Abstract
Regular physical activity (RPA) promotes cardiovascular health but its effects on blood flow and rheology characteristics remain unclear. This study compared hemorheological parameters between healthy adults who regularly jogged (n = 35) and sedentary controls (n = 35). Participants in the RPA group showed significantly lower fibrinogen levels, C-reactive protein concentrations, blood viscosity, plasma viscosity, and red blood cell aggregation (all p < 0.05). They also had higher hematocrit and albumin concentrations. Blood viscosity decreased by around 30% (native) and 49% (hematocrit-adjusted) in active individuals. These findings suggest regular jogging improves blood flow, potentially protecting against cardiovascular diseases.
Similar content being viewed by others
Introduction
Hemorheology is the subject of blood viscosity and contributes to clinical diagnosis and treatment decision in medicine. Viscosity is defined as the ratio of shear stress to shear rate which is reflect the fluidity of blood in the circulatory system1. There are many factors such as hematocrit, plasma viscosity, and attraction force between RBCs would influence blood resistance. Abnormal viscosity was found in a range of diseases, including cardiovascular disorders2,3, cerebral ischemia4, arterial hypertension5 and diabetes mellitus6. Blood fluidity can be measured using.
tradition rheometer7,8, which directly estimate the physical properties of blood.
Regular physical activity (RPA) is known to reduce cardiovascular risk in healthy individuals and improve blood fluidity potentially. Exercise programs have been shown to normalize pathological hemorheological status within the normal range9, and RPA is widely accepted as a therapeutic approach for cardiovascular patients7,10. Previous studies have also linked RPA to improve exercise self-efficacy and resilience and overall quality of life11,12, as well as enhanced cardiorespiratory and muscle metabolism through increased oxygen supply and cardiac output13,14.
Physical activity is characterized by intensity, duration, type, and frequency, with intensity being closely related to energy expenditure and oxygen consumption15. The World Health Organization (WHO) recommends that adults engage in at least 2.5 h of moderate-intensity physical activity per week16. RPA exercise has been associated with cardiovascular risk reduction, the specific effects of RPA on blood rheology in this population remain understudied. While some studies have explored the relationship between RPA and hemorheological parameters in patient populations or isolated physiological settings, few have systematically examined these effects in a healthy adult cohort17,18,19.
This study aims to compare the hemorheological profiles of healthy adults with and without long-term regular physical activity. It seeks to quantify differences in key parameters, including blood viscosity, RBC aggregation, and fibrinogen levels. The study tests the hypothesis that healthy adults engaging in regular activity exhibit lower blood viscosity and improved hemorheological profiles than sedentary individuals.
Materials and methods
Equity, diversity and inclusion statement
Our study aimed to include a diverse cohort of participants representing various demographics relevant to our research focus. The study population comprised healthy adults of different genders, ages, and socioeconomic backgrounds from urban and suburban areas of Taiwan. We recruited participants through various channels, including community centers and online platforms, to ensure broad representation.
Our research team consisted of 10 members, including cardiovascular doctors, anesthetists, nurses, academic professors from biomedical engineering, and graduate and undergraduate students. This diversity in academic and professional backgrounds ensured a range of perspectives throughout the research process. The team’s expertise spanned various fields, including exercise physiology, hematology, cardiovascular health, and biostatistics, bringing diverse viewpoints to the study design and data interpretation.
