Abstract
Relative Fat Mass (RFM) is an emerging body fat measurement index that more accurately reflects the distribution of visceral fat. This study aimed to investigate the association between RFM and female infertility. We utilized data from the National Health and Nutrition Examination Survey (NHANES) spanning from 2017 to 2020, enrolling 1487 female participants aged 20–44. The relationship between RFM and infertility was analyzed using multivariate Logistic regression, and the dose-response relationship was assessed with Restricted Cubic Splines (RCS). The study found a significant association between RFM and infertility. After adjusting for confounders such as age, ethnicity, and BMI, each unit increase in RFM was associated with a 6% increase in the risk of infertility (OR = 1.06, 95%CI = 1.01–1.12, P = 0.019), with stable results across different subgroups. RFM is significantly associated with female infertility and may serve as a useful tool in infertility screening. Future studies should further validate its potential for clinical application.
Similar content being viewed by others
Introduction
Infertility is defined as the inability of a couple to conceive after one year of unprotected intercourse and regular sexual activity, affecting approximately 10–15% of couples worldwide1,2,3. This reproductive health issue not only significantly impacts the physical and mental health of the couple but also imposes a substantial economic burden on families and society4,5,6,7. The causes of infertility are complex and diverse, including physiological abnormalities of the reproductive system, endocrine disorders, immune system diseases, and various lifestyle factors8,9,10,11,12,13. In recent years, with the acceleration of societal pace and changes in lifestyle, the relationship between obesity and metabolic disorders and infertility has attracted widespread attention14,15.
Female infertility can typically be attributed to ovarian dysfunction, tubal disease, endometriosis, and polycystic ovary syndrome (PCOS)16,17,18. Among these, PCOS is one of the most common endocrine diseases in women of reproductive age, characterized by impaired ovarian follicular function, hyperandrogenism, and insulin resistance, with these metabolic abnormalities being particularly pronounced in obese women19,20,21. Therefore, obesity and abnormal body fat distribution not only affect metabolic health but also directly interfere with female reproductive capacity22. In recent years, research on infertility has gradually shifted focus from single physiological factors to comprehensive metabolic health issues. A growing body of evidence indicates a significant positive correlation between obesity and the risk of infertility23,24,25. Obesity not only may lead to ovulatory disorders in women but also increases the risk of infertility through mechanisms such as chronic inflammation and insulin resistance26,27,28,29. Additionally, infertility may coexist with other metabolic disorders such as diabetes, hypertension, and cardiovascular diseases, which can further affect women’s reproductive health and pregnancy outcomes16.
Moreover, infertility is closely related to psychological health issues. Infertile patients often have higher levels of psychological stress, anxiety, and depression, and these psychological issues may further exacerbate the condition of infertility, indicating the need for comprehensive intervention and management from both physiological and psychological perspectives for infertile patients30,31,32. Therefore, a comprehensive assessment integrating metabolic health, psychological health, and reproductive health, along with personalized interventions for infertile patients, has become a new trend in modern infertility treatment33.
Assessing female infertility necessitates an understanding of ovulation and ovarian reserve biomarkers. Anti-Müllerian Hormone (AMH), which reflects the size of the primordial follicle pool, has potential as a predictive marker for menopause; however, further studies are required to validate its clinical utility34. Research on oocytes and granulosa cells has revealed that, in women over 38 years of age, luteinized granulosa cells exhibit various abnormalities associated with reduced fertility35. However, other studies suggest that ovarian reserve indicators, such as AMH and follicle-stimulating hormone (FSH), do not correlate with a decline in future reproductive potential. Due to this lack of correlation, caution is advised in using ovarian reserve biomarkers as predictive tools for future fertility outcomes36.
Relative Fat Mass (RFM) is a more effective measure than Body Mass Index (BMI) for assessing visceral fat, which plays a crucial role in metabolic disorders and infertility. Unlike BMI, which is a general indicator of body fat, RFM incorporates waist circumference, offering a more accurate reflection of visceral fat distribution. Visceral fat has a direct impact on ovarian function and fertility, particularly by influencing insulin resistance and chronic inflammation. These mechanisms, which are central to reproductive health, are more precisely captured by RFM compared to BMI. Numerous studies have demonstrated that RFM outperforms BMI in predicting metabolic diseases and reproductive health outcomes, including infertility. Consequently, RFM is a more reliable tool for screening fertility risk, particularly in women with normal BMI but elevated visceral fat, where traditional BMI may fail to identify those at higher risk37,38,39,40.
