Abstract
Two types of bauxite lenses and horizons are found in the Jajarm mining area: A-type and B-type. The B-type bauxite lenses and horizons occur as stratiform orebodies between the shallow-marine platform carbonate of the early–middle Triassic Elika Formation and the siliciclastic and molassic sedimentary rocks of the late Triassic Shahmirzad Formation from the Shemshak Group. Lithium concentrations in the ore samples vary significantly between profiles I and II, ranging from 7.9 to 3780 ppm (mean 593.6 ppm) in profile I and 94.8 to 723 ppm (mean 392.9 ppm) in profile II. Ore samples with moderate Al2O3 contents (30–40 wt%) and moderate Al2O3/SiO2 ratios (< 2) show markedly higher Li and REE contents. This enrichment is likely due to the preferential uptake of REE and Li by clay minerals via isomorphic substitution and surface adsorption during kaolinization and early-stage lateritization. The Jajarm deposit is thus not only a major source of Al, but also holds significant potential as a strategic resource for critical metals, such as Li, TiO2, Ga, Nb, Ta, V, and REE. This highlights its importance as a key exploration target for future mining endeavors focused on these essential elements.
Similar content being viewed by others
Introduction
Increasing global demand for strategic metals has stimulated research on their prospecting, distribution, and enrichment mechanisms. In this context, karst-type bauxite deposits have been attracting attention as an important source of critical metals. Host of several critical elements, including Li, Ga, V, Ti, Zr, Hf, Nb, Ta, and rare earth elements (La–Lu, hereafter REE), gives further consideration to karst-type bauxite deposits, especially across the western Tethyan belt and Iran to the east1. Recent studies on karst bauxite deposits in China have led to valuable exploration strategies for Li2,3.
The Iranian bauxite deposits are hosted within karstic sinkholes developed in late Paleozoic and Mesozoic marine carbonate associated with the Palaeo- and Neo-Tethys Oceans4. According to the classification of bauxite deposits based on bedrock lithology and the global distribution of bauxite deposits by5 they are categorized as karst-type and belong to the Irano–Himalayan bauxite belt. These deposits have undergone a complex geodynamic evolution during greenhouse periods4. Recent studies reveal that the Iranian bauxite deposits are enriched in specific critical elements. For example, the Soleiman Kandi deposit in northwestern Iran is enriched in V, Co, Ga, and particularly Ta6; the Nasr-Abad deposit in the same region is enriched in V, Ga, and Ni7; and the Gano deposit in the eastern Alborz Mountains is enriched in Ga, V, and Nb8. These enrichments have been attributed to the contribution of mafic source rocks in the Soleiman Kandi and Nasr-Abad deposits, and to monzonitic rocks at the base of the Elika Formation in the Gano deposit. Despite the enrichment of critical metals in the Iranian bauxite deposits, there has been little attention given to large-scale metal extraction, which presents a significant opportunity for future economic exploitation.
The Alborz Mountains are a significant metallogenic zone, hosting large karst-type bauxite deposits, including the Jajarm and Gano deposits, along with several smaller karst bauxite deposits. Previous studies on the Jajarm deposit have primarily focused on the geochemical characteristics and ore genesis of the deposit9. In this study, we present new and comprehensive geochemical and mineralogical data from the Jajarm Li-rich bauxite deposit in northeastern Iran. The main aims of the research are to (1) define the distribution of critical metals, such as Ga, Nb, Ta, Li, and REE, within the bauxite horizons, (2) advance the understanding of the mineralization processes of these critical metals, and (3) identify factors controlling the concentration and enrichment of specific critical metals in the Jajarm ore samples. This study highlights the importance of clay-rich ore subunits at the Jajarm karst-type bauxite deposit for beneficial exploration of Li. From a resource-based perspective, such geochemical and mineralogical studies are expected to be valuable for similar future bauxite exploration projects in Iran and elsewhere.
Geological frameworks
Regional geology
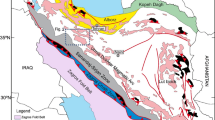
The Alborz Mountains in northern Iran represent a dynamic and complex geological region, which has undergone several tectono-metamorphic events and extensive deformations during the opening and closure of the Palaeo-Tethys and Neo-Tethys Oceans. Based on available paleomagnetic and paleontological data, the Iranian blocks were positioned along the northern margin of Gondwana during the Ediacara–late Paleozoic10,11. The opening of the Neo-Tethys Ocean across the Zagros suture during the late Paleozoic led to the detachment of the Iranian blocks, including the Central–East Iranian Microcontinent, the Alborz Mountains, and Northwestern Iran, from Gondwana12,13. The northward migration of the Cimmerian terranes to the southern margin of Eurasia resulted in the closure of the Paleo-Tethyan Ocean in the north during the middle–late Triassic Eo-Cimmerian orogeny, which led to the formation of the Alborz Mountains as a result of intense compressional forces12,13,14,15. This orogenic event transformed the Alborz Mountains from a passive margin into an underfilled (flysch-type) to an overfilled (molasse-type) peripheral foreland basin12. The Precambrian–middle Triassic rock units deposited along a passive continental margin setting, deformed during the Eo-Cimmerian orogeny13.
Geology of the deposit
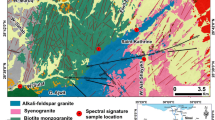
The Jajarm mining area, the largest bauxite deposit in Iran, is located 19 km northeast of Jajarm city in north Khorasan province, northeastern Iran. The deposit has a resource of 21 million tons with an Al2O3 grade of 46 wt% and an average Al2O3/SiO2 ratio of 3.6. This mining area is situated in the eastern Alborz Mountains, south of the Palaeo-Tethys suture (Fig. 1). The oldest rock units in the Jajarm mining area are gypsum and shale, with intercalations of sandstone of the early late Devonian Padeha Formation. These are followed by a 700 m-thick succession of brachiopod-bearing limestone, sandstone, and gray shale of the late Devonian Khosh Yeilagh Formation (Fig. 2). They are succeeded by the early Carboniferous Mobarak Formation, which consists of grayish, crinoid- and echinoid-bearing medium-bedded limestone and dolomitic limestone, along with yellowish, thick-bedded dolomite. The carbonate of the Mobarak Formation is capped by karst sinkholes and depressions accentuated by bauxite lenses and horizons. The A-type bauxite horizons occur as stratiform orebodies between the dark carbonate of the Mobarak Formation and the early–middle Triassic carbonate of the Elika Formation. The Triassic stratigraphic sequence includes a 200 m-thick succession of shale, red sandstone, and planolite-bearing thin-bedded limestone, followed by thin-bedded limestone and dolomitic limestone, and pea-colored, stromatolite-bearing, medium- to thick-bedded dolomite. Intraclastic dolomitic limestone of the Elika Formation suggests subaerial exposure and an intertidal environment. The carbonate of the Elika Formation has an NE–SW trend with an NW dip direction. At Jajarm, shallow-marine platform carbonate of the Elika Formation is also capped by karst sinkholes and depressions marked by bauxite lenses and horizons. The B-type bauxite lenses and horizons occur as stratiform orebodies between shallow-marine platform carbonate of the early–middle Triassic Elika Formation and coastal plain and shallow-marine shale interbedded with sandstone of the late Triassic Shahmirzad Formation from the Shemshak Group (Figs. 2, 3 and 4a). The B-type ores have an E–W trend and a total length of 26 km, with varying thickness from 10 to 75 m and colors ranging from light gray and off-white to deep red, multicolor, and off-white. From bottom to top, the Jajarm ore profile includes the lower bauxitic clay and clayey bauxite, Fe-rich bauxite, bauxite, and upper clayey bauxite (Fig. 4b).
