Abstract
Some β-lactam antibiotics are hydrolyzed by the enzyme New Delhi metallo-β-lactamase 1 (NDM-1), increasing antibiotic resistance and posing a significant threat to public health. Thus, there is an urgent need to develop and use potent inhibitors of NDM-1 and other β-lactamases. This study evaluated the effects of the flavonoid morin (3,5,7,2′,4′-pentahydroxyflavone) on the antimicrobial activity of β-lactams and the interaction between morin and NDM-1. New NDM-1 inhibitors were identified by the structure-based virtual screening of natural product libraries. The interaction between morin and NDM-1 was evaluated by molecular docking. The impact of morin on β-lactam activity was assessed in vitro using checkerboard tests, kill-time kinetics, and broth microdilution. The effect of morin on the anti-inflammatory activity of meropenem (MEM) in BALB/c mice infected with NDM-1-producing Escherichia coli was evaluated by ELISA. The impact of morin and MEM on mouse tissue damage was assessed by hematoxylin and eosin staining. The interaction mechanisms between morin and NDM-1 were examined using molecular dynamics simulations. Molecular docking showed that morin inhibited NDM-1. Morin alone did not inhibit the growth of NDM-1-producing E. coli. However, morin combined with β-lactams suppressed bacterial growth and reduced their MIC 4- to 32-fold. Morin combined with MEM decreased tissue damag Morin enhanced antibacterial activity of β-lactams against NDM-1-producing E. coli when combined with MEM. These findings establish the foundation for using morin in developing potent inhibitors for NDM-1 and other β-lactamases to reduce antibiotic resistance.
Similar content being viewed by others
Introduction
β-lactams have potent and broad-spectrum antibacterial activity, little toxicity to mammalian cells, and few adverse effects. Consequently, these antibiotics are extensively used in clinical medicine and animal husbandry1. However, antibiotic overuse and misuse have resulted in bacterial resistance. Furthermore, several genes related to resistance against β-lactams and carbapenems have been found2.Carbapenems are among the most effective β-lactams antibiotics, which are frequently regarded as the last line of defense against bacterial infections3. However, certain bacteria have developed resistance to carbapenem antibiotics. The ineffectiveness of antibiotics against superbugs is primarily due to the presence of genes that encode β-lactamases in most superbugs4. The β-lactamases has the ability to hydrolyze nearly all antibiotics that contain β-lactam rings, which are the most effective and widely utilized antibiotics5. Antibiotic resistance is caused by the emergence and rapid spread of multidrug-resistant bacteria and resistance genes, posing a threat to the environment, food security, and public health.
Bacterial β-lactamases hydrolyze β-lactam rings, decreasing the effectiveness of antibiotics and increasing bacterial resistance6. Based on the differences in the terminal amino acid sequences of the β-lactamases molecular structure, they can be divided into Serine-β-lactamases (SBLs) and Metallo-β-lactamase (MBLs).Sulbactam and clavulanic acid are effective inhibitors for SBLs and have been approved by FDA, which can effectively alleviate the generation of bacterial resistance7. However, compared with SBLs, the hydrolysis of MBLs is more extensive, and SBLs inhibitors have no inhibitory activity on MBLs, so the bacterial drug resistance caused by MBLs is more serious.
Based on the similarity of amino acid sequences and zinc coordination in binding pockets, MBLs have been classified into three subclasses: B1, B2, and B3. Most clinically relevant MBLs belong to subclass B18 .Among these, B1 subclass MBLs pose the greatest threat to clinical β-lactam antibiotic therapy due to their broad substrate hydrolysis capabilities, with New Delhi Metallo-β-lactamase-1 (NDM-1) being of particular concern9,10,11.However, there are no approved NDM-1 inhibitors for use as antibacterial agents, which remains imperative to develop novel NDM-1 inhibitors.
Currently, the strategic approaches for addressing infections caused by NDM-1-producing positive bacteria primarily encompass two main strategies. On one hand, the screening and subsequent combination of specific inhibitors that target the NDM-1 resistance enzyme with β-lactam antibiotics, which serves to safeguard the structural integrity of β-lactam antibiotics against degradation and enhances their antibacterial efficacy through a synergistic effect. On the other hand, the design of novel antibiotics that remain unaffected by NDM-1-mediated hydrolysis, although this approach is characterized by a prolonged development timeline and substantial financial investment. Given the favorable therapeutic index and minimal toxicity associated with β-lactam antibiotics, the pursuit of efficacious NDM-1 inhibitors for use in conjunction with these agents represents the most viable and cost-effective strategy.
