustion, reducing efficiency. The BTE for the H20 blend was lower than that of H40 and diesel due to the higher citronella content decreasing the blend’s overall energy content.
Result of BSEC
BSEC indicates the fuel’s ability to utilize energy effectively. The Fig. 5 showed that all test fuels used their energy more efficiently at top load than at other loads. This aligns with the findings of Suresh et al., who demonstrated that internal combustion engines generally exhibit better fuel energy consumption at full-load conditions due to enhanced air-fuel entrainment, increased peak pressure, and higher temperatures45. This phenomenon mirrors real-world driving practices. The Indian transportation government recommends quickly shifting to top gear to achieve better fuel economy46. Operating a vehicle in top gear reduces engine RPM, allowing it to function under higher load conditions with less frictional losses and improved combustion efficiency. Thus, there’s a direct correlation between engine efficiency at top load in controlled tests and the practical advice of using top gear in daily driving47. BSEC often mirrors BTE since fuel economy depends on energy conversion efficiency and energy losses. From the graph, it was identified that the H40 blend had better BSEC than all other test fuels, including diesel by 20–40%. This superior performance is attributed to the higher energy content of hydrogen and the efficient combustion process in the RCCI engine. However, when supplying H60, the BSEC was higher than those of diesel, H40, and H20. This indicates less efficient energy utilization and reduced thermal efficiency, likely due to excess hydrogen beyond 40% leading to quenching effects and incomplete combustion. Citronella oil had the highest BSEC and among all test fuels due to its minimal energy content. The H40 blend with 10% R-EGR exhibited a slight increase in BSEC compared to H40 without R-EGR by 11%. This suggests that excessive R-EGR may lead to charge dilution, reducing combustion efficiency and leading to incomplete combustion. Consequently, energy utilization becomes less effective48. Overall, the H40 blend without R-EGR proved to be the best-performing fuel in terms of both BTE and BSEC among all test fuels studied. It demonstrated superior energy utilization and fuel efficiency across the tested conditions, showing a 2% increase in BTE over diesel. These findings underscore the importance of optimizing R-EGR levels to enhance combustion processes without compromising engine efficiency. Balancing R-EGR is crucial for improving fuel reactivity and promoting complete combustion, especially at higher loads.
Emission test
The emission test was conducted to measure the HC, CO, CO2, NOx and Smoke versus brake power using six different test fuels: diesel, citronella, H20, H40, H60 and H40E. The detailed test fuel matrix is mentioned in the Table 2.
Results of HC
Hydrocarbon emissions in IC engines are influenced by various factors, particularly the type of test fuel used. These emissions can result from incomplete combustion caused by a lack of oxygen in the test fuel, improper air-fuel entrainment, a high hydrocarbon ratio of the test fuel, and cylinder wall quenching49. From the Fig. 6, it was confirmed that diesel fuel exhibited higher HC emissions compared to other test fuels, with an increase of about 13–65%. This observation suggests that diesel, due to its lower oxygen content, leads to higher HC formation through incomplete combustion. In contrast, when citronella fuel was used, the trend was reversed. Citronella, with its abundant oxygen content, resulted in better combustion efficiency compared to diesel. Additionally, the H20 and H40 blends demonstrated better HC emission performance than citronella, owing to improved air-fuel entrainment facilitated by the RCCI mode. Carbon-free hydrogen emitted lower HC levels, about 20–46% less than citronella. Notably, the H40 blend achieved the highest HC emission reduction among all test fuels due to several factors, including complete combustion with sufficient oxygen presence, better fuel atomization through RCCI mode, and cleaner combustion with adequate hydrogen presence. However, when supplying H60, the HC emissions were slightly higher than those of diesel, H40, and H20, likely due to excess hydrogen beyond 40%, leading to quenching effects and incomplete combustion. The H40 blend with 10% R-EGR exhibited a slight increase in HC emissions compared to H40 without R-EGR by 6%. This suggests that excessive R-EGR may lead to charge dilution, reducing combustion efficiency and resulting in incomplete combustion. Researchers Ahmad et al. proved and supports our investigation that diesel engines with biodiesel along with EGR dilution experience increased HC formation when EGR circulation exceeds 5%, due to charge dilution50. Overall, the H40 blend showed better HC emission performance than all other test fuels due to complete combustion.