(a) Simplified tectonic map of Iran showing the main tectonic domains16,17,18. (b) Simplified geological map of the Alborz Mountains13,19,20. The study area, marked with a yellow star, is located in the eastern Alborz Mountains. Map created by the authors using Adobe Illustrator CS6 (Version 10.0.0).
(a) General stratigraphic column of the eastern Alborz Mountains (modified after refs.14,20), and (b) local stratigraphy showing B-type bauxite lenses and horizons between carbonates of the Elika Formation and shale and sandstone of the Shahmirzad Formation (Shemshak Group) in the Jajarm deposit, North Khorasan Province, northeastern Iran. A = early Cimmerian unconformity; B = main Cimmerian unconformity.
The Shemshak Group consists of a 2050 m-thick succession of sandstone, grayish silty sandstone, light grayish siltstone, grayish shale, and fossiliferous limestone. Stratigraphically, the Shemshak Group includes the Shahmirzad, Alasht, Shirindasht, Fillzamin, and Dansirit formations of the Carnian–Bajocian age. The siliciclastic and molassic sedimentary rocks of the Shemshak Group, like carbonate of the Elika Formation, have a general NE–SW trend with a predominant NW dip direction. The slope angle of sediment strata of the Shemshak Group is variable, ranging from 30 to 70º. In the Jajarm area, siliciclastics of the Shahmirzad Formation are overlain by a 535 m-thick succession of silty sandstone and medium- to thick-bedded, litharenite sandstone, interbedded with siltstone of the Alasht Formation. These molasse-type sedimentary rocks are succeeded by shallow-marine sandstone and siltstone of the Shirindasht Formation, which are subsequently overlain by thick, deep-marine shale of the Fillzamin Formation during the Toarcian–Aalenian deepening pulse. The Dansirit Formation, the uppermost unit of the Shemshak Group, consists of 188 m of thick-bedded, fine-grained deltaic sandstone and, partly, thin-bedded siltstone. This succession records an upper delta-front regime, which slightly deepens upward14. The Dansirit Formation is disconformably underlain by shale of the Fillzamin Formation and disconformably overlain by a 250 m-thick succession of light greenish silty marl and alternating of medium-bedded ammonite-bearing limestone and greenish marl of the late Bajocian–middle Oxfordian Dalichai Formation during the Mid-Cimmerian orogeny. These disconformities reflect either rapid uplift and erosion at the base of the Dalichai Formation or a rapid subsidence pulse at its top, both of which indicate eustatic sea-level fluctuations14. The abrupt transition from shallow-marine sandstone of the Dansirit Formation to deep-marine marl of the Dalichai Formation signifies a marked increase in the subsidence rate, leading to a renewed phase of the late Bajocian–Bathonian deepening event12,14. These rock units are conformably overlain by chert-bearing, thick-bedded platform limestone and dolomitic limestone of the late Jurassic Lar Formation. These, in turn, are succeeded by the Cretaceous carbonate and the Quaternary deposits, including old and young terraces, river alluvium, and travertine.
Strike-slip faults with NW–SE and NE–SW trends and azimuths of less than 30º cause significant displacement of the bauxite ores as well as the Elika and Shahmirzad formations. These faults, predominantly dextral strike-slip faults, postdate thrust faults.
Sampling and analytical methods
Two bauxite profiles were chosen for sampling at an average vertical spacing of 0.5 m. In total, 20 samples were collected from profile I, while 19 samples were taken from profile II. The bulk samples were initially crushed to 60 mesh using a corundum jaw crusher, and then ground to less than 200 mesh with a tungsten carbide ring mill. Major and trace elements were analyzed at the ALS Chemex Laboratory in Guangzhou, China using Shimadzu 1800× X-ray fluorescence (XRF) and inductively coupled plasma mass spectrometry (ICP-MS), respectively. The powdered sample was placed in a Teflon bomb, moistened with a few drops of ultrapure water, and treated with 1.5 ml of HNO3 and 1.5 ml of HF. The sealed bomb was then heated at 190 °C for 48 h in an oven, followed by drying on a hotplate at ∼115 °C. The residue was subsequently dissolved in 1 ml of HNO3, evaporated to dryness once more, and then re-dissolved in 3 ml of 30% HNO3, with heating at 190 °C for an additional 24 h. The resulting solution was diluted with distilled water to 100 mg before analysis.
The mineralogical analysis of the powdered samples was conducted using an X’Pert PRO DY2198 diffractometer at the Geological and Environmental Institute of the China University of Geosciences, Wuhan. The operation conditions were 40 kV accelerating voltage, 40 mA beam current, Ni-filtered Cu-Kα radiation, a scanning range of 3° to 45° 2θ, a scan rate of 4° per minute, and a step size of 0.02° 2θ. Micromorphological and microchemical analyses were conducted using a MIRA3 field emission scanning electron microscope (FESEM) equipped with an energy-dispersive X-ray spectrometer (EDS) at the University of Kurdistan, Iran. The operation conditions were a 15 kV accelerating voltage, 1 nA beam current, and a beam diameter of 1 μm. Additionally, quantitative mineral abundances were determined using a TESCAN Integrated Mineral Analyzer (TIMA3 X GHM) system at the Nanjing Testech Technology Co., Ltd., China. This system combines a TESCAN MIRA3 Schottky field emission SEM with nine detectors, including four high-flux EDS detectors (EDAX Element 30) positioned at 90° intervals around the chamber. In this study, the dot mapping analysis mode was employed with X-ray counts set to 1200, a backscattered electron (BSE) pixel spacing of 3 μm, and an EDS dot spacing of 9 μm. Measurements were performed in a high-vacuum environment under the following conditions: 25 kV acceleration voltage, 9 nA beam current, and a working distance of 15 mm. The electricity and BSE signals were calibrated using a platinum Faraday cup, and EDS signals were calibrated with a manganese standard. TIMA automatically compared the measured BSE and EDS data for each phase against its database, distinguishing mineral assemblages and calculating their abundances.
Results
Mineralogical and textural features
The mineralogical composition of representative ore samples from the Jajarm bauxite deposit is summarized in Table 1 and shown in Fig. 5. While the overall mineral assemblage of the ores does not show significant variation, the mineralogical composition plays a key role in controlling the distribution and enrichment of certain critical metals. Diaspore, hematite, kaolinite, illite, and chlorite are the main constituents of representative ore samples, dependent to the geochemical and mineralogical characteristics of the ore subunits, accounting for more than 70% of the total chemical composition of the ores. Boehmite, anatase, analcime, calcite, and pyrite are present as accessory mineral phases in the Jajarm ore samples. In detail, from the bottom toward upward the profile I, the main constituents of representative ore samples change from illite and chlorite in the bauxitic clay samples, through kaolinite and hematite in the clayey bauxite samples, to diaspore in the bauxite samples as weathering degree intensifies. Anatase is present in all representative samples except sample J1, with content ranging from 1.89 to 7.12 wt%. An increase in diaspore content correlates with a decrease in kaolinite and an increase in anatase, suggesting that samples with higher diaspore content generally exhibit more intense weathering, along with elevated Al and Ti contents. Analcime with a content of up to 5.83 wt% occurs merely in the low-grade clayey bauxite and bauxitic clay samples at the basal parts of profile I. Calcite with as much as 35.44 wt% is found exclusively in the high-grade bauxite and low-Fe bauxite samples. On the other hand, pyrite occurs only in sample J20 with a significant content of 12.45 wt%.
