Abstract
The association between androgen deprivation therapy (ADT) and dementia including Alzheimer’s disease remains debatable. Moreover, the evidence regarding the risk of vascular dementia and Parkinson’s disease associated with ADT is limited. A population-based cohort study was conducted using the national health insurance data (2012–2022) from South Korea. Eligible patients newly diagnosed with prostate cancer who underwent ADT were compared with those who did not receive ADT. We applied propensity score matching and performed Cox proportional hazards regression analysis to calculate the adjusted hazard ratios (aHR) and 95% confidence intervals (CI). Of 163,723 patients with prostate cancer between 2013 and 2017, 24,456 were eligible. After 1:1 propensity score matching, 10,168 patients were included in each group. The risk of overall dementia was not significantly higher in ADT users compared with that of non-users (aHR = 1.07; 95% CI: 0.97–1.19); however, the elevated risk of Alzheimer’s disease was observed (aHR = 1.39; 95% CI: 1.21–1.59). ADT was not associated with the risk of vascular dementia (aHR = 1.14; 95% CI: 0.70–1.94) and Parkinson’s disease (aHR = 1.01; 95% CI: 0.75–1.35). Our updated evidence reinforces previous findings, indicating a positive association between ADT and the risk of Alzheimer’s disease. However, it is unlikely that ADT increases the risk of vascular dementia and Parkinson’s disease.
Similar content being viewed by others
Introduction
Prostate cancer is the most prevalent cancer in men worldwide, with 1,414,259 incident cases (14.1% of the total new male cases) and 375,304 deaths reported in 20201. The incidence of prostate cancer is increasing globally; the incidence rate has increased by 3% annually since 2014 in the United States and, notably, by 5.6% annually from 2015 to 2020 in Korea2,3. Since the discovery in the 1940s, androgens play a key role in the progression of prostate cancer; androgen deprivation therapy (ADT) has remained a crucial treatment option for high-risk localized or locally advanced disease as a combination or adjuvant therapy with definitive treatment, as well as metastatic status4. ADT controls prostate cancer by blocking the production of androgens or by inhibiting androgen receptors5. Approximately half of the patients diagnosed with prostate cancer has undergone ADT at some stage6.
Although ADT has been used for prostate cancer for several decades owing to its clinical benefits, oncologists and urologists are concerned about its potential adverse effects. Previous studies have reported that ADT is associated with musculoskeletal, cardiovascular, and metabolic disorders; sexual dysfunction; and fatigue7. The association between ADT and cognitive impairment, particularly dementia, including Alzheimer’s disease, which can crucially impair the quality of life of patients, has been investigated in numerous studies as androgens have neuroprotective effects8,9. Recent observational studies have produced controversial results10,11,12and most of them did not consider clinically important confounders such as primary treatments and the presence of metastatic cancer. Furthermore, the association between ADT and the risk of other cognitive dysfunctions, such as vascular dementia and Parkinson’s disease, remains largely unaddressed.
In this study, we aimed to evaluate the risk of dementia, including Alzheimer’s disease, vascular dementia, and Parkinson’s disease, in patients with prostate cancer exposed to ADT compared to those who were not in routine clinical practice using nationwide real-world data.
Materials and methods
Study design and data source
We conducted a population-based cohort study using the Korean National Health Insurance (NHI) data from Jan 1, 2012 to Sep 30, 2022 (Study number: M20230508001). Under the single-insurer system, the NHI data cover almost all citizens (approximately 97% of the population) residing in Korea. These data encompass demographic characteristics of patients, such as sex, age, type of insurance, and clinical information, including diagnosis coded with the International Classification of diseases, tenth revision (ICD-10), medical procedures, administration, and prescription of drugs. A previous validity study reported that the positive predictive values for major clinical outcomes in the Korean NHI data were 71.5–92.0%13. The use of the data and the research method were approved by the Institutional Review Board (IRB) of Ajou University (IRB Number: 202305-HB-EX-001). The need for informed consent was waived because no patient-identifying information was provided. All methods were performed in accordance with the relevant guidelines and regulations and the recommendations for longitudinal study design using healthcare databases [14].