Natural bioactive compounds have high structural diversity and multiple modes of action, allowing screening for novel antimicrobial agents and activities12. morin is a potential treatment for Dox-resistant breast cancer and that it does so by inducing DNA damage and modulating the LKB1/AMPK/mTORC1 pathway, along with regulating the MAPK, and Akt/GSK3αβ signaling pathways13,14. Morin can serve as potential inhibitors of Histidinol-Phosphate Aminotransferase in Drug-Resistant Acinetobacter baumannii15.Morin (3,5,7,2′,4′-pentahydroxyflavone) is a polyphenol isolated from the aerial parts of plants of the Moraceae family, including mulberries and Figs16. Morin has antibacterial17,18,19anti-inflammatory20,antioxidant21,22,glucose-lowering23and neuroprotective24 activity. Further, morin could treat chronic obstructive pulmonary disease by regulating autophagy25. Nonetheless, the effectiveness of morin in inhibiting NDM-1 is unknown.
The objective of this study was to utilize computer-aided drug design technology for the virtual screening of NDM-1 potential inhibitors through molecular docking. The NDM-1 inhibitor morin was verified by assessing the enzyme activity of the candidate inhibitors and the antibacterial activity both in vitro and in vivo when combined with MEM. The efficacy and synergistic effect of the combination of morin in vitro and in vivo was targeted in this study. This study provided a research basis and a novel strategy for the further development of specific inhibitors for “superbug” NDM-1 and the prevention and treatment of drug-resistant bacteria which held significant theoretical and practical value.
Materials and methods
Bacterial strains and chemicals
The NDM-1-positive E. coli isolates originated from Laboratory of phage Research Center, Liaocheng University. MEM (≥ 95% pure) and other ligand molecules (≥ 98% pure) were purchased from Target Mol, USA. Mouse IL-2 ELISA KIT and Mouse IL-6 ELISA KIT were purchased from Shanghai Enzyme Linked Biotechnology Co., Ltd.
Virtual screening and molecular docking
We established a virtual screening platform based on molecular docking by searching a natural compound library with over 30,000 ligands. Novel NDM-1 inhibitors were screened using the crystal structure of NDM-1 as the target protein. Molecular docking was performed using Glide (Glide, Schrödinger, LLC, New York, NY, USA) and Maestro (Maestro, Schrödinger, LLC, New York, NY, USA) to evaluate the strength of protein-ligand interactions. Initially, we employed computer simulation technology to screen a group of candidate ligands with potential antibacterial activity based on their chemical structures and physical properties. Subsequently, we utilized molecular docking techniques to assess the interactions between the candidate ligands and the active site of the NDM-1 enzyme, evaluating both the strength and affinity of these interactions. Binding free energy was calculated utilizing G scores and the following equation:
where Site is the polar interaction at the active site, Lipo is the hydrophobic interaction, Metal is the metal-binding term, Evdw is the van der Waals force, and Ecoul represents the electrostatic interaction.
Antimicrobial susceptibility testing
Three representative β-lactams—ampicillin sodium, ceftiofur sodium, and MEM—were selected based on structural classification and clinical application. The minimum inhibitory concentrations (MICs) against NDM-1-producing E. coli were determined by broth microdilution following Clinical and Laboratory Standards Institute guidelines. The synergistic effects between morin and antibiotics were assessed using checkerboard assays. The fractional inhibitory concentration index (FICI) was measured using the formula FICI = [MIC (morin + compound A)/MIC compound A] + [MIC (morin + compound B)/MIC compound B]. FICI values were scored as ≤ 0.5 (synergism), 0.5-2.0 (no effect), and > 2.0 (antagonism)26.
Time-kill assays
Isolated colonies of NDM-1-positive E. coli were inoculated in Mueller-Hinton broth and grown at 37 °C for 6–8 h. The culture was collected, and cell concentration was adjusted to 106 CFU/mL. The experimental setup included samples containing antibiotics (antibiotic group), no antibiotics (control group), morin alone (morin group), and morin combined with antibiotics (combination group). The antibiotic and control groups were maintained at 37 °C with identical bacterial concentrations. The culture medium from each treatment was collected, diluted, and plated at 0, 1, 3, 5, 7, 9, and 11 h. Colonies were counted after an incubation period of 18–24 h at 37 °C. Time-killing curves were generated by plotting the logarithm of the number of colonies on the Y-axis and the incubation period on the X-axis.
Animal experiments
Specific-pathogen-free, 6-week-old female BALB/c mice were purchased from Jinan Pengyue Experimental Animal Breeding Co., Ltd. (Jinan, China). Female mice were used to maintain consistency with prior studies on NDM-1–mediated resistance and to minimize hormonal effects on immune responses27,28 .The mice were housed in an animal facility with a relative humidity of 55–65% and a temperature of 20–25 °C under a 12-h/12-h light/dark cycle with ad libitum access to food and water. The animal experiments were approved by the Animal Care and Use Committee of Liaocheng University. After a 7-day acclimation period, mice were randomly assigned to five groups (five animals per group) as follows: (1) healthy control (not infected with E. coli), (2) infection control (infected with E. coli), (3) morin-treated group (infected and then treated with morin), (4) MEM-treated group (infected and then treated with MEM alone), and (5) morin and MEM-treated group (infected and then treated with morin and MEM). All groups, except healthy controls, were intraperitoneally injected with 100 µL of a sublethal concentration of NDM-1-positive E. coli (1.0 × 107 CFU/mL). The bacterial counts in vital organs were assessed at 2 h post-infection to confirm infection establishment before therapy initiation. Two hours after infection, morin (100 µL, 10 mg/kg) or morin + MEM (10 mg/kg) was injected intraperitoneally, and MEM (10 mg/kg) was injected subcutaneously for a total of five doses at 12 h intervals29. Healthy controls received 100 µL of sterile saline intraperitoneally. After 72 h of infection, mice were anesthetized with ketamine and xylazine, and blood was collected from the retro-orbital plexus. Then, mice were humanely euthanized through cervical dislocation.