PXRD patterns of representative ore samples from profile I of the Jajarm bauxite deposit, north Khorasan province, eastern Alborz Mountains, northeastern Iran. Major diffraction peaks are shown for phase identification. Y-axis intensities are omitted to avoid visual clutter, due to overlapping spectra. Ant = anatase, Bhm = boehmite, Cal = calcite, Chl = chlorite, Dsp = diaspore, Hem = hematite, Ilt = illite, Kln = kaolinite.
Geochemistry
Major oxides
Whole-rock major and trace element analyses of 39 ore samples from two profiles of the Jajarm deposit, along with elemental ratios, are given in Table 2. The ore samples from profile I are generally classified into four main categories: (i) clayey bauxite, (ii) bauxitic clay, (iii) low-Fe bauxite, and (iv) bauxite, which formed under moderate to strong lateritization. The bauxitic clays and clayey bauxites are situated adjacent to the underlying carbonate bedrocks of the Elika Formation and at the basal parts of the molassic sedimentary rocks of the late Triassic Shahmirzad Formation of the Shemshak Group, respectively. In profile II, the ore samples are categorized into four main categories: (i) bauxitic clay, (ii) clayey bauxite, (iii) bauxite, and (iv) Fe-rich bauxite, which have undergone week to strong lateritization and kaolinitization, as illustrated in the SiO2–Al2O3–Fe2O3 ternary diagram of5 in Fig. 6. The clayey bauxites in profile II are situated adjacent to the underlying carbonate bedrocks of the Elika Formation.
SiO2–Al2O3–Fe2O3 ternary diagram of5 showing the geochemical classification of the studied samples from two selected profiles of the Jajarm bauxite deposit, northeastern Iran.
The Jajarm ore samples predominantly consist of Al2O3, Fe2O3, and SiO2, which together account for more than 70 wt% of the total chemical composition in most samples, consistent with mineralogical analyses. The Al2O3 content ranges from 29.26 to 59.32 wt% in the ore samples from profile I and from 28.51 to 64.04 wt% in the ore samples from profile II. The Fe2O3 and SiO2 contents in the ore samples from profile I range from 0.79 to 27.37 wt% and 0.40 to 40.46 wt%, respectively, while they in the ore samples from profile II range from 1.41 to 40.02 wt% for Fe2O3 and 3.44 to 45.96 wt% for SiO2. TiO2 is the next most abundant component, ranging from 3.40 to 6.58 wt% in the ore samples from profile I and from 1.35 to 8.40 wt% in the ore samples from profile II. Other major oxides, including Na2O, K2O, CaO, MgO, MnO, and P2O5, together make up to 9.46 wt% of the chemical composition of the ore samples from profile I, except for sample J13 (21.3 wt%), and up to 10 wt% of the chemical composition of the ore samples from profile II. In profile I, SiO2 content in the bauxitic clays (40.46 wt%) and clayey bauxites (23.14–34.12 wt%, mean 30.35 wt%) is more than four times higher than in the Fe-rich bauxites (10.44 wt%), bauxites (3.89–9.67 wt%, mean 6.12 wt%), and low-Fe bauxites (0.40–8.78 wt%, mean 4.59 wt%), reflecting a high clay mineral content during the early stages of weathering (Fig. 7). Similarly, in profile II, SiO2 content in the bauxitic clays (41.17–45.96 wt%, mean 43.29 wt%) and clayey bauxites (21.2–38 wt%, mean 30.35 wt%) is more than double that in the bauxitic iron ores (15.07 wt%), bauxites (4.30–15.09 wt%, mean 9.70 wt%), low-Fe bauxites (8.29 wt%), and Fe-rich bauxites (3.44–15.20 wt%, mean 8.08 wt%). Conversely, the bauxites, Fe-rich bauxites, and low-Fe bauxites in both profiles exhibit higher Al2O3 and TiO2 contents, reflecting advanced weathering and bauxitization. The highest Fe2O3 content is observed in the Fe-rich bauxites of profile I (22.02 wt%) and the bauxitic iron ores of profile II (40.02 wt%) (Fig. 7).
Critical metals
Contents of Ga, Nb, and Ta in the Jajarm ore samples are in the range of 38.5–77.1 ppm Ga (mean 59.4 ppm), 103.5–208 ppm Nb (mean 156.4 ppm), and 6.2–12.5 ppm Ta (mean 9.3 ppm) across the entire profile I and 35.1–94.8 ppm Ga (mean 57.7 ppm), 26.7–264 ppm Nb (mean 145.1 ppm), and 1.9–15.8 ppm Ta (mean 8.8 ppm) across the entire profile II. In profile I, the Fe-rich bauxites, bauxites, and low-Fe bauxites exhibit slightly higher concentrations of these critical metals compared to the bauxitic clays and clayey bauxites. Similarly, in profile II, the bauxites and low-Fe bauxites have higher Ga, Nb, and Ta contents than the Fe-rich bauxites, bauxitic iron ores, bauxitic clays, and clayey bauxites (Fig. 8).
Lithium contents range from 7.9 to 3780 ppm (mean 593.6 ppm) in profile I and 94.8–723 ppm (mean 392.9 ppm) in profile II, both exceeding the cut-off grade of 0.05% and the industrial-grade cut-off of 300 ppm23. In profile I, the average Li content in the bauxitic clays (3780 ppm) and clayey bauxites (531–1025 ppm, mean 720.1 ppm) is significantly higher than in the Fe-rich bauxites (287 ppm), bauxites (68–324 ppm, mean 159.4 ppm), and low-Fe bauxites (7.9–201 ppm, mean 104.5 ppm). Similarly, in profile II, the clayey bauxites (438–631 ppm, mean 548.5 ppm) and bauxitic clays (94.8–723 ppm, mean 433 ppm) show higher Li concentrations than the Fe-rich bauxites (115.5–680 ppm, mean 308.9 ppm), bauxites (154–343 ppm, mean 248.5 ppm), bauxitic iron ores (232 ppm), and low-Fe bauxites (168.5 ppm) (Fig. 9). Lithium enrichment is predominantly observed in the clayey bauxites and bauxitic clays at the top and bottom portions of the studied bauxite profiles.
LREE (La–Sm) in the Jajarm ore samples vary, ranging from 146.45 to 1223.80 ppm in profile I and 110.72–753.90 ppm in profile II. In contrast, HREE (Eu–Lu) occur in lower concentrations, ranging from 5.70 to 126.10 ppm in profile I and 13.10–85.58 ppm in profile II. REE content is relatively high, ranging from 152.15 to 1349.90 ppm (mean 550.34 ppm) in profile I and 123.82–839.48 ppm (mean 480.91 ppm) in profile II. Notably, 26 samples exceed the industrial-grade cut-off of 300 ppm23. REE show an unevenly downward increasing trend across profile I, but follows an erratic pattern in profile II. In the profile I, the lower bauxitic clays and clayey bauxites exhibit higher concentrations of LREE, HREE, and REE contents compared to the Fe-rich bauxites, bauxites, and low-Fe bauxites. Conversely, in profile II, the clayey bauxites and low-Fe bauxites have higher concentrations of LREE, HREE, and REE than the bauxitic clays, Fe-rich bauxites, bauxites, and bauxitic iron ores (Fig. 9). The Ce anomaly values range from 0.32 to 21.60 in profile I and 0.75–6.23 in profile II. The (LREE/HREE)N and (La/Yb)N ratios range from 6.19 to 29.98 and 2.11 to 20.77, respectively, in profile I. In profile II, these ratios range from 4.50 to 10.95 and 2.29–21.45, respectively. Generally, the bauxitic clays in profile I exhibit higher (La/Yb)N and lower (LREE/HREE)N ratios compared to the clayey bauxites, Fe-rich bauxites, bauxites, and low-Fe bauxites. Similarly, in profile II, the clayey bauxites and bauxitic clays have higher (La/Yb)N ratio compared to other ore types, while the low-Fe bauxites and bauxitic iron ores exhibit the highest (LREE/HREE)N ratio (Fig. 10).