Inclusion and exclusion criteria
Patients diagnosed with prostate cancer (ICD-10: C61) between Jan 1, 2013 and Dec 31, 2017, were included (cohort entry). To identify incident cases of prostate cancer and rule out the potential confounding effects of other cancers, we excluded patients who were diagnosed with prostate cancer and other cancers within one year before cohort entry. We also excluded patients who did not have registration codes for prostate cancer (V027, V193, and V194) within 90 d of the cohort entry and billing codes for prostate biopsy (C8551 and C8552) between one year before and 90 d after the entry date to minimize misclassification14,15. In Korea, a registration program is operating to alleviate economic burden of patients with rare and intractable diseases including cancer. The sensitivity and positive predictive value of prostate cancer were 95.3 and 93.7%, respectively, when both registration program claims and primary diagnoses were used, which were higher than those when only the primary diagnosis was used16. To focus on the effects of chemical ADT and minimize confounding by indication, patients who underwent orchiectomy from one year prior to the index date through the end of follow-up were excluded (0.8% of incident cases).
Patients who received ADT at least once during the study period were classified as ADT users, while those without any record of ADT use were considered non-users. ADT included gonadotropin-releasing hormone (GnRH) agonists (leuprolide, goserelin, and triptorelin), GnRH antagonist (degarelix), and first-generation nonsteroidal antiandrogens (bicalutamide, flutamide, and cyproterone). The index date was the first ADT prescription date for ADT users. For non-users, 23 d (median time from the first diagnosis of prostate cancer to the first ADT use for ADT users) after cohort entry was defined as the index date to avoid immortal time bias17. Patients aged 40–79 years at the index date were included, as treatment patterns may differ in elderly patients older than 80 years. Patients with metastatic prostate cancer were also included, since ADT is a standard treatment option regardless of disease stage. To identify incident cases, we excluded patients who had a history of outcomes of interest (dementia or Parkinson’s disease) within one year before the index date. Patients diagnosed with dementia or Parkinson’s disease within 180 d of the index date were excluded to reduce the potential for reverse causation by excluding latent diagnoses. Since cerebrovascular diseases are strongly correlated with cognitive impairment18 we excluded patients with a record of cerebrovascular events, including stroke, from 1 year before to 180 d after the index date. The inclusion and exclusion schemes for this study are shown in Supplementary Fig. 1.
Definition of outcomes and covariates
We defined dementia (F00-F03, F051, G30, and G31) and Parkinson’s (G20) as the outcomes of interest. Dementia was classified as Alzheimer’s disease (F00 and G30), vascular dementia (F01), and other dementia (F02-03, G31, and F051), and each event was identified as a secondary outcome19,20. As an intention-to-treat approach was applied, patients were followed until the occurrence of outcome events, death, or the end of the study period (September 30, 2022), whichever occurred first.
Age and insurance type (health insurance and Medicaid/veteran) of patients at the index date; Charlson Comorbidity Index (CCI) score calculated with medical history identified during 1 year before the index date; prior treatment for prostate cancer (radical prostatectomy, robot-assisted radical prostatectomy, and radiotherapy) received during 6 months after index date; comorbidities including hypertension, diabetes, dyslipidemia, cardiovascular diseases, peripheral vascular disease, chronic obstructive pulmonary disease, asthma, and liver diseases; and use of medication within 1 year before the index date including statins, antiplatelets, anticoagulants, anticholinergics, antidepressants, and antipsychotics was treated as baseline covariates. Detailed diagnostic codes for comorbidities, ingredient codes for medications, and procedure codes for the treatment of prostate cancer are provided in Supplementary Table 1 in Supplementary Materials.