Results of CO
The formation of carbon monoxide (CO) is also dependent on the lack of oxygen and incomplete combustion in the IC engine’s combustion chamber51. Diesel fuel, unfortunately, exhibits higher CO formation compared to other test fuels, ranging from about 6–37%, due to the absence of oxygen in its composition. The author Shrivastava et al. compared the combustion characteristics of diesel and biodiesel in CI engines. They confirmed and supports our investigation that biodiesel consistently achieves more complete combustion than diesel but also experiences higher ignition delay and combustion duration. These factors contribute to the higher CO formation observed with diesel fuel52. The Fig. 7 report that H20 and H40 blends demonstrated better CO emission performance than citronella by 22% and 29% respectively, owing to improved air-fuel entrainment facilitated by the RCCI mode. Carbon-free hydrogen emitted lower CO levels, about 12–29% less than citronella. Notably, the H40 blend achieved the highest CO emission reduction among all test fuels due to several factors, including complete combustion with sufficient oxygen presence, better fuel atomization through RCCI mode, and cleaner combustion with adequate hydrogen presence. However, when supplying H60, the CO emissions were slightly higher than those of diesel, H40, and H20, likely due to excess hydrogen beyond 40%, leading to quenching effects and incomplete combustion. The H40 blend with 10% R-EGR exhibited a slight increase in CO emissions compared to H40 without R-EGR by 2.8%. This suggests that excessive R-EGR may lead to charge dilution, reducing combustion efficiency and resulting in incomplete combustion. Overall H40 blend had better CO formation. This superior emission reduction is largely owing to the higher energy content from hydrogen and the efficient combustion process facilitated by the RCCI engine configuration. Hydrogen’s high reactivity and fast flame speed improve combustion kinetics, enhancing overall CO emission rate.
Results of CO2
The formation of CO2 emissions from diesel engines is based on complete combustion. CO2 emissions follow an opposite trend compared to HC and CO emissions, which are formed due to incomplete combustion. Typically, complete combustion is achieved through the presence of sufficient oxygen, better air/fuel mixing rates, and optimal pressure and temperature53,54. Research by Rangabashiam et al. confirmed and supports our investigation that diesel has higher carbon dioxide emissions compared to the B100 blend. This is likely because the 100% diesel contains lower viscosity and minimal ignition delay, which enhances the combustion rate55. For all test fuels, CO2 increased with rising load. Notably, higher CO2 was observed at top load compared to middle load, likely due to enhanced combustion resulting from better air-fuel mixing and increased cylinder temperatures at higher loads. From the Fig. 8, it was confirmed that diesel fuel exhibited higher CO2 emissions compared to citronella, with an increase of about 2%. Additionally, the H20 and H40 blends demonstrated better CO2 emission performance than citronella, owing to carbon-free hydrogen emitted lower CO2 levels, about 5.4–6.5% less than citronella. Notably, the H60 blend achieved the lowest CO2 emission than those of diesel, H40, and H20, likely due to excess hydrogen beyond 40%, leading to quenching effects and incomplete combustion. R-EGR involves recirculating a portion of reformed exhaust gases enriched with hydrogen back into the combustion chamber. This process is dilute the intake charge. The H40 blend with 10% R-EGR exhibited a lower in CO2 emissions compared to all the hydrogen blends. On compare to diesel CO2 emission were lower for all the test fuels 0.8% for citronella, 1.5% for H60, 6.3% for H20, 7.4% for H40, and 10.4% for H40E. This suggests that R-EGR may lead to charge dilution, reducing combustion efficiency and resulting in incomplete combustion.