Discussion
Geochemical insights into the distribution of critical elements (Ga, nb, ta, and Li)
The Fe-rich bauxites, bauxites, and low-Fe bauxites in profile I, along with the bauxites and low-Fe bauxites in profile II, which are characterized by the elevated Al and Ti content, exhibit significantly higher concentrations of Ga, Nb, and Ta compared to other ore types. This enrichment is indicative of a higher degree of weathering, with the concentrations of these critical elements increasing progressively during weathering processes. In karst bauxite deposits, Ga shows a strong positive covariance with Al, particularly in low-water activity environments24,25. The stability of Ga under weathering conditions, combined with its ionic radius and charge being similar to those of Al, facilitates the enrichment of Ga in Al-rich mineral phases, such as diaspore, boehmite, and Al-rich hematite. This phenomenon has been consistently reported in previous studies5,8,26,27,28,29. The presence of Ga in these phases is further corroborated by strong positive correlations between Ga and Al in the Jajarm bauxite samples (r ≥ 0.79), suggesting that Al (oxyhydr)oxides are the primary hosts for Ga in this deposit.
Niobium and Ta exhibit a different geochemical behavior compared to Ga. These elements are predominantly associated with Ti-rich accessory minerals, such as rutile and anatase, which are resistant to intense weathering1,6,24,26,29. In the Jajarm bauxite deposit, strong positive correlations between Nb and Ta, and Ti (r ≥ 0.88) highlight the role of Ti-rich mineral phases, particularly anatase, in controlling the distribution of Nb and Ta. According to the literature29 the covariance of Ti with Nb and Ta represents that these critical metals are generally retained as less mobile elements during intense weathering and bauxitization. High concentrations of Ga, Nb, and Ta in the Jajarm bauxite deposit emphasize its potential as a valuable source of these critical elements. Specifically, diaspore-rich high-grade horizons are promising for Ga exploration, while anatase-rich horizons hold significant potential for Nb and Ta.
Higher Li content in the clayey bauxites and bauxitic clays in the Jajarm deposit aligns with mineralogical analyses, which show that these subunits contain more clay minerals than other ore types. As weathering progresses, Li content decreases from the clayey bauxites and bauxitic clays to the bauxitic iron ores, low-Fe bauxites, bauxites, and Fe-rich bauxites. This indicates that Li concentration generally diminishes with advancing weathering. The bauxitic clays at the base of profile I, near carbonate bedrocks, exhibit high Li contents compared to other ore types. In contrast, Li content along the profile II of the Jajarm bauxite deposit displays an irregular pattern of increase and decrease with depth. This erratic distribution of Li in bauxite deposits can be attributed to the heterogeneous chemical composition of parent rock(s), different type of clay minerals and variable contents of clay minerals, and the degree of weathering intensity and bauxitization processes. In profile I, Li distribution closely mirrors the SiO2 distribution, unlike profile II. In profile I, Li content increases proportionally with SiO2 content. However, in profile II, Li content initially increases with SiO2, plateaus between 15 and 40 wt% SiO2, and then decreases sharply beyond 40 wt% SiO2. Additionally, Li content in both profiles initially increases with Al2O3 content, reaching a peak at 30–40 wt% Al2O3, before decreasing unevenly. Notably, Li is concentrated in samples with low SiO2 contents (5–15 wt%), moderate Al2O3 contents (30–40 wt%), and moderate Al2O3/SiO2 ratios (less than 2; Fig. 11). High Li content in the clay-rich subunits of the Jajarm deposit is likely due to isomorphic substitution and the preferential adsorption of Li onto clay minerals during the early stages of weathering, when parent rocks decomposed under chemical weathering processes. This conclusion is supported by mineralogical evidence, which indicates limited Si leaching and the concurrent formation of kaolinite, a dominant mineral in most ore samples, during the early stages of bauxitization. The weathered materials subsequently accumulated in depressions and sinkholes within the carbonate bedrocks of the Elika Formation. They underwent intense lateritization, forming high-grade bauxite and low-Fe bauxite ores. However, the effects of alternating climatic conditions, seasonal groundwater fluctuations, and abundant rainfall during bauxitization must also be considered, as these factors significantly influenced the intensity of leaching processes, as noted by28. In tropical and subtropical climates, under warm and humid conditions, continuous desilicification and alumina enrichment lead to the decomposition of Li-bearing clay minerals into Al (oxyhydr)oxides, releasing Li from primary minerals into weathering solutions23. As a result, strongly weathered samples with high Al2O3 contents, high Al2O3/SiO2 ratios, and low Fe2O3 and SiO2 contents—such as the low-Fe bauxites and bauxites—are less viable for Li exploitation. Conversely, high Li content in the low-grade clayey bauxites and bauxitic clays of the Jajarm deposit makes these subunits suitable candidates for economically beneficial Li extraction.
A weak negative correlation between Li and Al2O3 in the ore samples from the studied profiles (r = − 0.16 for profile I and r = − 0.11 for profile II) suggests that Al (oxyhydr)oxides are unlikely to be significant host minerals for Li in the Jajarm mining area. Similarly, the negative correlations between Li and TiO2 (r = − 0.88 for profile I and r = − 0.02 for profile II) and between Li and Fe2O3 (r = − 0.88 for profile I and r = − 0.06 for profile II) indicate that Ti and Fe oxides also do not play a major role in hosting Li in the Jajarm ore samples. As weathering intensifies, Al content and Al (oxyhydr)oxides increase, while Fe content and Fe oxides decrease. This process correlates with a decrease in Li content in the Jajarm ore samples. However, the relationship between Li and K2O is more complex. When K2O content exceeds 2 wt%, Li content increases in the ore samples from profile I, but decreases in those from profile II. These variations, along with the observed correlations between Li and K2O or MgO, suggest that clay minerals like kaolinite and chlorite may act as host minerals for Li in certain ore samples. Post-bauxitization processes might also affect the distribution of Li in the Jajarm ore samples. In karst-type bauxite deposits, various clay minerals, including hectorite, smectite, kaolinite, montmorillonite, and Li-bearing chlorite (e.g., cookeite), are known to host Li23,30,31. In the Jajarm deposit, strong correlations between Li and clay minerals like illite and kaolinite in the low-grade, high-Li bauxitic clay and clayey bauxite samples highlight the complexity of Li uptake mechanisms.