Statistical analysis
Means with standard deviations (std), medians with interquartile ranges (Q1 and Q3) for continuous variables, and frequencies with percentages for categorical variables were computed to report the descriptive statistics of baseline characteristics. The absolute standardized difference (aSD) was calculated to determine whether significant imbalance exists between ADT users and non-users. We applied 1:1 propensity score matching using calipers of 0.1 which was 0.2 of the standard deviation of the logit of the propensity score21. A logistic model for the propensity score included age, insurance type, comorbidities, and recent 1-year use of statins, antiplatelets, and anticoagulants. The incidence rates were computed using the number of events and person-years. Finally, we calculated hazard ratios (HRs) and their 95% confidence intervals using a Cox proportional hazards regression model. The Kolmogorov-type supremum test was used to assess the proportional hazards assumption. To adjust for potential confounders, the following variables were included: age, primary treatment for prostate cancer, metastatic status, medications for metastatic cancer (taxanes, androgen receptor-targeted agents, and others), and recent 1-year use of specific medications (anticholinergics, antidepressants, and antipsychotics). Additionally, an analysis treating death as a competing risk was performed using Fine and Gray’s sub-distribution hazard model.
Subgroup and sensitivity analysis
Subgroup analyses were conducted to examine the potential heterogeneous effects of ADT across specific subsets of the populations stratified by age (under and over 70 years); concomitant use of medications for lower urinary tract symptoms (anticholinergics and imipramine/duloxetine), which are considered risk factors for cognitive decline, during the six months following the index date;22,23 and presence of metastasis from one year before to 180 days after the index date. All outcomes were assessed from the index date to avoid immortal time bias. We also investigated the dose-response relationship with duration (< one year and ≥ one year) and defined daily dose (DDD) [below and above the median DDD (1391.68)] of ADT. Sensitivity analyses were also performed to assess the robustness of the major results, including a full cohort analysis with adjustment for matching variables and with the application of a competing risk model.
Results
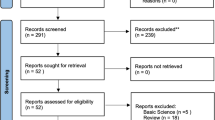
Among the 163,723 patients diagnosed with prostate cancer between 2013 and 2017, 36,162 were identified as incident cases: 52,873 with a history of prostate cancer, 15,432 with a history of other cancers, 47,771 without a registration code, and 11,485 without a biopsy procedure code were excluded (Fig. 1). We also excluded ineligible patients (289 with orchiectomy; 4,208 aged under 40 or over 80; five with a death record before the index date; 3,041 with a history of dementia or Parkinson’s disease; 4,152 with a history of cerebrovascular diseases) and then identified 24,456 remaining patients, consisting of 13,277 ADT users and 11,179 non-users. After propensity score matching, 10,168 patients were included in each group.
Before matching, the median age of the ADT group was higher than that of the non-ADT group (71 vs. 66 years, aSD = 0.507). The median CCI score in both groups was 4 (range: 3–5). Half the patients in both groups had hypertension as a comorbidity (53.3% and 49.6%, respectively). Anticholinergics were most frequently used in the two groups in the year before the index date (60.7% and 59.4%, respectively), followed by antidepressants (49.5% and 45.9%, respectively). After the index date, more patients in the non-user group used drugs for dysuria than those in the ADT group (anticholinergic: 47.8% vs. 72.3%; imipramine/duloxetine: 13.8% vs. 21.6%). The proportion of patients who had previously undergone radical prostatectomy with or without robotic assistance was higher in the non-use group than that in the ADT group (33.1% vs. 83.8%), whereas the proportion of patients with a history of radiotherapy was higher in the ADT group. In the ADT group, more patients had a metastatic status than non-users (9.7% vs. 5.7%, aSD = 0.149). The percentage of patients who used drugs for metastatic cancer was low and no significant difference was observed between the two groups (taxane: 0.47% vs. 0.015, aSD = 0.093; ARTA: 0.06% vs. 0.01, aSD = 0.031) (Table 1). After matching, the median follow-up duration was 68.6 months (range: 50.5–87.4) for ADT users and 77.8 months (range: 64.3–95.5) for non-users.