Subjects enrollment
Seventy participants were enrolled in this study to investigate the effects of moderate-intensity activity focused on jogging as the primary form on blood rheology. They were divided into two groups based on their lifestyle and exercise habits. Participants eligible for the regular exercise group were healthy adults aged between 20 and 40 years who performed jogging approximately 3000 m per session, 3 to 5 times per week, for at least one year. Their weekly average exercise time exceeded 180 min, meeting the WHO recommended exercise standard of at least 150 min per week. The exercise status and weekly jogging distances of participants in this group were verified through face-to-face interviews and confirmed by exercise logs maintained prior to enrollment. Conversely, participants included in the sedentary group were healthy adults aged between 20 and 40 years who engaged in minimal physical activity and no structured exercise or training within the past year. Their daily activities were predominantly limited to routine work-related tasks. The sedentary status of these participants was verified using detailed self-report questionnaires, specifically the validated Global Physical Activity Questionnaire (GPAQ), and confirmed by follow-up telephone interviews. Physical activity levels of all participants were evaluated using the Global Physical Activity Questionnaire (GPAQ, version 2, 2012), developed by the WHO20. The GPAQ quantifies physical activity in the metabolic equivalent task (MET)-minutes per week and records sedentary behavior in minutes per day. The GPAQ scoring system adhered to WHO guidelines, facilitating comparability with other studies using the standardized GPAQ instrument.
Participants in both groups were excluded if they smoked, had been diagnosed with cardiovascular, metabolic, inflammatory, or hematologic diseases, experienced recent acute illness or infection within the past month, or were taking medications known to affect cardiovascular or hematological parameters. This study protocol was approved by the Institutional Review Boards of Tri-Service General Hospital (1-104-05-032) and Academia Sinica (AS-IRB01-15037). All participants provided written informed consent prior to participation. All methods were carried out in accordance with the relevant guidelines and regulations.
Protocol
All participants were diagnosed as clinically healthy through annual health examinations and were not taking any medications. All trials were conducted during participants’ rest periods, not immediately following exercise. After obtaining informed consent, venous blood samples were collected into vacutainers (BD, Franklin Lakes, NJ, USA) containing ethylenediaminetetraacetic acid (EDTA) and trisodium-citrate at room temperature (24 ± 1 °C). All experiments were performed within two hours of blood collection21. Blood pressure and heart rate were measured and recorded using a sphygmomanometer. A questionnaire collected relevant information, including body mass index (BMI), exercise type, frequency, and lifestyle habits. Additionally, biochemical parameters such as fibrinogen, C-reactive protein (CRP), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were investigated and analyzed. To minimize potential confounding factors such as fasting, fatigue, and postprandial effects, all trials were conducted between 8:30 AM and 11:00 AM.
Blood rheological measurement
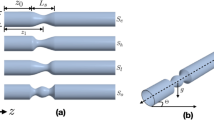
Whole Blood Viscosity (WBV) was measured using a stress-controlled Physica Rheometer MCR 501 (Anton-Paar, Graz, Austria), equipped with a titanium Concentric-Cylinder Measuring System (CC-MS). This rheometer employs a conventional rotation sweep method for viscosity measurement. The blood sample was contained in a cylindrical pot with a concentrically mounted bob in the measuring system, as shown in Fig. 1. Hemorheological measurements adhered to the standard protocol outlined by Baskurt et al.22. Blood viscosity was measured across a shear rate range of 0.1 to 1000 s− 1, with all measurements conducted at a constant temperature of 37 °C. To account for the effect of varying hematocrit levels on native blood viscosity, we calculated the WBV at a standardized hematocrit level of 45% using an empirical formula developed by Matrai et al.23,24. This adjusted viscosity, denoted as ηrel(45), eliminates the influence of individual hematocrit variations on native blood viscosity measurements.
RBC aggregation estimation
Blood microstructure at rest was characterized using optical microscopy (Nikon E100; Nikon Corp, Japan) with 10x magnification in a narrow-gapped slide chamber25. Images were captured within 5 min of sample preparation using a microscope connected to a CCD camera. Each blood sample underwent centrifugation for 10 min at 2000 rpm, after which RBCs were resuspended in autologous plasma at 1% hematocrit. Digital microstructure images of the diluted RBC suspension were analyzed using ImageJ software to obtain information on the morphology and size of RBC aggregates26,27. The average aggregation size (AAS) was used as the quantitative measure for this analysis.