White blood cell (WBC) count was measured using an auto hematology analyzer (BC-2800-Vet, Mindray, Shenzhen, China). Serum samples were centrifuged at 5000 rpm for 10 min at 4 °C. The serum levels of inflammatory cytokines TNF-α, IL-2, and IL-6 were measured by enzyme-linked immunosorbent assay (ELISA). The liver was excised under sterile conditions, and morphological changes were examined. The tissues were homogenized in three volumes of ice-cold saline using a tissue homogenizer. Homogenates were sonicated for 1 min and centrifuged at 12,000 rpm for 2 min at 4 °C. The supernatants were diluted serially and plated on LB agar plates containing 2 µg/mL MEM. The samples were cultured for 24 h at 37 °C, and bacterial colonies were counted.
Histological analysis
For histopathological, liver, spleen and intestinal tissues were fixed in 10% formaldehyde, embedded in paraffin, serially sectioned at a thickness of 4 μm, and stained with hematoxylin and eosin (H & E)30.
MD simulations
MD simulations based on the interaction between morin and NDM-1 were performed using the GROMACS 2018 molecular dynamics package. NDM-1 had the lowest free energy. A simulation box (10 × 10 × 10 nm2) was prepared using the TIP3P water model, and a minimum distance of 12 Å was kept between the box boundary and the NDM-1 protein surface. High-energy-density molecules in their native conformation were removed using a steepest descent algorithm over 50,000 steps to minimize energy. The system was equilibrated in a canonical ensemble (NVT) followed by an isothermal-isobaric ensemble (NPT) to maintain stable temperature and pressure conditions during the simulations. Electrostatic interactions during equilibration were controlled using the particle mesh Ewald method with a cutoff distance of 1 nm for Coulomb and van der Waals forces.
A temperature of 300 K and a pressure of 1 bar were used in 100 ns simulations. Temperature and pressure were maintained constant using the V-rescale and Berendsen method, respectively. A threshold of 10 Å was set for non-bonded interactions to improve computational efficiency. To assess structural stability, local flexibility, and changes in secondary and tertiary structures, GROMACS modules were used to generate plots of root mean square deviation (RMSD), radius of gyration (Rg), root mean square fluctuation (RMSF), and secondary structure. Protein-ligand binding free energies were computed using the Gmx_MMPBSA tool.
Statistical analysis
Statistical analysis was performed using SPSS version 22 (IBM Corp., Armonk, NY, USA). Data were expressed as means ± standard deviations. The significance of differences between means was assessed by one-way analysis of variance or an unpaired t-test. A p-value of less than 0.05 indicated statistical significance.
Results
Identification of morin as an NDM-1 inhibitor by virtual screening
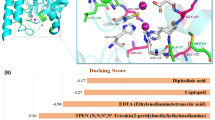
By comparing their inhibitory effects on NDM-1 enzyme activity, we identified morin as the primary focus of this study. The free binding energy of morin and NDM-1 was less than − 10.0 Kcal/mol. Morin binds to zinc ions in the active site of NDM. The stability of the protein-ligand complex is consistent with the 3D structure of the active site, allowing strong interactions with hydrophobic amino acids in the active site (Fig. 1A, B).
Morin enhances the antibacterial activity of β-lactams
The effect of morin on the antibacterial activity of β-lactams against NDM-1-positive E. coli was assessed by broth microdilution (Table 1). The MIC of morin, MEM, ceftiofur sodium, and ampicillin sodium was 512, 64, 128, and 256 µg/mL. Morin alone exhibited a high MIC of 512 µg/mL, indicating negligible standalone antibacterial activity at clinically relevant concentrations. However, morin combined with β-lactams suppressed bacterial growth and reduced their MIC 4- to 32-fold. Bacterial growth was inhibited by morin combined with MEM, ceftiofur sodium, or ampicillin sodium, with FICI values of 0.094, 0.375, and 0.156 (Table 1).
Morin enhances the antibacterial efficacy of β-lactams
The synergistic effect of morin and β-lactams on bacterial growth inhibition was evaluated using checkerboard and time-kill assays (Fig. 2A, B). The results showed that MEM, ceftiofur sodium, and ampicillin sodium inhibited bacterial growth in a concentration-dependent manner. morin dose-dependently potentiated this effect (Fig. 2A). MEM (2 µg/mL), ceftiofur sodium (32 µg/mL), and ampicillin sodium (8 µg/mL) had the strongest inhibitory effect at 5 h after culture, and 32–64 µg/mL morin enhanced this effect (Fig. 2B). These results indicate that morin potentiates the antimicrobial activity of β-lactams.