Although the etiology of infertility is complex, the role of obesity and abnormal body fat in infertility has been widely recognized41,42,43,44. To better understand the relationship between obesity and infertility, researchers have recently begun to focus on more accurate body fat measurement methods. Relative Fat Mass (RFM), as a new body fat measurement index, has gradually been applied in clinical research45,46,47,48. Compared to the traditional Body Mass Index (BMI), RFM, which combines the ratio of waist circumference to height, more accurately reflects the accumulation of visceral fat. RFM has been proven to be highly correlated with metabolic diseases, but its relationship with female reproductive health, especially infertility, has not been sufficiently studied.
Based on this, the aim of this study is to explore the association between RFM and female infertility using a large-sample cross-sectional dataset. We hypothesize that higher levels of RFM may be associated with an increased risk of infertility. The results of this study will provide a new perspective for the prediction of infertility risk and may offer insights for clinical management and health policy formulation.
Materials and methods
Study design
This study is a cross-sectional analysis using data from the National Health and Nutrition Examination Survey (NHANES) conducted between 2017 and 2020. NHANES is an ongoing survey representing the civilian, non-institutionalized U.S. population, aimed at assessing the health and nutritional status of U.S. residents. The data is publicly available, and all information can be accessed through the Centers for Disease Control and Prevention (CDC) website (https://www.cdc.gov/nchs/nhanes). The NHANES protocol has been approved by the National Center for Health Statistics Institutional Review Board, and all participants provided written informed consent.
Study population
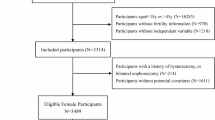
The study subjects were women aged 20 to 44 years. A total of 3274 women were initially included in the analysis. After excluding those with missing infertility information, unavailable RFM data, and a history of hysterectomy or oophorectomy, 1487 participants were ultimately included in the study. The specific study flow is shown in Fig. 1.
Exposure variable: relative fat mass (RFM)
The primary exposure variable in this study is Relative Fat Mass (RFM), a body fat percentage index calculated from waist circumference and height, which more accurately reflects body fat distribution compared to the traditional Body Mass Index (BMI). The formula for calculating RFM is as follows: RFM = 64 − (20 × height/waist circumference) + (12 × gender), where the gender value for females is 145.
Outcome variable: infertility
Based on previous research results49, this study used questionnaire items including: “Have you tried to get pregnant for at least a year without success?” or “Have you seen a doctor or other healthcare provider because you were unable to get pregnant?” Women who answered “yes” were categorized as the infertile group.
Covariates
Based on clinical experience and previous literature50,51,52,53, this study selected age, ethnicity, education level, marital status, household income, BMI, alcohol consumption, smoking status, sleep disorders, menstrual cycle regularity, and history of treatment for pelvic inflammatory disease or pelvic infection as covariates.
Statistical analysis
In this study, continuous variables were expressed as means and standard errors [SE] when normally distributed, and categorical variables were expressed as frequencies and percentages. The variance inflation factor (VIF) was used to assess multicollinearity among all variables. Covariates were included in the final model if they changed the estimate of RFM’s association with infertility by > 10%, or based on clinical judgment, or if they were significantly associated with infertility in univariate analysis (P < 0.1). A multivariate logistic regression model was constructed to assess the correlation between RFM and infertility. In Model I, only slight adjustments were made for sociodemographic variables. In Model II, all variables were adjusted. Additionally, RFM was divided into quartile groups for linear trend testing, and subgroup analyses were performed using stratified multiple regression analysis to test the stability of the association. All analyses were conducted using R software version 4.4.1, and P < 0.05 was considered statistically significant.
Results
Participant characteristics
A total of 1487 women aged 20–44 were included in this study, with an average age of 31.9 years (standard deviation 7.1 years). Among them, 200 participants (13.5%) were diagnosed with infertility, and 1287 participants (86.5%) did not have infertility issues. Table 1 summarizes the differences in baseline characteristics between infertile and non-infertile groups. Significant differences were observed in age, marital status, household income, smoking status, sleep disorders, and RFM (P < 0.05). The average age of the infertile group was higher (P < 0.001), more infertile women reported being married or cohabiting with a partner (P < 0.001), and the RFM values of the infertile group were higher than those of the non-infertile group (P < 0.001).