The risks of overall dementia and Parkinson’s disease were not higher in the ADT group compared with those in the non-user group (dementia: adjusted HR = 1.07, 95% confidence interval (CI) = 0.97–1.19; Parkinson’s disease: adjusted HR = 1.01, 95% CI: 0.75–1.35) (Table 2). However, when examined by the type of dementia, ADT use was associated with a higher risk of Alzheimer’s disease (adjusted HR = 1.39, 95% CI: 1.21–1.59). The Kaplan–Meier curves for overall dementia, Alzheimer’s disease, vascular disease, and Parkinson’s disease are shown in Fig. 2. A higher risk of Alzheimer’s disease was consistently observed in all subsets of the population (Fig. 3). The use of ADT was likely to be associated with a higher risk of vascular dementia in patients with metastatic cancer (adjusted HR = 3.54, 95% CI: 0.37–33.97); however, no statistical significance was observed. For Parkinson’s disease, no significant increase was found in any subgroup. Additionally, a dose-response relationship was not observed in the analyses of duration and DDD (Supplementary Table 2). Although ADT use for less than 12 months or with a cumulative dose below the median DDD was associated with an increased risk of dementia (adjusted HR = 1.22, 95% CI: 1.06–1.42 for the < 12-month group; adjusted HR = 1.32, 95% CI: 1.17–1.48 for the low DDD group), this risk was not observed in the ≥ 12-month and high DDD groups. The results of sensitivity analyses were consistent with the major results (Supplementary Table 3).
Discussion
In this nationwide cohort study, the use of ADT in patient with prostate cancer was associated with a higher risk of Alzheimer’s disease. The risk of vascular dementia, other dementias, and Parkinson’s disease did not increase with ADT use. The increased risk of Alzheimer’s disease was consistently observed regardless of sex, metastatic status, or concomitant use of anticholinergics or imipramine/duloxetine. The sensitivity analyses confirmed the robustness of the major results.
Our findings regarding the overall risk of dementia are consistent with those of previous observational studies. A study using population data of the United Kingdom (adjusted HR = 1.02, 95% CI: 0.85–1.23) and Taiwan (adjusted HR = 1.12, 95% CI: 0.87–1.43) showed no association between the overall dementia and ADT in both populations11. Another study conducted in UK with a cohort of patients with nonmetastatic prostate cancer also revealed that ATD use was not associated with an elevated risk of any dementia (adjusted HR = 1.02, 95% CI: 0.87–1.19)24. However, several US studies have reported an increased risk of dementia in ADT users compared with non-users12,25. This discrepancy could be led by differences in race or environmental factors among the populations. A meta-analysis revealed differential dementia risk associated with ADT by geographic regions (America, Europe, and Asia), suggesting that genetic and socioeconomic factors may contribute to the modified risk of cognitive decline in patients with prostate cancer26.
We also identified an elevated risk of Alzheimer’s disease in patients who underwent ADT compared with non-users. Several studies have reported a positive association between ADT and Alzheimer’s disease15,25. Conversely, some studies demonstrated a null association; however, they had a small sample size27did not include diverse clinical variables in the model28or limited study subjects to patients who received definitive radiation therapy29. Previous in vitro and in vivo studies demonstrated that testosterone exerted a neuroprotective effect by reducing beta-amyloid protein accumulation30enhancing synaptic plasticity30and preventing neuronal death31. Therefore, the blockage of testosterone can increase the risk of Alzheimer’s disease from a pathophysiological perspective.
ADT was not associated with the risk of vascular dementia in the present study, although it increases the risk of cardiovascular diseases32. Only a few studies have investigated the risk of vascular dementia as an adverse event of ADT and have reported a consistent null association11,31. Regarding the risk of Parkinson’s disease in relation to ADT use, we found no association, consistent with previous observational studies15,28. Our study provides current evidence that corroborates existing findings. Considering the relatively high mortality risk in patients with prostate cancer, we also performed a competing risk analysis using the Fine-Gray subdistribution hazard model. The results were similar to those from the primary Cox regression analysis, although the estimated effects were slightly attenuated. This may reflect the influence of death as a competing event before the onset of neurodegenerative outcomes.