Results of NOx
The formation of nitrogen oxides (NOx) in internal combustion engines can be primarily explained by the Zeldovich mechanism. NOx is produced at high peak cylinder temperatures during the combustion process56. Various factors contribute to the creation of these peak temperatures, with test fuels such as oxygen-enriched biofuels and hydrogen playing significant roles57. The Figure 9 indicates that NOx formation increases with engine load due to a gradual rise in in-cylinder peak temperatures. The output of NOx was 4.6 g/kWh for diesel, 5.2 g/kWh for Citronella, 4.9 g/kWh for H40, 5 g/kWh for H20, 4.75 g/kWh for H60 and 4.2 g/kWh for H40E respectively at top load. Citronella fuel exhibited approximately 13% higher NOx emissions than diesel. This increase may be due to the enhanced oxygen presence in the biofuel, which improves combustion and leads to higher in-cylinder peak temperatures and, consequently, NOx formation. Research by Kumar et al. confirmed and supports our investigation that diesel has lower nitrogen oxide emissions compared to the B100 blend. This is likely because biofuel contains sufficient oxygen, which enhances the combustion rate58. The H20 and H40 blends had lower NOx formation than citronella about 4% and 6% respectively but higher than diesel. Suresh et al. also confirmed that hydrogen-enriched fuel had higher NOx formation than diesel due to the high flame temperature of hydrogen, which promotes NOx formation. However, the H60 blend had the lowest NOx emissions compared to Citronella, H40, and H20. This is likely due to the excess hydrogen beyond 40%, leading to quenching effects that lower the in-cylinder peak temperature. R-EGR involves recirculating a portion of reformed exhaust gases enriched with hydrogen back into the combustion chamber. This process dilutes the intake charge, lowering NOx formation. The H40 blend with 10% R-EGR exhibited lower NOx emissions compared to all the hydrogen blends by 13–19%. This suggests that R-EGR may cause charge dilution, reducing combustion efficiency and resulting in lower peak cylinder temperatures.
Results of smoke
Smoke emissions from internal combustion engines are primarily the result of incomplete combustion, leading to the formation of particulate matter (PM) and soot. The type of fuel and combustion conditions significantly influence the level of smoke emissions produced59. The Fig. 10 indicates that smoke emissions decrease with increasing engine load when using alternative fuels such as oxygen-enriched biofuels and hydrogen blends. Citronella fuel demonstrated a reduction in smoke emissions by approximately 3.5% compared to diesel. This reduction is attributed to the higher oxygen content in the biofuel, which enhances combustion efficiency and promotes the complete oxidation of fuel, thereby reducing soot formation [63]. Research by Ellappan et al. supports our findings, showing that diesel produces higher smoke emissions compared to the B100 biofuel blend. The inherent oxygen in biofuels facilitates better combustion, reducing the tendencies for soot formation60. The H20 and H40 hydrogen blends exhibited even lower smoke emissions than citronella fuel by 11% and 13% respectively. The addition of hydrogen improves combustion characteristics due to hydrogen’s higher flame speed and diffusivity, which leads to more complete combustion and lower soot production. However, the H60 blend showed the lowest smoke emissions among all tested fuels. The excess hydrogen in the H60 blend enhances the combustion process significantly, effectively minimizing the formation of particulate matter. The implementation of Reformed Exhaust Gas Recirculation (R-EGR) further reduces smoke emissions. R-EGR involves recirculating a portion of reformed exhaust gases enriched with hydrogen back into the combustion chamber. This process not only dilutes the intake charge but also introduces active hydrogen species that promote soot oxidation. The H40 blend with 10% R-EGR exhibited the lowest smoke emissions compared to all other fuel blends by 3–20%. This suggests that R-EGR effectively reduces particulate matter emissions by enhancing combustion efficiency and oxidizing residual soot particles. These findings highlight the potential of using oxygen-enriched biofuels, hydrogen blends, and R-EGR technology to mitigate hazardous tailpipe emissions in internal combustion engines. By optimizing fuel composition and combustion strategies, it is possible to achieve cleaner engine operation with reduced environmental impact.
Combustion test
The combustion test was conducted to measure the ICP and HRR using six different test fuels: diesel, citronella, H20, H40, H60 and H40E. The detailed test fuel matrix is mentioned in the Table 2.