Effect of meropenem (MEM) and morin on inflammatory responses induced by bacterial infections in mice. (A) Schematic representation of the therapy experimental procedure in five groups, illustrating the timeline and key steps including treatment administration and subsequent analyses. (B) Effect of MEM and morin on white blood cell counts in mice intraperitoneally injected with NDM-1-producing Escherichia coli (n = 3). (C) Effect of MEM and morin on the serum levels of inflammatory markers (n = 3). (D) Impact of MEM and morin on bacterial burden in the mouse liver (n = 5). ns: not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001.
Morin enhances the therapeutic effects of MEM on inflammatory responses induced by bacterial infections in vitro.
The effect of morin on inflammatory responses was evaluated in vitro (Fig. 3A). The intraperitoneal injection of NDM-1-positive E. coli (107 CFU/mL) significantly increased WBC counts in BALB/c mice. In contrast, MEM in the presence or absence of morin reversed this effect (Fig. 3B). Bacterial infection elevated the serum levels of inflammatory markers TNF-α, IL-2, and IL-6; MEM mitigated this impact, while morin enhanced the antibacterial activity of MEM (Fig. 3C). Moreover, MEM in the presence or absence of morin reduced bacterial load in the liver of infected mice (Fig. 3D).
Morin and MEM reduce tissue damage
The effects of treatments on the morphology and histology of the mouse liver, spleen, and intestine 72 h after infection are shown in Fig. 4A and B. Bacterial infection decreased liver pigmentation and caused necrotic lesions; morin and MEM reversed these effects (Fig. 4A). In healthy controls, liver cells were arranged regularly and densely. The spleen had regularly arranged oval-shaped cells and a clear border between the white and red pulp. Intestinal villi were well-defined and intact. In infected mice, neutrophil infiltration caused liver damage and necrosis. The border between the white and red pulp in spleen tissues was ill-defined, with decreased cell-cell adhesion. Furthermore, intestinal tissues presented epithelial cell shedding and necrosis. MEM in the presence or absence of morin reduced these effects (Fig. 4B). These results indicate that morin combined with MEM reduced tissue damage in mice by decreasing WBC count, serum inflammatory markers, tissue necrosis, and bacterial load in infected organs.
MD simulations of morin and NDM-1
The interaction between morin and NDM-1 and the dynamic stability of protein-ligand complexes were evaluated using MD simulations. Rg values were maintained at approximately 1.67 nm during the simulations, indicating no significant alterations in protein compactness (Fig. 5A). RMSD values initially fluctuated but then stabilized at approximately 0.11 nm. These results suggest that the morin-NDM-1 complex is in equilibrium without notable conformational changes.
Molecular docking simulations of the morin-NDM-1 complex. (A) The complex’s hydrogen bonding, radius of gyration, root mean square fluctuation, and root mean square deviation. (B) Secondary structure of the complex. (C) Binding mechanism of morin to NDM-1 at 100 ns. (D) Protein-ligand binding free energy on a per-residue basis. (E) Establishment of the molecular dynamics simulation system.
The flexibility of amino acid residues was analyzed within the protein. The RMSF values of most amino acid residues were less than 0.5 nm, demonstrating secondary structure stability. Conversely, the RMSF values of residues 38 to 42 were comparatively higher, suggesting structural flexibility or dynamic conformational changes in this region, shifting from a β-turn to a bent conformation and returning to a β-turn (Fig. 5B). The number of hydrogen bonds between morin and NDM-1 varied from 0 to 2, indicating the successful formation of a protein-ligand complex.
The binding free energy of the protein-ligand complex was computed to evaluate the strength and stability of the interactions. The total free energy was − 22.82 ± 4.96 kcal/mol, demonstrating strong interactions. Furthermore, the histidine residues 93, 160, and 191 greatly influenced the binding free energy (Fig. 5D).
The binding mechanism and interaction between morin and NDM-1 are shown in Fig. 5C and E. Morin interacted with NDM-1 by binding to histidine residues 93, 160, and 191 in the active site. These results indicate that morin decreases the enzymatic activity of NDM-1 and competes with antibiotics for amino acids at the active site of NDM-1.
Discussion
β-lactam antibiotics are extensively utilized in clinical medicine and animal husbandry due to their potent antibacterial properties and low toxicity31. However, the overuse and misuse of these antibiotics have led to an increasingly severe problem of bacterial resistance, particularly resistance mediated by NDM-1, which poses a significant threat to public health32. NDM-1 is capable of hydrolyzing a wide range of β-lactam antibiotics, including carbapenems, thereby substantially diminishing the effectiveness of conventional antibiotic therapies. Consequently, the development of effective NDM-1 inhibitors to restore and enhance the antibacterial efficacy of β-lactam antibiotics represents a critical focus in contemporary antibiotic research and development.