The distribution of dementia subtypes observed in our study was generally consistent with those reported in previous Korean population-based studies33,34. However, the pattern differs from that reported in a study based on postmortem pathological data35, which showed different proportions of vascular and neurodegenerative pathologies. These differences may be explained by variations in ethnicity, genetic background, and diagnostic methods across studies. In particular, our study relied on clinical diagnosis codes to define dementia subtypes, which may not fully correspond to pathology-based classifications. Such factors should be considered when interpreting the results and assessing their generalizability.
The overall risks for dementia and Parkinson’s disease in the subgroups were similar to those in the major results. Consistent results in subpopulations according to metastatic status may indicate that the effect of ADT on cognitive impairment is not influenced by disease severity. Although ADT is the recommended standard of care for metastatic prostate cancer, a recent real-world study has shown that a considerable proportion of patients do not receive ADT-based therapy. A U.S. claims-based study reported that 38–48% of patients with metastatic castration-sensitive prostate cancer remained untreated or deferred treatment, indicating substantial variation in treatment patterns in clinical practice36. These findings help contextualize approximately 6% of metastatic patients in the non-ADT group in our study. Moreover, the overall ADT usage in our cohort (54.3%; 13,277 out of 24,456 patients) is generally comparable to a previous Korean study, which reported a rate of 66.4%15. This suggests that the ADT usage observed in our study aligns with treatment patterns in Korean clinical settings.
We did not observe significant differences in neurodegenerative outcomes according to concomitant use of anticholinergics or imipramine/duloxetine. These medications are commonly used short-term to manage urinary symptoms after radical prostate treatment, due to their well-known side effects, including cognitive impairment37. In our study, anticholinergic use was prevalent in both groups (47.4% of ADT users and 72.9% of non-users). The incidence of overactive bladder or lower urinary tract symptoms after radical prostate treatment varies but has been reported to reach up to 80%38. A Korean study reported that 82.9% of patients experienced urinary incontinence six months after robot-assisted laparoscopic prostatectomy39. The higher use of anticholinergics in the no-ADT group, where radical treatment was more frequent, likely reflects this clinical pattern. We also did not observe a dose-response relationship between ADT and dementia or Parkinson’s disease. However, as ADT exposure was defined by cumulative dose and duration during follow-up and not treated as a time-varying covariate, this may reflect methodological limitations. A time-dependent Cox model could better account for changes in exposure and should be considered in future research40.
Hormone replacement therapy (HRT) and ADT have fundamentally opposing endocrine effects, potentially leading to divergent neurological outcomes. HRT, which is primarily used in postmenopausal women, has been associated with potential neuroprotective effects by mediating through the modulation of amyloid metabolism, enhancement of cerebral blood flow, and reduction of neuroinflammation41,42. In contrast, ADT significantly lowers testosterone levels, which may negatively impact brain health by depriving neurons of androgens essential for survival, synaptic plasticity, and cognitive function43. Testosterone also plays a regulatory role in neurotransmitter systems such as dopamine and acetylcholine, and its deficiency has been linked to increased oxidative stress and impaired neurogenesis43. These differences in hormonal mechanisms suggest that sex-specific hormone environments may play an important role in neurological outcomes. Our results, which show a higher risk of Alzheimer’s disease in patients receiving ADT, support this idea and indicate that cognitive risks related to hormonal treatments should be considered on an individual basis44.
This study had some limitations that must be acknowledged. Due to the nature of observational studies, potential misclassifications in patient selection or identification of exposure and outcomes may exist. We aimed to minimize this misclassification by applying various inclusion criteria (records of diagnosis codes, registration codes, and prostate biopsies) when selecting patients with prostate cancer. Additionally, the risk of dementia could be underestimated because patients with mild dementia symptoms may not have been diagnosed with dementia, especially during cancer treatment. However, we assume that this would not alter our results because underreporting of outcomes may not occur differentially between the two groups. Confounding by indication is another potential limitation of this study. Men who received curative treatments such as prostatectomy are usually healthier and may be less likely to receive ADT. Although we adjusted for comorbidity using the CCI, some unmeasured factors related to treatment selection could remain. To mitigate this bias, we excluded patients who underwent orchiectomy from one year before the index date to the end of follow-up. In addition, because ADT exposure was defined using prescription records from claims data, we could not assess treatment adherence, early discontinuation, or intermittent use. Furthermore, Finally, residual confounding factors may remain, although we attempted to include and control for many clinical variables.