Result of ICP and HRR
In-cylinder pressure (ICP) and heat release rate (HRR) are pivotal parameters that profoundly influence the performance, efficiency, and emission characteristics of internal combustion engines. ICP measures the pressure within the combustion chamber during the engine cycle, directly affecting mechanical power output and thermal efficiency. HRR denotes the rate at which energy is released during combustion, impacting how effectively the engine converts fuel into work. From the Fig. 11, it was confirmed that the use of citronella affects both ICP and HRR due to variations in combustion properties. Oxygen-enriched citronella fuel enhances combustion efficiency through their higher oxygen content, leading to a more rapid heat release and elevated peak in-cylinder pressures compared to diesel. The additional oxygen facilitates more complete combustion, increasing the HRR and improving energy conversion efficiency. Hydrogen-enriched blends, such as H20 and H40, markedly impact ICP and HRR because hydrogen’s high flame speed and diffusivity accelerate the combustion process. These blends exhibit higher peak pressures and heat release rates than both diesel and citronella fuel. The rapid combustion associated with hydrogen addition results in a sharper pressure rise and enhanced thermal efficiency. However, the H60 blend presents a unique behavior; the excessive hydrogen content beyond 40% induces a quenching effect, lowering the in-cylinder peak temperature and pressure, which in turn reduces the HRR. This quenching occurs because surplus hydrogen absorbs heat during combustion, moderating the overall energy release and slowing down the combustion rate. The implementation of Reformed Exhaust Gas Recirculation (R-EGR) further influences ICP and HRR. R-EGR recirculates a portion of reformed exhaust gases enriched with hydrogen back into the combustion chamber, diluting the intake mixture and introducing reactive hydrogen species. This process enhances combustion efficiency while reducing peak combustion temperatures and pressures. The H40 blend with 10% R-EGR demonstrates a balanced reduction in ICP and HRR compared to the H40 blend without R-EGR, potentially reducing mechanical stress on engine components and decreasing NOx emissions by mitigating high combustion temperatures without significantly compromising engine performance.
Sankey validation report
The Sankey tool was used to validate the experimental results against the expected reference output. This validation of the experimental output is significant for ensuring accuracy and repeatability. Validation helps with the reproduction and improvement of results, which is a crucial pillar of research. Hence, the validation highly supports the research framework of this study through the use of SankeyMATIC. The Sankey diagrams effectively express the energy distribution and emission formation, making it easier to validate the investigational outputs61,62.
In this study, the results of the investigation were validated by Sankey diagrams for diesel, citronella, and hydrogen fuels. The diesel output showed a brake thermal efficiency (BTE) of 31.9%, CO emissions of 2.92 g/kWh, HC emissions of 0.26 g/kWh, CO₂ emissions of 0.82 g/kWh, NOx emissions of 5.2 g/kWh, and smoke emissions of 72.50%. These results were almost identical to the expected outcomes, which were validated by the Sankey diagram shown in Fig. 12.
Likewise, for citronella fuel, the investigation outcomes showed HC emissions of 0.23 g/kWh, CO emissions of 2.75 g/kWh, NOx emissions of 0.61 g/kWh, and an energy conversion factor of 31%. The results for biodiesel followed a similar pattern, as displayed in the Sankey diagram on energy and emissions, which corroborated the experimental findings illustrated in Fig. 13.
In the case of hydrogen fuel, low emissions were observed with HC and CO emissions at 0.3 g/kWh each, CO2 emissions at 0.2 g/kWh, NOx emissions at 1 g/kWh, and an energy conversion factor of 55%. The Sankey diagram for hydrogen effectively represented these values, highlighting the clean combustion characteristics and high efficiency of hydrogen as a fuel, as shown in Fig. 14.
Sankey diagrams proved to be highly effective tools for visualizing and validating experimental data, clearly delineating areas of efficiency and environmental impact for each type of fuel. A comprehensive comparison reveals that Sankey diagrams serve as an essential basis for comparing diverse fuels by providing transparent performance metrics. Figure 15 shows that Overview of LTC Engines.