In this study, morin was identified from a natural product library as a potential NDM-1 inhibitor using structure-based virtual screening and molecular docking techniques. The molecular docking results indicated that Morin can bind tightly to the active site of NDM-1, with a free binding energy lower than − 10.0 Kcal/mol, suggesting a potential inhibitory effect. Further in vitro experiments demonstrated that morin enhanced antibacterial activity when combined with β-lactam antibiotics, reducing the minimum inhibitory concentration (MIC) by 4 to 32 times, with the synergy index (FICI) confirming a synergistic effect. These findings suggested that Morin can not only inhibited NDM-1 activity but also enhanced the antibacterial efficacy of β-lactam antibiotics against NDM-1-producing bacteria. Morin’s ability to bind to Zn2+ in NDM-1 was a key mechanism through which it inhibits the enzyme, thereby reducing bacterial resistance to MEM. Compared to other studies, research on Morin reveals that it inhibits enzyme activity by binding to the active site of NDM-1 and enhances the effects of β-lactam antibiotics. For instance, Aspergillus fumigatus has also been shown to inhibit NDM-1 and exhibits significant synergistic effects when combined with MEM27. Additionally, other studies have highlighted compounds identified through virtual screening and molecular docking technologies, such as DPA and its derivatives, also demonstrate similar inhibitory effects33.
Through time-kill experiments and checkerboard assays, this study elucidated the synergistic mechanism between Morin and β-lactam antibiotics. Morin has been shown to enhance the inhibitory effect of β-lactam antibiotics on NDM-1-positive E. coli in a dose-dependent manner. The combination of Morin and MEM achieved a stronger inhibitory effect in a shorter duration. The result suggested that Morin may inhibit the hydrolysis of the β-lactam by binding to the active site of NDM-1, thereby preserving the structural integrity of the antibiotic and enhancing its antibacterial activity. When administered alone, Morin exhibited a negligible antibacterial effect, which possible reason was that Morin only inhibits the NDM-1 activity of bacteria, but cannot directly kill bacteria. However, existing literature indicated that β-lactamase inhibitors typically function by preventing the hydrolysis of β-lactam antibiotics by enzymes, rather than directly killing bacteria. Thus, Morin’s mechanism of action seems to enhance the efficacy of existing antibiotics rather than provide direct killing. Further research is needed to determine whether Morin can directly kill bacteria34,35.
In the BALB/c mouse model, the combination of Morin and MEM reduced tissue damage and inflammatory responses in mice infected with NDM-1 -producing bacteria. Specifically, it is manifested as a decrease in white blood cell count, serum inflammatory marker levels, tissue necrosis, and bacterial load. These results indicated that Morin not only exhibits good synergistic antibacterial effects in vitro experiments, but also shows improvement in inflammatory responses and tissue damage in in vivo models, further confirming the potential of Morin as an NDM-1 inhibitor28.
Compared with other studies, verifying the effects of morin in animal models has research value. Morin combined with cisplatin reduces cisplatin-induced liver and kidney damage by alleviating oxidative stress, inflammation, and cell apoptosis36,37,38. It has been found that beta-lactamase inhibitors such as sulbactam, when used in combination with cephalosporin antibiotics, show significant synergistic antibacterial effects in animal models39. In addition, the anti-inflammatory effects of Morin observed in in vivo models are similar to those seen in other studies40.
Molecular dynamics simulation results provided valuable research into the interaction between morin and NDM-1, which demonstrated that the morin-NDM-1 complex remained stable throughout the simulation period. Both the radius of gyration (Rg) and root mean square deviation (RMSD) values indicated that the protein-ligand complex was in equilibrium, exhibiting no significant conformational changes. The stability of RMSD value in molecular dynamics simulation is an important index to evaluate the stability of protein-ligand complex. Azhar Salari-jazi evaluated the binding stability of NDM-1 to different ligands through molecular dynamics simulations and found that most complexes reached an equilibrium state within the last 10 ns of the simulation and the RMSD value stabilized in the low range. This is consistent with the fact that the RMSD value of the morin-NDM-1 complex remains stable, indicating that its binding is stable41. The root mean square fluctuation (RMSF) values of amino acid residues revealed that the secondary structure of most residues remained stable, while some residues exhibited higher flexibility, potentially related to conformational changes induced by Morin binding. A higher root-mean-square fluctuation (RMSF) value generally signifies that the residue exhibits greater flexibility or has experienced more substantial conformational changes during the simulation. Based on Wang Hong’s RMSF analysis of the anti-inflammatory component of Ganoderma lucidum and its protein complex, several residues displayed elevated RMSF values, suggesting significant conformational alterations in these regions throughout the simulation. This phenomenon may be associated with the conformational change at the NDM-1 active site induced by morin binding42. Additionally, variations in the number of hydrogen bonds and the calculated binding free energy further corroborated the formation of a stable complex between morin and NDM-1, with specific histidine residues playing a key role in contributing to the binding free energy. With regard to the analysis of hydrogen bond number and binding free energy, these parameters can further reveal the interaction mechanism between morin and NDM-1. Amit Dubey investigated the inhibition of NDM-1 by natural products of Marine bacteria through molecular dynamics simulations and free energy analysis. The results show that specific histidine residues play a key role in inhibitor binding at the active site of NDM-1. This is consistent with the idea that Morin inhibits NDM-1 enzyme activity by binding to histidine residues43.These findings indicated that morin binds to the histidine residue in the active site of NDM-1, reduced the enzymatic activity of NDM-1, and competed with antibiotics for amino acids in the active site, thereby exerting an inhibitory effect.