Despite these limitations, this study has several strengths. We used a nationwide database covering a large population to ensure the higher external validity of the results. Furthermore, we generated up-to-date, real-world evidence of the cognitive safety of ADT. It could be essential to ensure the risk of adverse events using the latest data that reflect changes in patient characteristics and treatment environment, such as the introduction of novel drugs (e.g., androgen receptor-targeted agents). We also applied propensity score matching and controlled for diverse variables to enhance comparability between ATD users and non-users. Previous studies have not considered a wide range of drugs used and primary treatments for prostate cancer, which could confound this association. In addition, we included medications commonly used for patients with prostate cancer, which may influence the risk of cognitive dysfunction, as a covariate in the model.
Conclusions
In conclusion, this population-based cohort study supports previous findings that ADT is associated with a higher risk of Alzheimer’s disease. However, ADT is unlikely to increase the risk of vascular dementia or Parkinson’s disease. An increased risk of Alzheimer’s disease in ADT users was observed regardless of the metastatic status. This updated evidence on the adverse cognitive effects of ADT will encourage physicians to cautiously monitor patients with prostate cancer undergoing ADT.
Data availability
The data that support the findings of this study are available from the Health Insurance Review and Assessment Service (HIRA) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of HIRA (opendata@hira.or.kr).
Abbreviations
- ADT:
-
Androgen deprivation therapy
- aHR:
-
Adjusted hazard ratio
- CI:
-
Confidence interval
- CCI:
-
Charlson Comorbidity Index
- DDD:
-
Defined daily dose
- HR:
-
Hazard ratio
- ICD-10:
-
International Classification of Diseases, tenth revision
- NHI:
-
National Health Insurance
- aSD:
-
Absolute standardized difference
- ARTA:
-
Androgen receptor-targeted agents
- COPD:
-
Chronic obstructive pulmonary disease
- PY:
-
Person-years
References
Cancer IAfRo. Cancer Today; Data visualization tools for exploring the global cancer burden in 2020, (2024).
Kang, M. J. et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2020. crt 55(2), 385–399. https://doi.org/10.4143/crt.2023.447 (2023).
Siegel, R. L. et al. Cancer statistics, 2023. CA Cancer J. Clin. 73(1), 17–48 (2023).
Perlmutter, M. A. & Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 9(Suppl 1), S3–8 (2007).
Desai, K., McManus, J. M. & Sharifi, N. Hormonal therapy for prostate cancer. Endocr. Rev. 42(3), 354–373. https://doi.org/10.1210/endrev/bnab002 (2021).
Gilbert, S. M., Kuo, Y. F. & Shahinian, V. B. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol. Oncol. 29(6), 647–653. https://doi.org/10.1016/j.urolonc.2009.09.004 (2011).
Nguyen, P. L. et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 67(5), 825–836. https://doi.org/10.1016/j.eururo.2014.07.010 (2015).
Son, S. W. et al. Testosterone depletion increases the susceptibility of brain tissue to oxidative damage in a restraint stress mouse model. J. Neurochem. 136(1), 106–117. https://doi.org/10.1111/jnc.13371 (2016).
Bianchi, V. E. et al. Androgen therapy in neurodegenerative diseases. J. Endocr. Soc. 4(11). https://doi.org/10.1210/jendso/bvaa120 (2020).
Kim, J. W. et al. Androgen deprivation therapy in patients with prostate cancer is associated with the risk of subsequent alzheimer’s disease but not with vascular dementia. World J. Mens Health. 40(3), 481–489. https://doi.org/10.5534/wjmh.210019 (2022).