Statistics analysis
Data were presented as mean ± standard deviation (SD) or median with interquartile range, depending on the normality of data distribution. Normality was assessed using the Shapiro-Wilk test. Statistical differences between groups were evaluated using the two-tailed Student’s t-test for normally distributed data or the Mann-Whitney U test for non-normally distributed data. Spearman’s rank correlation coefficients (r) were computed using the entire sample to assess the associations between hematocrit-adjusted blood viscosity (ηrel(45)) and biochemical parameters. A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, NY, USA).
Results
To fully understand the physiological benefits of physical activity, we compared hemorheological parameters between individuals with active and sedentary lifestyles. Table 1 presents a comparative analysis of laboratory parameters related to complete blood count (CBC) and biochemical profiles for the regular exercise (n = 35) and sedentary (n = 35) groups. there was no difference on blood pressure and heart rate. Table 1 also summarizes the baseline characteristics of the two groups. the regular-exercise group and the sedentary group were comparable in age (31.23 ± 5.21 vs. 29.68 ± 10.63 years, p = 0.68), BMI (22.09 ± 2.21 vs. 23.31 ± 3.09 kg/m², p = 0.77), height (170.2 ± 7.3 vs. 167.8 ± 6.9 cm, p = 0.24), and weight (63.9 ± 8.7 vs. 67.4 ± 9.5 kg, p = 0.52). Gender distribution was also similar (20 men/15 women vs. 16 men/19 women). these data indicate that the two cohorts were well matched for basic variables the laboratory data encompass a spectrum of biomarkers, including fibrinogen, hematocrit levels, C-reactive protein (CRP), and lipid profiles, specifically low-density lipoprotein (LDL) and high-density lipoprotein (HDL). the fibrinogen and CRP concentrations are significantly lower in the regular exercise group (p < 0.01). Additionally, hematocrit and albumin levels were significantly higher in the regular exercise group (p < 0.01). However, LDL and HDL concentrations and total protein levels did not show statistically significant differences between the two groups.
As depicted in Table 2, the GPAQ quantified physical activity in terms of Metabolic Equivalent of Task (MET) minutes per week, providing a comprehensive assessment of participants’ engagement in various forms of physical activity. Notably, the regular exercise group reported significantly higher levels of vigorous-intensity sports activities than the sedentary group. Figure 2 illustrates the microstructure of RBC aggregates in both groups. The regular exercise group exhibits smaller and more dispersed RBC aggregates, with individual cells and small clusters clearly visible. In contrast, the sedentary group shows larger and more compact aggregates, forming extended chain-like structures. The average aggregation size (AAS), indicated by the white arrow in the left image, is visibly smaller in the regular exercise group.
Figure 3 illustrates the results from whole blood viscosity (WBV) measurements conducted using a rheometer. These measurements were taken at native hematocrit (HCT) levels for both the regular exercise and sedentary groups across a range of shear rates. Notably, the regular exercise group exhibited a statistically significant reduction in WBV compared to the sedentary group at shear rates less than 0.5 s− 1.
Figure 4 depicts the hematocrit-adjusted viscosity (ηrel(45)) measurements for both groups, standardized to a reference hematocrit of 45% to eliminate individual variations in hematocrit levels. The adjustment was achieved using a specific correction formula, ensuring that the reported viscosity values are directly comparable regardless of each subject’s native hematocrit. As shown in Fig. 4, at lower shear rates (\(\:\dot{\gamma\:}\) < 2 s− 1), the sedentary group exhibited a significantly higher viscosity compared to the regular exercise group. These results indicate a difference in blood viscosity at low shear conditions between the two groups. Conversely, no significant difference in viscosity was observed between the two groups at shear rates higher than 2 s−1. This observation at elevated shear rates can be attributed to the shear-thinning behavior of RBCs, a typical rheological phenomenon wherein RBCs align and deform under shear stress, resulting in reduced resistance to flow.