Geochemical insights into the distribution of REE
Leaching and precipitation of REE in bauxite deposits depends on the pH of the weathering solutions. During weathering processes, the leaching and precipitation of these elements occur at low and high pH, respectively32,33. The rising groundwater level and the dissolution of the carbonate bedrock, promoting the pH of weathering solutions, can cause precipitation of these critical elements. Conversely, the fall of the underground water table and downward-percolating meteoric waters can facilitate the leaching of REE. It is believed that LREE are less likely to form fluid complexes compared to HREE, are instead retained in weathering products, such as phyllosilicates34. Mobilization and fractionation of REE in bauxite deposits occur under intensive chemical weathering and strong drainage conditions, typically in humid, tropical, or subtropical climates26. The distribution of REE within bauxite deposits is a crucial factor in evaluating an ore deposit as a potential REE resource35,36. During chemical weathering, REE released from primary minerals of the parent rock tend to be absorbed by certain mineral phases, including clay minerals, Fe and Mn (oxyhydr)oxides, and Ti oxides. Additionally, REE can be hosted by phosphate minerals and REE-bearing minerals. It is believed that the preferential adsorption of REE onto secondary minerals is highly dependent on the composition of soil solutions. As a result, the partitioning of REE between soil solutions and mineral surfaces is largely governed by adsorption and desorption processes, which are controlled by pH6,27,29,37.
In the bivariate plot of REE versus Al2O3, a distinct spike in REE contents is observed within the range of 30–40 wt% Al2O3 for the Jajarm ore samples. Specifically, samples with moderate Al2O3 contents (30–40 wt%) and Al2O3/SiO2 ratios (less than 2) show significantly higher REE contents compared to other ore types, suggesting the efficient role of clay minerals in concentration of REE (Fig. 12). Furthermore, the backscattered electron images and corresponding EDS elemental maps of the Jajarm ore samples reveal the spatial distribution of independent REE minerals, such as xenotime and monazite, within the clay matrix (Figs. 13 and 14). These findings emphasize that, even at low concentrations, authigenic REE minerals can significantly influence the distribution and fractionation patterns of REE in bauxite ores and should not be overlooked. Despite the high capacity of organic matter in the upper parts of the weathered profile to adsorb and chelate REE38 the successive decomposition of organic matter in the uppermost parts of the weathered profiles creates acidic conditions that promote the continuous downward leaching of REE from the parent rocks. Additionally, pyrite-bearing black shale and coal seams in the overlying molasse sediments contribute to the development of an acidic environment, thereby facilitating the leaching of metals. Although clay minerals are present in the uppermost portion of profile I, acidic solutions likely percolated from the REE-depleted ore subunits, formed by intense chemical weathering, towards the REE-enriched ore subunits formed during the early stages of weathering. These solutions eventually encountered the underlying carbonate of the Elika Formation. A gradual increase in soil pH, through continuous water–regolith interaction, favors the adsorption of REE onto clay minerals, particularly kaolinite, as previously outlined by39,40. At greater depths, near the underlying carbonate, the higher pH of soil solutions accelerates the complexation of REE with carbonate ligands, resulting in the retention and fixation of REE in the weathering environment40. Due to high content of clay minerals in the lower bauxitic clays and bauxitic clays, REE can be effectively absorbed onto clay minerals at the basal parts of profile I. LREE are more readily absorbed onto clay minerals than HREE, due to the larger ion radii of LREE41. Non-complexed HREE migrate downward toward the underlying carbonate of the Elika Formation, leading to concentration and enrichment of HREE at the basal parts of profile I. As the degree of weathering intensifies, with associated desilicification and alumina enrichment, the content of clay minerals gradually decreases, and REE concentrations in most Jajarm ore samples typically decrease. According to42 the erratic distribution of REE across profile II can be attributed to fluctuations in the groundwater table during bauxitization, accompanied by pH changes in the weathered environment, which influence the distribution and fractionation of REE28. noted that seasonal variations in precipitation during the rainy and dry seasons contribute to fluctuations in the groundwater table level.
The false-colored mineral phase map of sample J2 from the clayey bauxite subunit of profile I is shown in Fig. 15. TIMA analysis reveals that Ce is primarily concentrated in rutile (Fig. 15b and c), highlighting a significant correlation between LREE and Ti-bearing minerals. Furthermore, Sm + HREE are predominantly enriched in hematite (Fig. 15d–g). Geochemical data from the Jajarm profiles (I and II) further corroborate this observation, showing correlations between (Sm + HREE) and Fe2O3 (r = 0.58 in profile I and r = 0.48 in profile II). These results underscore the role of Fe oxides as a major carrier of Sm and HREE. This conclusion aligns with findings by43 who reported a similar association in bauxitic claystone at the base of the Longtan Formation in Zunyi, Guizhou Province of Southwest China, during the late Permian. Similarly44, demonstrated that Fe2O3 exhibited the strongest correlation with HREE enrichment and served as a key predictive factor in their model, emphasizing the critical role of Fe oxides in the geochemical behavior of HREE. The principles of cation exchange and adsorption mechanisms provide further insight into this phenomenon. The adsorption capacity of REE is directly proportional to their ionic radii. However, due to their smaller ionic radii, HREE are less readily adsorbed by mineral surfaces compared to LREE23. Instead, HREE preferentially form stable inner-sphere complexes, driven by their higher hydrolytic tendencies and stronger affinity for adsorption sites suited to smaller ionic radii1,23. Consequently, the enrichment of HREE is likely facilitated by inner-sphere complexation processes, which distinguish their geochemical behavior from that of LREE.
TIMA false-colored mineral phase map of sample J2 from the clayey bauxite subunit of profile I. (a) The full image of the polished thin section showing mineral assemblage of the bauxite ore, (b-c) close-up image showing Ce enrichment in Ti minerals, (d-e) close-up images showing Eu enrichment in hematite, (f-g) close-up images showing Er enrichment in hematite.
High (LREE/HREE)N and (La/Yb)N ratios reflect fractionation of LREE from HREE, driven by the preferential retention of LREE, which occurs concurrently with the extensive leaching of HREE. According to existing literature, fractionation of REE in karst bauxite deposits is primarily influenced by the Eh–pH conditions of the weathering environment. This process involves the leaching of REE from the parent rock(s) followed by the retention and fixation of these elements into secondary mineral phases and REE minerals40. The elevated (LREE/HREE)N and (La/Yb)N ratios observed in the Jajarm ore samples suggest a higher mobility of LREE compared to HREE during the weathering processes.
The studied profiles exhibit an irregular increase in Ce anomaly upward, indicating the preferential retention of Ce and the consequent fractionation and enrichment of Ce relative to other LREE. Specifically, the bauxitic iron ores, Fe-rich bauxites, low-Fe bauxites, and bauxites in the upper portions of the profiles display pronounced positive Ce anomalies compared to the clayey bauxites and bauxitic clays at the lower sections. Positive Ce anomalies in karst bauxite deposits can be attributed to several processes, including the scavenging of Ce by Fe or Mn (oxyhydr)oxides40 the precipitation of cerianite, due to the oxidation of Ce3+ to Ce4+—resulting in the decoupling of Ce from other REE40,45,46,47,48—and the precipitation of monazite-(Ce) under diagenetic conditions38. The oxidation of the depositional environment, coupled with the development of oxic fronts, is likely influenced by seasonal fluctuations in the groundwater Tables40,45,47. The enrichment of REE and the presence of negative Ce anomalies in the basal sections of profile I can be attributed to the scavenging of REE by Fe oxides from Ce-depleted percolating solutions. Additionally, the remobilization of Ce as fluoride complexes under acidic conditions in the upper sections of the profile, followed by the precipitation of Ce3+ near underlying carbonate bedrocks under alkaline pH conditions, further contributes to this phenomenon29,49. Furthermore, at the base of profile I, the reduction of Ce4+ to Ce3+ is likely linked to a decrease in Eh, potentially associated with a rising groundwater Table48. Conversely, positive Ce anomalies in the lower sections of profile II may result from the precipitation of parisite-(Ce)50.