Conclusion
This study confirms the potential of integrating oxygen-enriched biofuels, hydrogen supplementation, and recirculated exhaust gas (R-EGR) to reduce harmful tailpipe emissions and enhance combustion in internal combustion engines. Among the tested fuels, the H40 blend (40% hydrogen-enriched fuel) exhibited the most favorable results across multiple parameters:
-
It achieved significant reductions compared to diesel: HC by 13–65%, CO by 6–37%, NOx by 4–6%, CO₂ by 7.4%, and smoke emissions by 13%.
-
Compared to citronella-based fuel, H20 and H40 blends recorded 11% and 13% lower smoke emissions, respectively, while the H60 blend yielded the lowest smoke emissions overall due to its higher hydrogen concentration.
-
The addition of 10% R-EGR to the H40 blend resulted in a slight increase in HC (6%) and CO (2.8%), but delivered a notable NOx reduction of 13–19% and smoke reduction of 3–20% compared to other hydrogen blends.
-
The H40 blend also demonstrated improved combustion efficiency, as evidenced by its 7.4% lower CO₂ emissions than diesel, supporting previous findings (e.g., by Suresh et al.) that attribute biodiesel’s lower CO₂ output to better oxygen availability and combustion quality. Additionally, the Sankey diagram analyses effectively validated these combustion trends and emission results, providing a visual confirmation of energy flow and clean combustion across fuel types.
In conclusion, the H40 blend with moderate R-EGR levels offers a balanced and cleaner combustion strategy, optimizing in-cylinder pressure and heat release rates while significantly lowering key emissions. These findings underscore that combining oxygenated biofuels, hydrogen enrichment, and R-EGR is a promising approach to achieving sustainable engine operation with reduced environmental impact.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Nayak, S. K., Behera, G. R. & Mishra, P. C. Exhaust from a dual-fuel engine using quinine nut oil and producer gas. Energy Sour. Part A Recover. Utilization Environ. Eff. 39 (2), 246–253 (2017).
Ramalingam, K. et al. Substitution of diesel fuel in conventional compression ignition engine with waste biomass-based fuel and its validation using artificial neural networks. Process Saf. Environ. Prot. 177, 1234–1248 (2023).
Nayak, S. K., Nayak, B., Mishra, P. C., Noor, M. M. & Nanda, S. Effects of biodiesel blends and producer gas flow on overall performance of a turbocharged direct injection dual-fuel engine. Energy Sour. Part A Recover. Utilization Environ. Eff. 46 (1), 4165–4184 (2024).
Munimathan, A. et al. ML techniques increasing the power factor of a compression ignition engine that is powered by Annona biodiesel using SATACOM. Sci. Rep. 15 (1), 11669. https://doi.org/10.1038/s41598-025-91162-1 (2025).
Nayak, S. K. & Mishra, P. C. Emissions from sawdust biomass and diesel blends fuels. Energy Sour. Part A Recover. Utilization Environ. Eff. 38 (14), 2050–2057 (2016).
Kanth, S., Sumita, D. & Biplab, D. Effect of hydrogen enrichment in the intake air of diesel engine fuelled with Honge biodiesel blend and diesel. Int. J. Hydrog. Energy 45 (56), 32521–32533 (2020).
Nayak, S. K., Behera, G. R. & Mishra, P. C. Physio-chemical characteristics of Punnang oil and rice husk-generated producer gas. Energy Sour. Part A Recover. Utilization Environ. Eff. 39 (3), 291–298 (2017).
Guo, H. et al. Transient lubrication of floating Bush coupled with dynamics and kinematics of cam-roller in fuel supply mechanism of diesel engine. Phys. Fluids. 36 (12), 123103. https://doi.org/10.1063/5.0232226 (2024).
Huynh, D. N. L. et al. Using hydrogen as potential fuel for internal combustion engines: a comprehensive assessment. Int. J. Renew. Energy Dev. 14 (1), 83–103 (2024).
Chen, L. et al. Raman spectral optimization for soot particles: a comparative analysis of fitting models and machine learning enhanced characterization in combustion systems. Build. Environ. 271, 112600. https://doi.org/10.1016/j.buildenv.2025.112600 (2025).