Despite the positive outcomes of this study, several limitations could be acknowledged. While the synergistic antibacterial and anti-inflammatory effects of morin have been demonstrated in mouse models, the efficacy of these effects in human patients requires further clinical trials for validation. Additionally, the combined effects of Morin with other β-lactam antibiotics, as well as its inhibitory effects on other metallo-β-lactamases, needed to be further explored. Notably, the study lacks direct biochemical characterization of morin’s inhibitory potency against NDM-1, such as the half-maximal inhibitory concentration (IC₅₀) and inhibition constant (Ki) values, which are critical for defining the enzyme-ligand interaction kinetics44. These parameters would complement the molecular docking and simulation data to mechanistically validate morin’s role as an NDM-1 inhibitor, and thus represent an important direction for future research.
In summary, this study systematically evaluated the potential of morin as an NDM-1 inhibitor through various methods such as virtual screening, molecular docking, in vitro experiments, in vivo models, and molecular dynamics simulations. The results showed that morin can effectively inhibit the activity of NDM-1, enhance the antibacterial effect of β-lactam antibiotics, and show an improvement in inflammatory response and tissue damage in an in vivo model. These findings provided important theoretical basis and experimental data for the development of new NDM-1 inhibitors, and are expected to provide new strategies and methods to solve the increasingly serious problem of antibiotic resistance. Future research will be dedicated to optimizing the drug properties of morin, exploring its application potential in clinical treatment, and further expanding its inhibitory effect on other drug resistance mechanisms.
Conclusion
Morin enhanced antibacterial activity of β-lactams against NDM-1-producing E. coli when combined with MEM. These findings establish the foundation for using morin in developing potent inhibitors for NDM-1 and other β-lactamases to reduce antibiotic resistance. Future research may explore morin’s efficacy against other metallo-β-lactamases and optimize its pharmacokinetic properties, thereby addressing the global challenge of multidrug-resistant bacterial infections.
Data availability
The datasets used for the current study are available from the corresponding author upon reasonable request.
References
Bush, K. & Bradford, P. A. β-Lactams and β-Lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 6 https://doi.org/10.1101/cshperspect.a025247 (2016).
Stewart, A. C. et al. Clinical variants of New Delhi Metallo-β-Lactamase are evolving to overcome zinc scarcity. ACS Infect. Dis. 3, 927–940. https://doi.org/10.1021/acsinfecdis.7b00128 (2017).
Papp-Wallace, K. M., Endimiani, A., Taracila, M. A. & Bonomo, R. A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 55, 4943–4960. https://doi.org/10.1128/aac.00296-11 (2011).
Wang, T. et al. Recent research and development of NDM-1 inhibitors. Eur. J. Med. Chem. 223, 113667. https://doi.org/10.1016/j.ejmech.2021.113667 (2021).
Acharya, Y., Bhattacharyya, S., Dhanda, G. & Haldar, J. Emerging roles of glycopeptide antibiotics: Moving beyond gram-positive bacteria. ACS Infect. Dis. 8, 1–28. https://doi.org/10.1021/acsinfecdis.1c00367 (2022).
Bush, K. Past and present perspectives on β-Lactamases. Antimicrob. Agents Chemother. 62 https://doi.org/10.1128/aac.01076-18 (2018).
Ambler, R. P. The structure of beta-lactamases. Philos. Trans. R Soc. Lond. B Biol. Sci. 289, 321–331. https://doi.org/10.1098/rstb.1980.0049 (1980).
Khan, A. U., Maryam, L., Zarrilli, R. & Structure Genetics and worldwide spread of New Delhi metallo-β-lactamase (NDM): A threat to public health. BMC Microbiol. 17, 101. https://doi.org/10.1186/s12866-017-1012-8 (2017).
Page, M. G. b-Lactamase inhibitors. Drug Resist. Updat 3, 109–125. https://doi.org/10.1054/drup.2000.0137 (2000).
Drawz, S. M. & Bonomo, R. A. Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 23, 160–201. https://doi.org/10.1128/cmr.00037-09 (2010).
Olsen, I. New promising β-lactamase inhibitors for clinical use. Eur. J. Clin. Microbiol. Infect. Dis. 34, 1303–1308. https://doi.org/10.1007/s10096-015-2375-0 (2015).