In summary, the distribution and concentration of critical elements, such as Ga, Nb, Ta, Li, and REE in the Jajarm karst bauxite deposit are controlled by a combination of factors. These include the physicochemical conditions of the weathering environment (Eh–pH), climatic conditions, seasonal groundwater fluctuations, the chemical properties of elements, processes, such as adsorption and isomorphic substitution, the presence of weathering-resistant minerals, the chemistry of weathering solutions, the intensity of weathering, as well as diagenetic and epigenetic processes. While this study provides valuable new insights into the geochemical characteristics and enrichment patterns of critical metals in the Jajarm bauxite deposit, certain aspects remain open for further investigation. For example, additional mineralogical data could enhance our understanding of the mechanisms responsible for Li enrichment. Nonetheless, the current dataset provides a solid foundation for understanding the distribution of critical metals in the Jajarm deposit.
Assessment of critical metals in the Jajarm bauxite deposit compared to karst bauxite deposits in Iran
Understanding the distribution of critical metals in bauxite deposits is essential for evaluating their economic potential and guiding exploration efforts. This study compares the concentrations of critical metals, including TiO2, Al2O3, V, Co, Ga, Ta, Nb, Hf, LREE, and HREE, in the Jajarm bauxite deposit with those in karst bauxite deposits from northwestern Iran (e.g., Arbanos, Amir-Abad, Kani Zarrineh, Kanirash, Soleiman Kandi, Darzi-Vali, Sheikh-Marut, Shahindezh, Kanigorgeh, and Badamlu deposits) and the Alborz Mountains (e.g., Zan, Gheshlagh, Siahrudbar, Separdeh, and Gano deposits). The analysis presented in Fig. 16 reveals several key trends. The third quartile values of TiO2, Nb, Ta, V, LREE, and HREE in the Jajarm bauxite ores exceed those in the Alborz deposits and most northwestern Iranian bauxites. Similarly, Al2O3 and Ga concentrations are higher in the Jajarm bauxite ores compared to both regions, except for the Gano deposit. The median values of Hf in the Jajarm bauxite ores are lower than the third quartile in the Alborz and northwestern Iranian bauxite deposits, indicating variability in its distribution. For Co, the third quartile in Jajarm exceeds that in several deposits (e.g., Gano, Siahrudbar, Badamlu, Kanirash, Sheikh-Marut, Soleiman Kandi, Darzi-Vali, and Arbanos) but is lower than in others, such as Zan, Kanigorgeh, Kani Zarrineh, and Amir-Abad. Although V is typically associated with Fe (oxyhydr)oxide horizons6,8,24,51 these horizons do not significantly affect its concentration in the Jajarm deposit. This explains why the median values of Fe2O3 in Jajarm are generally lower than those in most deposits from both regions. Overall, the Jajarm bauxite deposit is distinguished by its enrichment in critical metals, such as TiO2, Al2O3, Nb, Ta, V, Ga, and REE, making it a highly promising target for further exploration. As a result, diaspore-, anatase-, and clay-rich horizons seem to be the primary hosts for critical metals like Ga, Nb, Ta, and REE, highlighting their potential for future resource development strategies.
Box and whisker plots showing the minimum, median, maximum, first, and third quartiles for Fe2O3 and critical metals (TiO2, Al2O3, V, Co, Ga, Ta, Nb, Hf, LREE, and HREE) in the Jajarm bauxite deposit, along with several other bauxite deposits in northwestern Iran and the Alborz Mountains. Data for the Jajarm deposit are from: Darzi-Vali51, Zan52, Arbanos27, Kanirash53, Amir-Abad26, Sheikh-Marut, Kanigorgeh, and Shahindezh54, Badamlu55, Gheshlagh, Siahrudbar, and Separdeh56, Kani Zarrineh24, Soleiman Kandi6 and Gano8. Aa = Amir-Abad, Ab = Arbanos, Bd = Badamlu, Dv = Darzi-Vali, G = Gano, Gh = Gheshlagh, J = Jajarm, Kg = Kanigorgeh, Kr = Kanirash, Kz = Kani Zarrineh, Sd = Shahindezh, Sk = Soleiman Kandi, Sm = Sheikh-Marut, Sp = Separdeh, Sr = Siahrudbar, Z = Zan.
Conclusions
Two types of bauxite lenses and horizons are found in the Jajarm mining area: A-type and B-type. The B-type bauxite lenses and horizons are located between the shallow-marine platform carbonate of the early–middle Triassic Elika Formation and the siliciclastic and molassic sedimentary rocks of the late Triassic Shahmirzad Formation from the Shemshak Group. The clayey bauxite and bauxitic clay samples in the lower parts of the studied profiles exhibit higher Li contents compared to the Fe-rich bauxite, bauxitic iron ore, bauxite, and low-Fe bauxite samples in the upper sections. This pattern indicates a gradual decrease in Li concentration as the degree of weathering progresses. Lithium is particularly enriched in samples with moderate Al2O3 contents (30–40 wt% Al2O3) and Al2O3/SiO2 ratios (less than 2), as opposed to the low-Fe bauxite and bauxite samples characterized by higher Al contents. This highlights a pronounced concentration and enrichment of Li during the kaolinization and weak lateritization stages. Similarly, most clay-rich ore samples show elevated REE contents, likely due to the presence of independent REE minerals and/or the preferential uptake of REE by clay minerals through isomorphic substitution and surface adsorption during the initial stages of weathering. Elevated Ga, Nb, and Ta contents in the Fe-rich bauxites, bauxites, and low-Fe bauxites of profile I, as well as in the bauxites and low-Fe bauxites of profile II, correlate with increasing concentrations of Al (oxyhydr)oxides and Ti oxides. This association reflects a more intense degree of weathering, leading to higher Ga, Nb, and Ta contents in these ore subunits. Elemental enrichment is driven by weathering intensity, host mineral phases, and physicochemical conditions like Eh–pH and adsorption mechanisms. The comprehensive evaluation of Li, TiO2, Ga, Nb, Ta, V, and REE in the ore samples underscores the significance of the Jajarm karst bauxite deposit as a key exploration and mining area for critical metals, in addition to its potential as an Al ore resource in the future.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Mongelli, G., Mameli, P., Sinisi, R., Buccione, R. & Oggiano, G. REEs and other critical Raw materials in cretaceous Mediterranean-type bauxite: the case of the Sardinian ore (Italy). Ore Geol. Rev. 139, 104559. https://doi.org/10.1016/j.oregeorev.2021.104559 (2021).
Deng, X. S. et al. Discovery and significance of Shixi bauxite-type lithium deposit in Guizhou Province. Geol. Rev. 69, 1–15. https://doi.org/10.1016/j.chemgeo.2020.119503 (2023).
Ling, K. et al. Lithium-rich claystone in Pingguo area, guangxi, Southwest china: precursor kaolinite controls lithium enrichment. Min. Depos. 59, 329–340. https://doi.org/10.1007/s00126-023-01210-x (2024).
Khosravi, M., Vérard, C. & Abedini, A. Palaeogeographic and geodynamic control on the Iranian karst-type bauxite deposits. Ore Geol. Rev. 139, 104589. https://doi.org/10.1016/j.oregeorev.2021.104589 (2021).
Bárdossy, G. Karst Bauxites (Bauxite Deposits on Carbonate Rocks) (Elsevier Scientific, 1982).