Vijayakumar, V., Lewis, F., Mahdi, S. & Daniel, S. Creating a global hydrogen economy: review of international strategies, targets, and policies with a focus on Japan, Germany, South Korea, and California (2022).
Tsujimura, T. & Yasumasa, S. The utilization of hydrogen in hydrogen/diesel dual fuel engine. Int. J. Hydrog. Energy. 42 (19), 14019–14029 (2017).
Ikeuba, A. I. et al. A review on exploring the potential of liquid hydrogen as a fuel for a sustainable future. Sustain. Chem. One World 2024, 100022 (2024).
Singh, A. P. Assessment of india’s green hydrogen mission and environmental impact. Renew. Sustain. Energy Rev. 203, 114758 (2024).
Athia, N., Mukesh, P. & Seema, S. Evaluating the effectiveness of National green hydrogen mission in India. Environ. Dev. Sustain. 2024, 1–23 (2024).
Gupta, S., Rupesh, K. & Amit, K. Green hydrogen in india: prioritization of its potential and viable renewable source. Int. J. Hydrog. Energy. 50, 226–238 (2024).
Jumaat, N. A. S. & Azianabiha, A. H. K. A comprehensive review of challenges, prospects, and future perspectives for hydrogen energy development in Malaysia. Int. J. Hydrog. Energy. 55, 65–77 (2024).
Hassan, N. S. et al. Ismail. Recent review and evaluation of green hydrogen production via water electrolysis for a sustainable and clean energy society. Int. J. Hydrog. Energy 52, 420–441 (2024).
Zakaria, Z. et al. Energy scenario in malaysia: embarking on the potential use of hydrogen energy. Int. J. Hydrog. Energy. 48 (91), 35685–35707 (2023).
Shadidi, B., Gholamhassan, N. & Talal, Y. A review of hydrogen as a fuel in internal combustion engines. Energies 14, 6209 (2021).
Koten, H. Hydrogen effects on the diesel engine performance and emissions. Int. J. Hydrog. Energy. 43 (22), 10511–10519 (2018).
Thiyagarajan, S. et al. Effect of hydrogen on compression-ignition (CI) engine fueled with vegetable oil/biodiesel from various feedstocks: a review. Int. J. Hydrog. Energy. 47 (88), 37648–37667 (2022).
Chelladorai, P., Edwin, G. V. & Leenus, J. M. Synergistic effect of hydrogen induction with biofuel obtained from winery waste (grapeseed oil) for CI engine application. Int. J. Hydrog. Energy 43 (27), 12473–12490 (2018).
Duraisamy, B. et al. Impact of hydrogen addition on diesel engine performance, emissions, combustion, and vibration characteristics using a Prosopis Juliflora Methyl ester-decanol blend as pilot fuel. Int. J. Hydrog. Energy. 75, 12–23 (2024).
Shirneshan, A., Berna, K. & Guven, G. Experimental investigation and parametric modeling of the effect of alcohol addition on the performance and emissions characteristics of a diesel engine fueled with biodiesel-diesel-hydrogen fuel mixtures. Fuel 381, 133489 (2025).
Jayabal, R. Effect of hydrogen/sapota seed biodiesel as an alternative fuel in a diesel engine using dual-fuel mode. Process Saf. Environ. Protect. (2024).
Bakar, R. A. et al. Experimental analysis on the performance, combustion/emission characteristics of a DI diesel engine using hydrogen in dual fuel mode. Int. J. Hydrog. Energy. 52, 843–860 (2024).
Qian, F. et al. Ammonia energy fraction effect on the combustion and reduced NOX emission of ammonia/diesel dual fuel. Environ. Res. 261, 119530 (2024).
Shahid, M. I. et al. Hydrogen production techniques and use of hydrogen in internal combustion engine: a comprehensive review. Fuel 378, 132769 (2024).
Ramalingam, K. et al. An experimental and ANN analysis of ammonia energy integration in biofuel powered low-temperature combustion engine to enhance cleaner combustion. Case Stud. Therm. Eng. 63, 105284 (2024).
Ramalingam, K. et al. Energy, environmental, and economic benefits of hydrogen-enriched biofuel using ammonium hydroxide in reactivity-controlled compression ignition engines. Results Eng. 24, 103672 (2024).