Porras, G. et al. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem. Rev. 121, 3495–3560. https://doi.org/10.1021/acs.chemrev.0c00922 (2021).
Maharjan, S., Lee, M. G., Lee, K. S. & Nam, K. S. Morin overcomes doxorubicin resistance in human breast cancer by inducing DNA damage and modulating the LKB1/AMPK/mTORC1 signaling pathway. Biofactors 51, e2112. https://doi.org/10.1002/biof.2112 (2025).
Alla, N., Palatheeya, S., Challa, S. R. & Kakarla, R. Morin attenuated the global cerebral ischemia via antioxidant, anti-inflammatory, and antiapoptotic mechanisms in rats. Metab. Brain Dis. 39, 1323–1334. https://doi.org/10.1007/s11011-024-01410-y (2024).
Unsal, V., Cicek, M., Aktepe, N. & Oner, E. Morin attenuates arsenic-induced toxicity in 3T3 embryonic fibroblast cells by suppressing oxidative stress, inflammation, and apoptosis: In vitro and silico evaluations. Toxicol. Res. (Camb). 13, tfae113. https://doi.org/10.1093/toxres/tfae113 (2024).
Caselli, A., Cirri, P., Santi, A. & Paoli, P. A promising natural drug. Curr. Med. Chem. 23, 774–791. https://doi.org/10.2174/0929867323666160106150821 (2016).
Kang, S. S., Kim, J. G., Lee, T. H. & Oh, K. B. Flavonols inhibit sortases and sortase-mediated Staphylococcus aureus clumping to fibrinogen. Biol. Pharm. Bull. 29, 1751–1755. https://doi.org/10.1248/bpb.29.1751 (2006).
Xu, H. et al. Flavones inhibit the hexameric replicative helicase RepA. Nucleic Acids Res. 29, 5058–5066. https://doi.org/10.1093/nar/29.24.5058 (2001).
Kopacz, M., Woźnicka, E. & Gruszecka, J. Antibacterial activity of Morin and its complexes with La(III), Gd(III) and Lu(III) ions. Acta Pol. Pharm. 62, 65–67 (2005).
Khamchai, S. et al. Morin protects the blood-brain barrier integrity against cerebral ischemia reperfusion through anti-inflammatory actions in rats. Sci. Rep. 10, 13379. https://doi.org/10.1038/s41598-020-70214-8 (2020).
Lian, T. W., Wang, L., Lo, Y. H., Huang, I. J. & Wu, M. J. Fisetin, morin and myricetin attenuate CD36 expression and OxLDL uptake in U937-derived macrophages. Biochim. Biophys. Acta. 1781, 601–609. https://doi.org/10.1016/j.bbalip.2008.06.009 (2008).
Kim, J. M. et al. Morin modulates the oxidative stress-induced NF-kappaB pathway through its anti-oxidant activity. Free Radic Res. 44, 454–461. https://doi.org/10.3109/10715761003610737 (2010).
Vanitha, P. et al. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and β-cell function in streptozotocin-induced diabetic rats. Environ. Toxicol. Pharmacol. 37, 326–335. https://doi.org/10.1016/j.etap.2013.11.017 (2014).
Thangarajan, S., Vedagiri, A., Somasundaram, S., Sakthimanogaran, R. & Murugesan, M. Neuroprotective effect of morin on lead acetate- induced apoptosis by preventing cytochrome c translocation via regulation of Bax/Bcl-2 ratio. Neurotoxicol. Teratol. 66, 35–45. https://doi.org/10.1016/j.ntt.2018.01.006 (2018).
Liu, Z. et al. Treatment of chronic obstructive pulmonary disease by traditional Chinese medicine morin monomer regulated by autophagy. J. Thorac. Dis. 16, 6052–6063. https://doi.org/10.21037/jtd-23-1836 (2024).
Odds, F. C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52, 1. https://doi.org/10.1093/jac/dkg301 (2003).
Xiao, G., Li, J. & Sun, Z. The combination of antibiotic and non-antibiotic compounds improves antibiotic efficacy against multidrug-resistant bacteria. Int. J. Mol. Sci. 24. https://doi.org/10.3390/ijms242015493 (2023).
Sun, J., Ren, S., Yang, Y., Li, X. & Zhang, X. Betaxolol as a potent inhibitor of NDM-1-Positive E. coli that synergistically enhances the Anti-Inflammatory effect in combination with meropenem. Int. J. Mol. Sci. 24. https://doi.org/10.3390/ijms241713399 (2023).
Liu, S. et al. Pterostilbene restores carbapenem susceptibility in new Delhi metallo-β-lactamase-producing isolates by inhibiting the activity of new Delhi metallo-β-lactamases. Br. J. Pharmacol. 176, 4548–4557. https://doi.org/10.1111/bph.14818 (2019).
Soares, P. M. et al. Inflammatory intestinal damage induced by 5-fluorouracil requires IL-4. Cytokine 61, 46–49. https://doi.org/10.1016/j.cyto.2012.10.003 (2013).