Abedini, A., Mongelli, G. & Khosravi, M. Geochemistry of the early jurassic Soleiman Kandi karst bauxite deposit, Irano–Himalayan belt, NW iran: constraints on bauxite genesis and the distribution of critical Raw materials. J. Geochem. Explor. 241, 107056. https://doi.org/10.1016/j.gexplo.2022.107056 (2022).
Abedini, A., Khosravi, M. & Mongelli, G. Critical metals distribution in the late Triassic–early jurassic Nasr-Abad bauxite deposit, Irano–Himalayan karst bauxite belt, NW Iran. Geochemistry 84, 107030. https://doi.org/10.1016/j.chemer.2023.126039 (2024).
Khosravi, M., Yu, W., Abedini, A. & Zhou, J. Geochemical constraints on the Gano karst bauxite deposit, Eastern Alborz mountains, Northern iran: implications for provenance, elemental mobility, and distribution of critical metals. J. Geochem. Explor. 262, 107468. https://doi.org/10.1016/j.gexplo.2024.107468 (2024).
Esmaeily, D., Rahimpour-Bonab, H., Esna-Ashari, A. & Kananian, A. Petrography and geochemistry of the Jajarm bauxite ore deposit, Northeast iran: implications for source rock material and ore genesis. Turk. J. Earth Sci. 19, 267–284. https://doi.org/10.3906/yer-0806-15 (2010).
Ghavidel-Syooki, M. Peri-Gondwanan acritarchs, chitinozoans, and miospores from paleozoic succession in the high Zagros mountains, Southern iran: regional stratigraphic significance and paleogeographic implications. Rev. Palaeobot Palynol. 292, 104457. https://doi.org/10.1016/j.revpalbo.2021.104457 (2021).
Ghavidel-Syooki, M. & Vecoli, M. Palynostratigraphy of middle cambrian to lowermost ordovician strata sequences in the high Zagros mountains, Southern iran: regional stratigraphic implications, and palaeobiogeographic significance. Rev. Palaeobot Palynol. 150, 97–114. https://doi.org/10.1016/j.revpalbo.2008.01.006 (2008).
Wilmsen, M., Fürsich, F. T., Seyed-Emami, K., Majidifard, M. R. & Taheri, J. The Cimmerian orogeny in Northern iran: tectono-stratigraphic evidence from the foreland. Terra Nova. 21, 211–218. https://doi.org/10.1111/j.1365-3121.2009.00876.x (2009).
Zanchi, A. et al. The Eo-Cimmerian (Late? Triassic) orogeny in North Iran. In South Caspian to Central Iran Basins (eds. Brunet, M. F., Wilmsen, M. & Granath, J. W.) 312 (Geol. Soc. Lond. Spec. Publ., 2009). https://doi.org/10.1144/SP312.3.
Fürsich, F. T., Wilmsen, M., Seyed-Emami, K. & Majidifard, M. The Mid-Cimmerian tectonic event (Bajocian) in the Alborz mountains, Northern iran: evidence of the break-up unconformity of the South Caspian basin. Spec. Publ Geol. Soc. Lond. 312, 189–203. https://doi.org/10.1144/SP312.9 (2009).
Şengör, A. C. The cimmeride orogenic system and the tectonics of Eurasia. Geol. Soc. Am. https://doi.org/10.1130/SPE195-p1 (1984).
Berberian, M. The Southern caspian: a compressional depression floored by a trapped, modified oceanic crust. Can. J. Earth Sci. 20, 163–183. https://doi.org/10.1139/e83-015 (1983).
Berberian, M. & King, G. C. P. Towards a paleogeography and tectonic evolution of Iran. Can. J. Earth Sci. 18, 210–265. https://doi.org/10.1139/e81-019 (1981).
Stöcklin, J. & Nabavi, M. H. Tectonic Map of Iran 1:250,000 (Geological Survey of Iran GSI, 1973).
Allen, M. B., Ghassemi, M. R., Shahrabi, M. & Qureshi, M. Accommodation of late cenozoic oblique shortening in the Alborz range, Northern Iran. J. Struct. Geol. 25, 659–679 (2003). -8141(02)00064 – 0.
Fürsich, F. T., Wilmsen, M., Seyed-Emami, K. & Majidifard, M. Lithostratigraphy of the upper Triassic–Middle jurassic Shemshak group of Northern Iran. Geol. Soc. Lond. Spec. Publ. 312, 129–160. https://doi.org/10.1144/SP312.6 (2009).
Soheili, M. & Sahandi, M. R. 1:100,000 Geological Map of the Sankhast (Geological Survey of Iran GSI, 1999).
Vaziri, H. & Shafiee, A. 1:100,000 Geological Map of the Robat-e-Qarhbil (Geological Survey of Iran GSI, 2001).
Wang, Z., Li, Y., Algeo, T. J., Yu, W. & He, X. F. Critical metal enrichment in upper carboniferous karst bauxite of North China craton. Min. Depos. 59, 237–254. https://doi.org/10.1007/s00126-023-01207-6 (2023).
Abedini, A., Mongelli, G. & Khosravi, M. Geochemical constraints on the middle triassic Kani Zarrineh karst bauxite deposit, Irano–Himalayan belt, NW iran: implications for elemental fractionation and parental affinity. Ore Geol. Rev. 133, 104099. https://doi.org/10.1016/j.oregeorev.2021.104099 (2021).
Putzolu, F. et al. Geochemical characterization of bauxite deposits from the abruzzi mining district (Italy). Minerals 8, 298. https://doi.org/10.3390/min8070298 (2018).
Abedini, A. & Khosravi, M. Geochemical constraints on the Triassic–Jurassic Amir-Abad karst-type bauxite deposit, NW Iran. J. Geochem. Explor. 211, 106489. https://doi.org/10.1016/j.gexplo.2020.106489 (2020).
Abedini, A., Khosravi, M. & Calagari, A. A. Geochemical characteristics of the Arbanos karst-type bauxite deposit, NW iran: implications for parental affinity and factors controlling the distribution of elements. J. Geochem. Explor. 200, 249–265. https://doi.org/10.1016/j.gexplo.2018.09.004 (2019).
Bárdossy, G. & Aleva, G. J. J. Lateritic bauxites. In Developments in Economic Geology, vol. 27 (Elsevier Scientific Publication, 1990).
Mongelli, G., Boni, M., Buccione, R. & Sinisi, R. Geochemistry of the Apulian karst bauxites (southern Italy): chemical fractionation and parental affinities. Ore Geol. Rev. 63, 9–21. https://doi.org/10.1016/j.oregeorev.2014.04.012 (2014).
Zhang, J. Y. et al. Provenance and ore-forming process of permian lithium-rich bauxite in central Yunnan SW China. Ore Geol. Rev. 145, 104862. https://doi.org/10.1016/j.oregeorev.2022.104862 (2022).
Zhao, H. et al. Geochemical features of lithium-rich bauxite from the Benxi formation in Qinyuan county, shanxi, china: insights into their depositional environment and lithium enrichment. Ore Geol. Rev. 163, 105780. https://doi.org/10.1016/j.oregeorev.2023.105780 (2023).
Aubert, D., Stille, P. & Probst, A. REE fractionation during granite weathering and removal by waters and suspended loads: Sr and Nd isotopic evidence. Geochim. Cosmochim. Acta. 65, 387–406. https://doi.org/10.1016/S0016-7037(00)00546-9 (2001).