Liu, J. M. et al. Association between androgen deprivation therapy and risk of dementia in men with prostate Cancer. Cancers (Basel). 13(15). https://doi.org/10.3390/cancers13153861 (2021).
Lonergan, P. E. et al. Androgen deprivation therapy and the risk of dementia after treatment for prostate cancer. J. Urol. 207(4), 832–840. https://doi.org/10.1097/ju.0000000000002335 (2022).
Park, J. et al. Validation of diagnostic codes of major clinical outcomes in a National health insurance database. Int. J. Arrhythmia. 20(1), 5. https://doi.org/10.1186/s42444-019-0005-0 (2019).
Shim, M. et al. Risk of dementia and parkinson’s disease in patients treated with androgen deprivation therapy using gonadotropin-releasing hormone agonist for prostate cancer: A nationwide population-based cohort study. PLoS One. 15(12), e0244660. https://doi.org/10.1371/journal.pone.0244660 (2020).
Tae, B. S. et al. Correlation of androgen deprivation therapy with cognitive dysfunction in patients with prostate cancer: A nationwide population-based study using the National health insurance service database. Cancer Res. Treat. 51(2), 593–602. https://doi.org/10.4143/crt.2018.119 (2019).
Yang, M. S. et al. Validation of cancer diagnosis based on the National health insurance service database versus the National Cancer registry database in Korea. Cancer Res. Treat. 54(2), 352–361. https://doi.org/10.4143/crt.2021.044 (2022).
Suissa, S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 16(3), 241–249. https://doi.org/10.1002/pds.1357 (2007).
Kalaria, R. N. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke 43(9), 2526–2534. https://doi.org/10.1161/strokeaha.112.655803 (2012).
Hurh, K. et al. Increased risk of all-cause, Alzheimer’s, and vascular dementia in adults with migraine in Korea: a population-based cohort study. J. Headache Pain. 23(1), 108. https://doi.org/10.1186/s10194-022-01484-y (2022).
Kwon, H. S. et al. Comparing the characteristics of patients with newly diagnosed dementia before and after 2008 in Seoul, South Korea: A population-based study. J. Clin. Neurol. (Seoul Korea). 18(6), 711–713. https://doi.org/10.3988/jcn.2022.18.6.711 (2022).
Austin, P. C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 10(2), 150–161. https://doi.org/10.1002/pst.433 (2011).
Coupland, C. A. C. et al. Anticholinergic drug exposure and the risk of dementia: A nested case-control study. JAMA Intern. Med. 179(8), 1084–1093. https://doi.org/10.1001/jamainternmed.2019.0677 (2019).
Mo, M. et al. Antidepressant use and cognitive decline in patients with dementia: a national cohort study. BMC Med. 23(1), 82. https://doi.org/10.1186/s12916-025-03851-3 (2025).
Khosrow-Khavar, F. et al. Androgen deprivation therapy and the risk of dementia in patients with prostate cancer. J. Clin. Oncol. 35(2), 201–207 (2017).
Krasnova, A. et al. Risk of dementia following androgen deprivation therapy for treatment of prostate cancer. Prostate Cancer Prostatic Dis. 23(3), 410–418 (2020).
Hinojosa-Gonzalez, D. E. et al. Androgen deprivation therapy for prostate cancer and neurocognitive disorders: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 1–13 (2024).
Chung, S. et al. Androgen deprivation therapy did not increase the risk of Alzheimer’s and Parkinson’s disease in patients with prostate cancer. Andrology 4(3), 481–485 (2016).
Hong, J-H. et al. Different androgen deprivation therapies might have a differential impact on cognition-An analysis from a population-based study using time-dependent exposure model. Cancer Epidemiol. 64, 101657 (2020).
Ng, H. S. et al. Development of comorbidities in men with prostate cancer treated with androgen deprivation therapy: an Australian population-based cohort study. Prostate Cancer Prostatic Dis. 21(3), 403–410 (2018).
Robinson, D. et al. Androgen deprivation therapy for prostate cancer and risk of dementia. BJU Int. 124(1), 87–92 (2019).