Giurazza, R. et al. Emerging treatment options for multi-drug-resistant bacterial infections. Life (Basel) 11. https://doi.org/10.3390/life11060519 (2021).
Bogaerts, P., Verroken, A., Jans, B., Denis, O. & Glupczynski, Y. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect. Dis. 10, 831–832. https://doi.org/10.1016/s1473-3099(10)70278-4 (2010).
Sharma, S., Sharma, S., Singh, P. P. & Khan, I. A. Potential inhibitors against NDM-1 type metallo-β-Lactamases: An overview. Microb. Drug Resist. 26, 1568–1588. https://doi.org/10.1089/mdr.2019.0315 (2020).
McGeary, R. P., Tan, D. T. & Schenk, G. Progress toward inhibitors of metallo-β-lactamases. Future Med. Chem. 9, 673–691. https://doi.org/10.4155/fmc-2017-0007 (2017).
Faridoon Ul islam, N. An update on the status of potent inhibitors of Metallo-β-Lactamases. Sci. Pharm. 81, 309–327. https://doi.org/10.3797/scipharm.1302-08 (2013).
K, V. A. et al. Morin hydrate mitigates cisplatin-Induced renal and hepatic injury by impeding oxidative/nitrosative stress and inflammation in mice. J. Biochem. Mol. Toxicol. 30, 571–579. https://doi.org/10.1002/jbt.21817 (2016).
Wei, Z. et al. Renoprotective mechanisms of morin in cisplatin-induced kidney injury. Int. Immunopharmacol. 28, 500–506. https://doi.org/10.1016/j.intimp.2015.07.009 (2015).
Kaltalioglu, K. & Coskun-Cevher, S. Potential of morin and hesperidin in the prevention of cisplatin-induced nephrotoxicity. Ren. Fail. 38, 1291–1299. https://doi.org/10.1080/0886022x.2016.1209383 (2016).
Ma, H. R., Xu, H. Z., Kim, K., Anderson, D. J. & You, L. Private benefit of β-lactamase dictates selection dynamics of combination antibiotic treatment. Nat. Commun. 15, 8337. https://doi.org/10.1038/s41467-024-52711-w (2024).
Lee, K. M. et al. Neuroprotective and anti-inflammatory effects of Morin in a murine model of Parkinson’s disease. J. Neurosci. Res. 94, 865–878. https://doi.org/10.1002/jnr.23764 (2016).
Salari-Jazi, A., Mahnam, K., Sadeghi, P., Damavandi, M. S. & Faghri, J. Discovery of potential inhibitors against New Delhi metallo-β-lactamase-1 from natural compounds: In silico-based methods. Sci. Rep. 11, 2390. https://doi.org/10.1038/s41598-021-82009-6 (2021).
Xiaoying, M. et al. Elucidating the molecular mechanisms underlying anti-inflammatory effects of Morchella esculentain the arachidonic acid metabolic pathway by network pharmacology and molecular docking. Sci. Rep. 13, 15881. https://doi.org/10.1038/s41598-023-42658-1 (2023).
Dubey, A., Alanazi, A. M., Bhardwaj, R. & Ragusa, A. Identification of potential NUDT5 inhibitors from marine bacterial natural compounds via molecular dynamics and free energy landscape analysis. Mol. Divers. https://doi.org/10.1007/s11030-024-10950-5 (2024).
Georgakis, N. et al. Determination of half-maximal inhibitory concentration of an enzyme inhibitor. Methods Mol. Biol. 2089, 41–46. https://doi.org/10.1007/978-1-0716-0163-1_3 (2020).
Acknowledgements
We thank TopEdit (www.topeditsci.com) for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China (32372957) and the Project of Shandong Province Higher Educational Science and Technology Program(2022KJ287).
Author information
Authors and Affiliations
Contributions
R.Q., Data curation, Methodology, Software, Writing-original draft, Writing-reviewing & editing, Conceptualization; Z.B., Assist in collecting experimental materials, Writing-reviewing & editing; D.L., Visualization, Investigation,Writing-reviewing & editing; W.M., Data curation, methodology, Writing-reviewing & editing; W.T., Software, Visualization, Writing-reviewing & editing; Y.M., Collect experimental materials, Writing-reviewing & editing; Z.W., Writing-reviewing & editing; L.Y., Conceptualization, Methodology, funding acquisition, Software, Writing-reviewing & editing; L.X., Software, Validation, Writing-reviewing & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All protocols and procedures involving animals adhered to the Chinese Regulations for Laboratory Animals and received approval from the Animal Ethics Committee at Liaocheng University (permit number: LC2024–06).The study is reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, Q., Zhang, B., Wang, M. et al. Morin combined with meropenem is a potent inhibitor of NDM-1 against NDM-1-producing E. coli. Sci Rep 15, 24169 (2025). https://doi.org/10.1038/s41598-025-08532-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08532-y