Table 3 Summarizes the hemorheological properties and RBC aggregation characteristics for two group participants. Measurements include plasma viscosity at a high shear rate, native and hematocrit-adjusted viscosity at a low shear rate of 0.1 s− 1, as well as the average aggregation size (AAS) of rbcs. The data show that the regular exercise group displayed a significantly lower hematocrit-adjusted viscosity at 0.1 s− 1, approximately 25% lower than that of the sedentary group (p < 0.01). Similarly, plasma viscosity and the AAS of RBC aggregation were substantially reduced in the regular exercise group compared to the sedentary group (p < 0.01). These results indicate a less pronounced tendency for RBC aggregation in the regular exercise group. Finally, Table 4 presents the associations between hemorheological parameters and various biochemical markers from entire sample through spearman’s rank correlation analysis. A moderate positive correlation was observed between the relative viscosity at a standardized hematocrit of 45% (ηrel(45)) and fibrinogen concentration (r = 0.523, p < 0.001). Additionally, the aggregation size of rbcs.(AAS) also showed a statistically significant correlation with ηrel(45) to a lesser extent (r = 0.375, p < 0.001). However, correlations between ηrel(45) and other lipid profile components (CRP, LDL, HDL) were not statistically significant (p > 0.05).
Discussion
Our study demonstrates that RPA is associated with lower WBV and diminished RBC aggregation in young adults. This correlation appears to be linked to reduced fibrinogen concentrations observed in the regular exercise group. While previous research has explored the relationship between elevated viscosity in cardiovascular disease (CVD) patients and the benefits of physical activity28, our study examines the long-term effects of a physically active lifestyle on hemorheological parameters in healthy individuals. These findings suggest potential mechanisms by which regular endurance training may contribute to the reduced cardiovascular risk observed in regular physical activity individuals.
Our results are consistent with guidelines from the American Heart Association and the European Society of Cardiology, which recommend adults engage in at least 30 min of moderate-intensity physical activity (70–85% of maximal heart rate) three to five times per week29. Our study provides quantitative evidence of how long-term regular physical activity (RPA) modulates blood rheology, particularly in relation to viscosity and RBC aggregation. While previous research has demonstrated the benefits of short-term exercise interventions, fewer studies have systematically assessed these effects in individuals engaged in sustained, long-term exercise. These findings support the clinical importance of promoting long-term aerobic exercise as a non-pharmacological strategy to enhance microvascular circulation and reduce cardiovascular risk. This approach is supported by longitudinal studies showing that sustained RPA more effectively reduces cardiovascular morbidity and mortality30. Moreover, improvements in hemorheological parameters following prolonged RPA have been associated with a concomitant reduction in cardiovascular disease risk31. The present study examined healthy volunteers segregated into two distinct cohorts based on their engagement in long-term RPA. This research design addressed the limitations of previous studies that predominantly focused on patient populations32 or lacked defined duration and intensity of exercise protocols33.
Blood fluidity is modulated by many factors, primarily hematocrit level, fibrinogen concentration, and temperature24. This study demonstrated that subjects engaged in regular physical activity (RPA) exhibited significantly reduced blood and plasma viscosity compared to sedentary controls (p < 0.01), correlating with decreased plasma fibrinogen levels (r = 0.523, p < 0.001). Fibrinogen is a key determinant of both blood viscosity and RBC aggregation, and its reduction may serve as an upstream mechanism that influences these hemorheological parameters and improves blood flow. This finding aligns with prior research suggesting that an RPA lifestyle may enhance microvascular blood flow and nutrient delivery34. The native WBV was consistent with previous studies for healthy control subjects35. Interestingly, RPA subjects showed significantly elevated hematocrit and albumin levels (p < 0.01). Unlike endurance athletes who undergo significant plasma volume expansion as a long-term adaptation to prolonged high-intensity training, the regular physical activity individuals in our study may not have reached the exercise intensity or duration necessary to induce this effect. This increase is likely attributed to mild dehydration, resulting from chronic fluid loss during prolonged exercise36. It is important to note that the regular exercise group was recruited from an army school where all students adhere to a standardized diet. Consequently, dietary intake was uniform across the exercise group, minimizing the potential for diet-related confounding effects on the observed hemorheological parameters. Furthermore, moderate-intensity aerobic can lead to hemoconcentration and increased plasma protein levels due to fluid shifts and reduced plasma volume37. This adaptive response potentially serving as a protective mechanism against fluid imbalance and maintaining vascular integrity.