Deka, R. et al. Association of androgen deprivation therapy with dementia in men with prostate cancer who receive definitive radiation therapy. JAMA Oncol. 4(11), 1616–1617 (2018).
Yan, X. S. et al. Protective mechanism of testosterone on cognitive impairment in a rat model of Alzheimer’s disease. Neural Regen Res. 14(4), 649–657. https://doi.org/10.4103/1673-5374.245477 (2019).
Choi, Y. J. et al. Prevalence of dementia in Korea based on hospital utilization data from 2008 to 2016. Yonsei Med. J. 62(10), 948–953. https://doi.org/10.3349/ymj.2021.62.10.948 (2021).
Jhoo, J. H. et al. Prevalence of dementia and its subtypes in an elderly urban Korean population: results from the Korean longitudinal study on health and aging (KLoSHA). Dement. Geriatr. Cogn. Disord. 26(3), 270–276. https://doi.org/10.1159/000160960 (2008).
Azarpazhooh, M. R. et al. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimer’s Dement. J. Alzheimer’s Assoc. 14(2), 148–156. https://doi.org/10.1016/j.jalz.2017.07.755 (2018).
Ryan, C. J. et al. Management of patients with metastatic castration-sensitive prostate cancer in the real-world setting in the United States. J. Urol. 206(6), 1420–1429. https://doi.org/10.1097/ju.0000000000002121 (2021).
Braun, A. E. et al. Association between common urologic medications and onset of alzheimer’s disease and related dementias in men with prostate cancer managed by different primary treatment modalities. Urology 182, 161–167 (2023).
Hoyland, K. et al. Post-radical prostatectomy incontinence: etiology and prevention. Rev. Urol. 16(4), 181–188 (2014).
Jo, J. K. et al. Urinary continence after robot-assisted laparoscopic radical prostatectomy: the impact of intravesical prostatic protrusion. Yonsei Med. J. 57(5), 1145–1151. https://doi.org/10.3349/ymj.2016.57.5.1145 (2016).
Cho, I. S. et al. Statistical methods for elimination of guarantee-time bias in cohort studies: a simulation study. BMC Med. Res. Methodol. 17(1), 126. https://doi.org/10.1186/s12874-017-0405-6 (2017).
Brinton, R. D. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol. Sci. 30(4), 212–222. https://doi.org/10.1016/j.tips.2008.12.006 (2009).
Maki, P. M. & Henderson, V. W. Hormone therapy, dementia, and cognition: the women’s health initiative 10 years on. Climacteric: J. Int. Menopause Soc. 15(3), 256–262. https://doi.org/10.3109/13697137.2012.660613 (2012).
Rosario, E. R. et al. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol. Aging. 32(4), 604–613. https://doi.org/10.1016/j.neurobiolaging.2009.04.008 (2011).
Nead, K. T. et al. Androgen deprivation therapy and future Alzheimer’s disease risk. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 34(6), 566–571. https://doi.org/10.1200/jco.2015.63.6266 (2016).
Acknowledgements
We sincerely thank the Health Insurance Review and Assessment Service for providing valuable data (study number: M20230508001).
Funding
This study was supported by a grant from the Korea Institute of Drug Safety and Risk Management in 2023. The funding source was not involved in the study design; collection, analysis, and interpretation of data; writing of the manuscript; or decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
Conceptualization: H-LJ, CWJ, and HL; Data curation: EC and JY; Formal analysis: H-LJ, EC, S-hJ and CWJ; Funding acquisition: HL; Resources: JY; Supervision: CWJ and HL; Writing – original draft: H-LJ and JY; Writing – review & editing: EC, S-hJ, CWJ and HL. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Ajou University (202305-HB-EX-001), which waived the requirement for informed consent as only de-identified data were used.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jeon, HL., Choo, E., Jeong, Sh. et al. Risk of Alzheimer’s disease and Parkinson’s disease following androgen deprivation therapy in a real world nationwide cohort. Sci Rep 15, 23490 (2025). https://doi.org/10.1038/s41598-025-08279-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08279-6