Rameez, P. V. & Ibrahim, M. M. Maximizing hydrogen utilization in CI engines: an investigation of dual fuel and RCCI combustion approaches under low and mid-load conditions in a medium duty automotive engine. Energy. Conv. Manag. 321, 119100 (2024).
Kumar, M. & Abhishek, P. Comparative evaluation of combustion, performance, exergy and emission characteristics in hydrogen-biodiesel dual fuel engine under RCCI mode. Energy Environ. 35 (7), 3418–3440 (2024).
Gharehlar, H. H., Mojtaba, E., Morteza, H. & Shabnam, H. Hydrogen/diesel RCCI engine performance assessment at low load. Int. J. Hydrog. Energy. 58, 200–209 (2024).
Kesharvani, S. et al. Enhancing diesel engine performance and emission reduction through hydrogen enrichment in algal biodiesel blends. Environ.Sci. Pollut. Res. 2024, 1–14 (2024).
Annamalai, B. & Parthasarathy, M. The combined effect of hydrogen enrichment and exhaust gas recirculation on the combustion stability, performance and emissions of CI engine energized by algae biodiesel. Int. J. Hydrog. Energy. 50, 524–546 (2024).
Loganathan, M., Thanigaivelan, V., Madhavan, V. M., Anbarasu, A. & Velmurugan, A. The synergetic effect between hydrogen addition and EGR on cashew nut shell liquid biofuel-diesel operated engine. Fuel 266, 117004 (2020).
Chaichan, M. T. Performance and emission characteristics of CIE using hydrogen, biodiesel, and massive EGR. Int. J. Hydrog. Energy. 43 (10), 5415–5435 (2018).
Kannappan, C., Sudhakar, S. & Rajappan, R. The combined effect of EGR and hydrogen addition on a Syzygium cumini (jamun) liquid biofuel engine. Biotechnol. Biofuels Bioprod. 16 (1), 105 (2023).
Lionus-Leo, G. M., Ravikumar, J., Das, M. C. & Arivazhagan, S. An experimental investigation on enhancing diesel engine performance and emissions with cashew nut shell oil biodiesel and hydrogen fumigation. Environ. Dev. Sustain. 2024, 1–23 (2024).
Suresh, A., Babu, A. V., Balaji, B. & Ranjit, P. S. Experimental investigation of NO x emission control using carbon nanotube additives and EGR configuration on CRDI engine fueled with ternary fuel. Biofuels 15 (10), 1315–1329. https://doi.org/10.1080/17597269.2024.2366446 (2024).
Ramalingam, K., Annamalai, K., Th, P. J., Joshua, S. C. & Production of eco-friendly fuel with the help of steam distillation from new plant source and the investigation of its influence of fuel injection strategy in diesel engine. Environ. Sci. Pollut. Res. 26, 15467–15480 (2019).
Dash, S. K. et al. Investigation on the adjusting compression ratio and injection timing for a DI diesel engine fueled with policy-recommended B20 fuel. Discover Appl. Sci. 6, 8. https://doi.org/10.1007/s42452-024-06076-w (2024).
Nayak, S. K., Mishra, P. C. & Behera, G. R. Characterization of coconut shell imitatived producer gas in adiesel engine. Energy Sourc. Part A: Recov. Utilization Environ. Effects 39 (16), 1718–1724 (2017).
Tiwari, P. K. et al. Influence of calophyllum inophyllum and Jojoba oil methyl ester blended with n-pentanol additive upon overall performance, combustion and emission characteristics of a TDI engine operated in natural aspirated mode. Fuel 288, 119576 (2021).
Nayak, S. K. & Mishra, P. C. Emission characteristics of jatropha oil blends using waste wood producer gas. Energy Sour. Part A Recover. Utilization Environ. Eff. 38 (14), 2153–2160 (2016).
Asaithambi, K., Krishnamoorthy, R. & Balasubramanian, D. A comparative assessment of tailpipe emission characteristics on diesel engine using nanofluid with R-EGR setup. No. 2020-28-0442. SAE Technical Paper (2020).