Current literature indicates that increased red blood cell (RBC) aggregation can negatively impact microcirculatory flow, particularly in microcapillaries where the luminal diameter is often smaller than RBCs, potentially impeding the passage of aggregated cells38. Our study found that individuals with an RPA lifestyle exhibited significantly reduced RBC aggregation (p < 0.01) compared to sedentary controls. Although blood viscosity and RBC aggregation are direct measures of blood fluidity, we emphasize fibrinogen levels as a potential mechanism because fibrinogen not only directly affects these parameters but is also an established marker of inflammation and thrombogenic risk. This quantitative evidence suggests that regular physical activity may influence the structural properties of RBC aggregates, potentially affecting blood flow characteristics. This reduction may contribute to enhanced blood fluidity and potentially optimize oxygen transport to musculature by reducing resistance to blood flow. This findings are consistent with previous studies reporting significant reductions in whole blood viscosity and RBC aggregation following moderate-intensity physical activity interventions in cardiovascular disease patients39. Nevertheless, there remains a paucity of research on the enduring impact of an RPA lifestyle on hemorheological properties32. Our investigation contributes by examining the implications of RPA on RBC aggregation. We observed lower RBC aggregation in the regular exercise group, likely facilitating more efficient blood transit through the microvasculature40. This observation is consistent with multiple studies that have established a correlation between cardiovascular risk and hemorheological abnormalities, such as increased RBC aggregation41,42. Heightened RBC aggregation has been associated with increased WBV and vascular resistance, potentially elevating the risk of cardiac events43,44. The Spearman correlation analysis showed that hematocrit-adjusted blood viscosity (ηrel(45)) exhibited middle to weaker correlations with fibrinogen (r = 0.523, p < 0.001), AAS (r = 0.375, p < 0.001), and CRP (r = 0.388, p = 0.091). This reduction in correlation strength may be attributed to the correction formula applied to adjust hematocrit to a standardized 45% (ηrel(45)), which effectively reduces inter-individual variability in hematocrit levels. Consequently, the adjusted viscosity values may not fully capture the natural hematocrit-dependent variations observed in WBV, potentially weakening the associations with hemorheological and biochemical parameters. Interestingly, despite the significant improvements in hemorheological parameters, we observed no significant differences in resting blood pressure between the groups. This dissociation supports the hypothesis that changes in blood viscosity and RBC aggregation may precede and possibly contribute to later increases in blood pressure.
This study provides insights into the potential mechanisms by which RPA contributes to cardiovascular health. We observed differences in blood rheology between regular exercise and sedentary individuals, suggesting that hemorheological adaptations may play a role in the cardiovascular benefits associated with long-term exercise habits. The 15% reduction in fibrinogen levels among physically active individuals suggests that changes in coagulation-related parameters may be linked to the cardiovascular benefits of physical activity. These findings support further research into the role of hemorheological markers as potential indicators of cardiovascular risk modification through moderate-intensity exercise interventions. In addition to lower fibrinogen levels, the regular exercise group also had about 30% lower native blood viscosity and nearly 50% lower hematocrit-adjusted viscosity than the sedentary group. These large differences suggest that long-term regular exercise may significantly reduce vascular resistance and subsequently improve blood fluidity. The limitation of this study is the self-reported questionnaires for quantifying physical activity levels, potentially introducing recall bias. Future research should consider incorporating objective measures such as heart rate monitoring and accelerometry to capture a more accurate representation of physical activity levels. Additionally, using a rheometer for blood viscosity measurements may not fully represent the complex in vivo environment of the circulatory system. Furthermore, the specific physiological mechanisms underlying the observed hemorheological changes should be further investigated45. While previous studies have only focused on specific populations (e.g., professional athletes46 and cardiovascular patients39), the generalizability of this study to broader populations remains limited. Moreover, this study was cross-sectional and cannot establish causal relationships between RPA and hemorheological adaptations. Therefore, longitudinal or intervention studies are required to confirm whether the hemorheological differences observed in this study are causally attributable to regular jogging behavior. Future studies would benefit from including participants with a wider range of training backgrounds and health conditions, using larger sample sizes to enhance statistical power, and exploring how different types and intensities of physical activity may affect hemorheological outcomes.