Patino, L. C., Velbel, M. A., Price, J. R. & Wade, J. A. Trace element mobility during spheroidal weathering of basalts and andesites in Hawaii and Guatemala. Chem. Geol. 202, 343–364. https://doi.org/10.1016/j.chemgeo.2003.01.002 (2003).
Nesbitt, H. W. Mobility and fractionation of rare Earth elements during weathering of a granodiorite. Nature 279, 206–210. https://doi.org/10.1038/279206a0 (1979).
Berger, A., Janots, E., Gnos, E., Frei, R. & Bernier, F. Rare Earth element mineralogy and geochemistry in a laterite profile from Madagascar. Appl. Geochem. 41, 218–228. https://doi.org/10.1016/j.apgeochem.2013.12.013 (2014).
Sanematsu, K., Kon, Y., Imai, A., Watanabe, K. & Watanabe, Y. Geochemical and mineralogical characteristics of ion-adsorption type REE mineralization in phuket, Thailand. Min. Depos. 48, 437–451. https://doi.org/10.1007/s00126-011-0380-5 (2013).
Deluca, F., Mongelli, G., Paternoster, M. & Zhu, Y. Rare Earth elements distribution and geochemical behaviour in the volcanic groundwaters of Mount vulture, Southern Italy. Chem. Geol. 539, 119503. https://doi.org/10.1016/j.gexplo.2022.106962 (2020).
Abedini, A. & Khosravi, M. REE geochemical characteristics of the Huri karst-type bauxite deposit, Irano–Himalayan belt, Northwestern Iran. Minerals 13, 926. https://doi.org/10.3390/min13070926 (2023).
Coppin, F., Berger, G., Bauer, A., Castet, S. & Loubet, M. Sorption of lanthanides on smectite and kaolinite. Chem. Geol. 182, 57–68. https://doi.org/10.1016/S0009-2541(01)00283-2 (2002).
Li, M. Y. H., Zhou, M. F. & Williams-Jones, A. E. Controls on the dynamics of rare Earth elements during subtropical hillslope processes and formation of regolith-hosted deposits. Econ. Geol. 115, 1097–1118. https://doi.org/10.5382/econgeo.4727 (2020).
Maksimović, Z. & Pantó, G. Y. Contribution to the geochemistry of the rare Earth elements in the karst-bauxite deposits of Yugoslavia and Greece. Geoderma 51, 93–109. https://doi.org/10.1016/0016-7061(91)90067-4 (1991).
Mongelli, G., Buccione, R., Gueguen, E., Langone, A. & Sinisi, R. Geochemistry of the Apulian allochthonous karst bauxite, Southern italy: distribution of critical elements and constraints on late cretaceous Peri-Tethyan palaeogeography. Ore Geol. Rev. 77, 246–259. https://doi.org/10.1016/j.oregeorev.2016.03.002 (2016).
Zhou, J. et al. Provenance change and continental weathering of late permian bauxitic claystone in Guizhou province, Southwest China. J. Geochem. Explor. 236, 106962. https://doi.org/10.1016/j.gexplo.2022.106962 (2022).
Buccione, R., Ameur-Zaimeche, O., Ouladmansour, A., Kechiched, R. & Mongelli, G. Data-centric approach for predicting critical metals distribution: heavy rare Earth elements in cretaceous Mediterranean-type karst bauxite deposits, Southern Italy. Geochemistry 84, 126026. https://doi.org/10.1016/j.chemer.2023.126026 (2024).
Braun, J. J. et al. Ce anomalies in lateritic profiles. Geochim. Cosmochim. Acta. 54, 781–795. https://doi.org/10.1016/0016-7037(90)90373-S (1990).
Gamaletsos, P. N. et al. Nano-mineralogy and -geochemistry of high-grade diasporic karst-type bauxite from Parnassos–Ghiona mines, Greece. Ore Geol. Rev. 84, 228–244. https://doi.org/10.1016/j.oregeorev.2016.11.009 (2017).
Ma, J., Wei, G., Xu, Y., Long, W. & Sun, W. Mobilization and re-distribution of major and trace elements during extreme weathering of basalt in Hainan island, South China. Geochim. Cosmochim. Acta. 71, 3223–3237. https://doi.org/10.1016/j.gca.2007.03.035 (2007).
Mongelli, G. Ce anomalies in the textural components of upper cretaceous karst bauxites from the Apulian carbonate platform (southern Italy). Chem. Geol. 140, 69–79 (1997).
Radusinović, S. et al. Content and mode of occurrences of rare Earth elements in the Zagrad karstic bauxite deposit (Nikšić area, Montenegro). Ore Geol. Rev. 80, 406–428. https://doi.org/10.1016/j.oregeorev.2016.05.026 (2017).
Wang, X. et al. REE mobility and Ce anomaly in bauxite deposit of WZD area, Northern Guizhou. J. Geochem. Explor. 133, 103–117. https://doi.org/10.1016/j.gexplo.2013.08.009 (2013).
Khosravi, M., Abedini, A., Alipour, S. & Mongelli, G. The Darzi-Vali bauxite deposit, West Azarbaidjan province, iran: critical metals distribution and parental affinities. J. Afr. Earth Sci. 129, 960–972. https://doi.org/10.1016/j.jafrearsci.2017.02.024 (2017).
Calagari, A. A., Farahani, F. K. & Abedini, A. Geochemical characteristics of a laterite: the jurassic Zan deposit, Iran. Acta Geodyn. Et Geomater. 12, 67–77. https://doi.org/10.13168/AGG.2015.0001 (2015).
Abedini, A., Habibi Mehr, M., Khosravi, M. & Calagari, A. A. Geochemical characteristics of the karst-type bauxites: an example from the Kanirash deposit, NW Iran. Arab. J. Geosci. 12, 475. https://doi.org/10.1007/s12517-019-4601-z (2019).
Abedini, A., Mongelli, G., Khosravi, M. & Sinisi, R. Geochemistry and secular trends in the middle–late permian karst bauxite deposits, Northwestern Iran. Ore Geol. Rev. 124, 103660. https://doi.org/10.1016/j.oregeorev.2020.103660 (2020).
Abedini, A., Khosravi, M. & Dill, H. G. Rare Earth element geochemical characteristics of the late permian Badamlu karst bauxite deposit, NW Iran. J. Afr. Earth Sci. 172, 103974. https://doi.org/10.1016/j.jafrearsci.2020.103974 (2020).
Kiaeshkevarian, M., Calagari, A. A., Abedini, A. & Shamanian, G. Geochemical and mineralogical features of karst bauxite deposits from the Alborz zone (Northern Iran): implications for conditions of formation, behavior of trace and rare earth elements and parental affinity. Ore Geol. Rev. 125, 103691. https://doi.org/10.1016/j.oregeorev.2020.103691 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1812402), the Iran Alumina Company (IAC), and the State Key Laboratory of GPMR. Special thanks are extended to Dr. Rafal Marszalek and the Associate Editor for their valuable suggestions and editorial assistance, as well as to two anonymous reviewers for their critical insights and constructive feedback on this manuscript.
Author information
Authors and Affiliations
Contributions
Author contributionsMaryam Khosravi: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Validation, Visualization, Supervision, Data curation, Formal analysis, Project administration.Wenchao Yu: Writing – review & editing, Methodology, Data curation.Ali Abedini: Writing – review & editing.Jintao Zhou: Methodology, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khosravi, M., Yu, W., Abedini, A. et al. Geochemical constraints on critical metals in the Jajarm bauxite deposit in Northeastern Iran. Sci Rep 15, 25256 (2025). https://doi.org/10.1038/s41598-025-08487-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08487-0