Nayak, S. K., Mishra, P. C., Kumar, A., Behera, G. R. & Nayak, B. Experimental investigation on property analysis of Karanja oil Methyl ester for vehicular usage. Energy Sour. Part A Recover. Utilization Environ. Eff. 39 (3), 306–312 (2017).
Ahmad, A., Ashok, K. Y. & Shifa, H. Enhancing efficiency and environmental impacts of a nano-enhanced oleander biodiesel-biohydrogen dual fuel engine equipped with EGR, through operational parameter optimization using RSM-MOGA technique. Int. J. Hydrog. Energy. 78, 1157–1172 (2024).
Rao, T. et al. Sustainable synthesis and advanced optimization of Prosopis juliflora biomass catalyst for efficient biodiesel production and environmental impact assessment. Sci. Rep. 15 (1), 4472 (2025).
Shrivastava, K., Thipse, S. S. & Patil, I. D. Optimization of diesel engine performance and emission parameters of Karanja biodiesel-ethanol-diesel blends at optimized operating conditions. Fuel 293, 120451 (2021).
Pullagura, G. et al. Enhancing performance characteristics of biodiesel-alcohol/diesel blends with hydrogen and graphene nanoplatelets in a diesel engine. Int. J. Hydrog. Energy 50, 1020–1034 (2024).
Chetia, B., Debbarma, S. & Das, B. An experimental investigation of hydrogen-enriched and nanoparticle blended waste cooking biodiesel on diesel engine. Int. J. Hydrog. Energy 49, 23–37 (2024).
Rangabashiam, D., Jayaprakash, V. & Rameshbabu, A. Emission, performance, and combustion study on nanoparticle-biodiesel fueled diesel engine. Energy Sour. Part A Recover. Utiliz. Environ. Eff. 45 (3), 8396–8407 (2023).
Zare, A. et al. Analysis of cold-start NO2 and NOx emissions, and the NO2/NOx ratio in a diesel engine powered with different diesel-biodiesel blends. Environ. Pollut. 290, 118052 (2021).
Nadanakumar, V. et al. Experimental investigation to control HC, CO & NOx emissions from diesel engines using diesel oxidation catalyst. Mater. Today: Proc. 43, 434–440 (2021).
Kumar, V. & Akhilesh, K. C. Assessment and usability of Jatropha biodiesel blend with phenolic antioxidant to control NOx emissions of an unmodified diesel engine. Environ. Sci. Pollut. Res. 30 (49), 108051–108066 (2023).
Selleri, T. et al. An overview of lean exhaust denox aftertreatment technologies and nox emission regulations in the european union. Catalysts 11, 404 (2021).
Ellappan, S. & Silambarasan, R. A comparative review of performance and emission characteristics of diesel engine using eucalyptus-biodiesel blend. Fuel 284, 118925 (2021).
Butrymowicz, D. et al. Experimental validation of new approach for waste heat recovery from combustion engine for cooling and heating demands from combustion engine for maritime applications. J. Clean. Prod. 290, 125206 (2021).
Stainchaouer, A., Christopher, S., Christoph, W., George, S. & Hartmut, S. Evaluating long-term operational data of a very large crude carrier: assessing the diesel engines waste heat potential for integrating ORC systems. Appl. Therm. Eng. 255, 123974 (2024).
Dhairiyasamy, R., Bunpheng, W., Kit, C. C. & Hasan, N. Comparative performance and emission analysis of soybean and algae biodiesels in low heat rejection engines. Energy Sci. Eng. https://doi.org/10.1002/ese3.2090 (2025).
Author information
Authors and Affiliations
Contributions
K.R, M.Z.A developed the idea, conducted the experiments and wrote the manuscript, E.P.V wrote the manuscript, M.V.R, Y.Y, P.S, K.P.K edited the manuscript, all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ramalingam, K., Abdullah, M.Z., Elumalai, P.V. et al. Production and utilization of hydrogen enriched fifth generation biofuel in LTC engines with reformed EGR. Sci Rep 15, 25922 (2025). https://doi.org/10.1038/s41598-025-08259-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08259-w