Conclusions
This study provides evidence that long-term engagement in regular physical activity is associated with favorable hemorheological adaptations in healthy young adults, including reduced blood viscosity, decreased RBC aggregation, and lower concentrations of fibrinogen and CRP (p < 0.01). Specifically, native and hematocrit-adjusted blood viscosity were approximately 30% and 50% lower, respectively, in the regular exercise group compared to sedentary controls. These differences highlight the substantial hemorheological benefits of sustained physical activity. However, further research is needed to establish causal relationships and elucidate the underlying mechanisms.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Baskurt, O. K. & Meiselman, H. J. Blood rheology and hemodynamics. In Seminars in thrombosis and hemostasis. Copyright© 2003 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New … 5–450.
Dintenfass, L. Clinical applications of blood viscosity factors and functions: especially in the cardiovascular disorders. Biorheology 16, 69–84 (1979).
Koenig, W. et al. Plasma viscosity and the risk of coronary heart disease: results from the MONICA-Augsburg cohort study, 1984 to 1992. Arterioscler. Thromb. Vasc. Biol. 18, 768–772 (1998).
Fisher, M. & Meiselman, H. J. Hemorheological factors in cerebral ischemia. Stroke 22, 1164–1169 (1991).
Presti, R. L., Hopps, E. & Caimi, G. Hemorheological abnormalities in human arterial hypertension. Korea-Australia Rheology J. 26, 199–204 (2014).
Le Devehat, C., Vimeux, M. & Khodabandehlou, T. Blood rheology in patients with diabetes mellitus. Clin. Hemorheol. Microcirc. 30, 297–300 (2004).
Reinhart, W. H., Dziekan, G., Goebbels, U., Myers, J. & Dubach, P. Influence of exercise training on blood viscosity in patients with coronary artery disease and impaired left ventricular function. Am. Heart J. 135, 379–382 (1998).
Thurston, G. B. Viscoelastic properties of blood and blood analogs. Adv. Hemodynamics Hemorheology. 1, 1–30 (1996).
Nikkilä, E. A., Viikinkoski, P., Valle, M. & Frick, M. Prevention of progression of coronary atherosclerosis by treatment of hyperlipidaemia: a seven year prospective angiographic study. Br. Med. J. (Clin Res. Ed). 289, 220–223 (1984).
Durstine, J. L., Gordon, B., Wang, Z. & Luo, X. Chronic disease and the link to physical activity. J. Sport Health Sci. 2, 3–11 (2013).
Pescatello, L. S. et al. Exercise and hypertension. Med. Sci. Sports Exerc. 36, 533–553 (2004).
Painter, P., Carlson, L., Carey, S., Paul, S. M. & Myll, J. Physical functioning and health-related quality-of-life changes with exercise training in Hemodialysis patients. Am. J. Kidney Dis. 35, 482–492 (2000).
Duncker, D. J. & Bache, R. J. Regulation of coronary blood flow during exercise. Physiol. Rev. 88, 1009–1086 (2008).
Vander, A. J., Sherman, J. H. & Luciano, D. S. Human physiology: the mechanisms of body function. New York, US: McGraw-Hill, ; 1998. (1990).
Courneya, K. S. & McAuley, E. Are there different determinants of the frequency, intensity, and duration of physical activity? Behav. Med. 20, 84–90 (1994).
Haskell, W. L. et al. Physical activity and public health: updated recommendation for adults from the American college of sports medicine and the American heart association. Circulation 116, 1081 (2007).
Nelson, A. J., Juraska, J. M., Musch, T. I. & Iwamoto, G. A. Neuroplastic adaptations to exercise: neuronal remodeling in cardiorespiratory and locomotor areas. Journal Appl. Physiology 99, 2312-2322 (2005).
Cakir-Atabek, H., Atsak, P., Gunduz, N. & Bor-Kucukatay, M. Effects of resistance training intensity on deformability and aggregation of red blood cells. Clin. Hemorheol. Microcirc. 41, 251–261 (2009).
Immanuel, S., Bororing, S. & Dharma, R. The effect of aerobic exercise on blood and plasma viscosity on cardiac health club participants. Acta Med. Indones. 38, 185–188 (2006).
Bull, F. C. & Maslin, T. S. Armstrong tjjopa, health: global physical activity questionnaire (GPAQ): nine country reliability and validity study. Journal of Physical Activity and health 6, 790–804. (2009).
Mvere, D. & Vinelli, E. Manual on the Management, Maintenance and Use of Blood Cold Chain Equipment (World Health Organization, 2005).
Baskurt, O. et al. Meiselman HJCh, microcirculation: New guidelines for hemorheological laboratory techniques. 42:75–97. (2009).
Matrai, A., Whittington, R., Ernst, E. J. C. H. & Microcirculation A simple method of estimating whole blood viscosity at standardized hematocrit. Clinical Hemorheology and Microcirculation 7, 261–265 (1987).
Wu, Y-F., Hsu, P-S., Tsai, C-S., Pan, P-C. & Chen, Y-L. Significantly increased low shear rate viscosity, blood elastic modulus, and RBC aggregation in adults following cardiac surgery. Sci. Rep. 8, 7173 (2018).
Assayag, E. B. et al. Erythrocyte aggregation as an early biomarker in patients with asymptomatic carotid stenosis. Dis. Markers. 24, 33–39 (2008).
Foresto, P., Arrigo, D., Carreras, M., Cuezzo, L. & Valverde, R. E. J, Rasia RJM-BA-: evaluation of red blood cell aggregation in diabetes by computarized image analysis. MEDICINA-BUENOS AIRES- 60:570–572. (2000).
Foresto, P. et al. Rasia rjjch, microcirculation: comparative analysis of aggregate shapes by digitized microscopic images. Application Hypertens. 26, 137–144 (2002).
Reinhart, W. H. et al. Influence of exercise training on blood viscosity in patients with coronary artery disease and impaired left ventricular function. American Heart Journal. 135, 379–382 (1998).
Levine, G. N., O’Malley, C. & Balady, G. J. J. T. A. Exercise training and bleed viscosity in patients with ischemic heart disease. The American journal of cardiology. 76, 80–81 (1995).
Ford, E. S. et al. Explaining the decrease in US deaths from coronary disease, 1980–2000. N. Engl. J. Med. 356, 2388–2398 (2007).
Kannel, W. B., D’Agostino, R. B. & Belanger, A. J. Fibrinogen, cigarette smoking, and risk of cardiovascular disease: insights from the Framingham study. Am. Heart J. 113, 1006–1010 (1987).
Dintenfass, L. & Lake, B. J. E. S. R. Exercise fitness, cardiac work and blood viscosity factors in patients and normals. European Surgical Research. 8, 174–184 (1976).
Brun, J-F., Varlet-Marie, E., Connes, P. & Aloulou, I. J. B. Hemorheological alterations related to training and overtraining. Biorheology. 47, 95–115. (2010).
Rosengren, A. et al. Social influences and cardiovascular risk factors as determinants of plasma fibrinogen concentration in a general population sample of middle aged men. British Medical Journal. 300, 634–638 (1990).
Chien, S., Usami, S. & Bertles, J. F. Abnormal rheology of oxygenated blood in sickle cell anemia. J. Clin. Investig. 49, 623–634 (1970).
Ernst, E. Changes in blood rheology produced by exercise. JAMA 253, 2962–2963 (1985).
Salmon, A. H. & Satchell, S. C. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J. Pathol. 226, 562–574 (2012).
Vicaut, E., Hou, X., Decuypere, L., Taccoen, A. & Duvelleroy, M. Red blood cell aggregation and microcirculation in rat cremaster muscle. Int. J. Microcirculation. 14, 14–21 (1994).
Sandor, B. et al. Effects of moderate aerobic exercise training on hemorheological and laboratory parameters in ischemic heart disease patients. PloS One. 9, e110751 (2014).
Pries, A., Secomb, T. W. & Gaehtgens, P. J. C. Biophysical aspects of blood flow in the microvasculature. Cardiovascular research 32, 654–667 (1996).
Kesmarky, G. et al. Hemorheological and oxygen free radical associated alterations during and after percutaneous transluminal coronary angioplasty. Clin. Hemorheol. Microcirc. 24, 33–41 (2001).
Marton, Z. et al. Follow-up of hemorheological parameters and platelet aggregation in patients with acute coronary syndromes. Clin. Hemorheol. Microcirc. 29, 81–94 (2003).
Fatah, K. et al. Haemostasis: proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thrombosis and haemostasis, 76, 535–540 (1996).
Lawrence, M. J. et al. Fractal dimension: a novel clot microstructure biomarker use in ST elevation myocardial infarction patients. Atherosclerosis. 240, 402–407. (2015).
Cossette, S., Frasure-Smith, N. & Lespérance, F. Clinical implications of a reduction in psychological distress on cardiac prognosis in patients participating in a psychosocial intervention program. Psychosom. Med. 63, 257–266 (2001).
Ernst, E. Influence of regular physical activity on blood rheology. Eur. Heart J. 8, 59–62 (1987).
Acknowledgements
The authors gratefully acknowledge Professor Yeng-Long Chen of the Physics of Active and Living Matter Group, Institute of Physics, Academia Sinica. He provided valuable guidance and support during the development of this work.
Funding
This work was supported by grants from the National Science and Technology Council [NSTC-112-2314-B-016-052, NSTC-113-2314-B-016-024 to P-S Hsu], and Tri-Service General Hospital, National Defense Medical Center in Taiwan [TSGH-C105-029, TSGH-D-113057, TSGH-D-114055 to P-S Hsu, and TSGH-D-112175 to J-L Chen], Taiwan, Republic of China. In addition, it was also supported by grants from Ming Chuan University Faculty Internal Research Project.
Author information
Authors and Affiliations
Contributions
Conception and design: Po-Shun Hsu, Chien-Sung Tsai Analysis and interpretation: Po-Shun Hsu, Jia-Lin Chen, Yi-Fan Wu, Wei-En HongData collection: Po-Shun Hsu, Yi-Fan Wu Investigation: Po-Shun Hsu, Jia-Lin Chen, Wei-En HongMethodology: Po-Shun Hsu Project administration: Po-Shun Hsu, Jia-Lin Chen, Resources: Po-Shun HsuSoftware: Po-Shun Hsu, Yi-Fan WuWriting the article: Yi-Fan Wu, Po-Shun Hsu Critical-revision and final approval of the article: Po-Shun Hsu.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statements and declarations
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Ethics approval
This study protocol was approved by the Institutional Review Boards of Tri-Service General Hospital (1-104-05-032) and Academia Sinica (AS-IRB01-15037). All participants provided written informed consent prior to participation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, YF., Chen, JL., Tsai, CS. et al. Effects of regular exercise on blood rheology. Sci Rep 15, 26128 (2025). https://doi.org/10.1038/s41598-025-08337-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08337-z