Association between RFM and infertility
Logistic regression analysis showed a significant positive correlation between Relative Fat Mass (RFM) and infertility. In the crude model not adjusted for any covariates, each unit increase in RFM was associated with a 4% increase in the risk of infertility (OR = 1.04, 95% CI = 1.02–1.07, P < 0.001). In Model I, adjusted for age and ethnicity, the relationship between RFM and infertility remained significant (OR = 1.04, 95% CI = 1.01–1.07, P = 0.002). In Model II, after adjusting for all covariates, RFM remained a significant predictor (OR = 1.06, 95% CI = 1.01–1.12, P = 0.019). When RFM was divided into quartiles, further analysis showed that the highest quartile group (Q4) compared to the lowest quartile group (Q1) had a significantly increased risk of infertility. After adjusting for all covariates, the risk of infertility in the highest RFM quartile group was 2.38 times that of the lowest quartile group (OR = 2.38, 95% CI = 0.99–5.70, P = 0.005). Additionally, trend analysis indicated that as RFM increased, the risk of infertility gradually increased (P for trend < 0.05) (Table 2).
Restricted cubic splines analysis
To further assess the nonlinear relationship between RFM and infertility, this study used restricted cubic splines (RCS) analysis (Fig. 2). The results showed a significant positive linear association between RFM and infertility, with the risk of infertility showing a continuous increasing trend as RFM increased (P for nonlinearity = 0.588).
Restricted cubic spline (RCS) analysis for the association between the RFM and Infertility. Red lines represent odds ratios, and pink areas represent 95% confidence intervals. The model was adjusted for age, race, education level, marital status, family income, body mass index, drink status, smoking status, trouble sleeping, menstrual cycle regularity and ever treated for a pelvic infection/PID.
Subgroup analysis
To explore the association between RFM and infertility in different populations, subgroup analyses stratified by ethnicity, education level, household income, BMI, alcohol consumption, smoking status, sleep disorders, menstrual cycle regularity, and history of treatment for pelvic inflammatory disease or pelvic infection indicated that the influence of RFM was relatively stable across all subgroups (Fig. 3).
Adjusted for age, race, education level, marital status, family income, body mass index, drink status, smoking status, trouble sleeping, menstrual cycle regularity and ever treated for a pelvic infection/PID except the stratification factor itself.
Discussion
This study is the first to explore the association between Relative Fat Mass (RFM) and female infertility based on NHANES data from 2017 to 2020. Our main finding indicates a significant positive correlation between RFM and female infertility, which remains significant after adjusting for various confounding factors (OR = 1.06, 95% CI = 1.01–1.12, P = 0.019), and the results are stable across different subgroups.
RFM is an emerging body fat measurement index that combines waist circumference and height, providing a better reflection of visceral fat distribution46. Compared to the traditional Body Mass Index (BMI), RFM can more effectively predict metabolic disorders and cardiovascular diseases47,54,55, which may also be one of the important reasons for its association with infertility. Excessive accumulation of obesity, especially visceral fat, may affect female fertility through multiple mechanisms. First, obesity is closely related to insulin resistance and chronic inflammation56. Insulin resistance and inflammation may interfere with ovarian function, leading to ovulatory disorders57,58. Second, excessive visceral fat may lead to hormonal imbalances, increasing androgen levels, thereby affecting the reproductive system59. Our finding of a positive correlation between RFM and infertility supports the above mechanisms. After adjusting for age, ethnicity, education level, marital status, BMI, and other confounding factors, the significant association between RFM and infertility still exists, indicating that RFM may be a reproductive health prediction index independent of traditional risk factors.
Consistent with previous studies, our study found a significant association between obesity and infertility60,61. However, most past studies have relied on BMI as an indicator of obesity, which cannot fully reflect the differences in fat distribution. This study further reveals the potential impact of visceral fat on infertility by using RFM, a more accurate indicator, which is consistent with the findings of Kuang et al. regarding the association between age-adjusted visceral adiposity index (AVAI) and female infertility62. In addition, our study used restricted cubic splines analysis to further explore the nonlinear relationship between RFM and infertility, finding that as RFM increases, the risk of infertility shows a linearly increasing trend, but without statistical significance (P for nonlinearity = 0.588). Zhu et al.‘s study showed the nonlinear association between relative fat mass and other metabolic diseases45. The results of this study have important clinical significance. RFM, as a simple and easily accessible index, may become a powerful tool for infertility risk screening, especially in women with normal weight but excessive visceral fat. Given the complexity and multifactorial nature of infertility, RFM can provide a new perspective for the early identification and intervention of infertile women.
Although this study reveals an important association between RFM and infertility, there are some limitations. First, due to the cross-sectional design of this study, causality cannot be determined. Future longitudinal studies should further verify the temporal dynamic association between RFM and infertility. Second, despite controlling for various confounding factors, there may still be some unconsidered confounding factors that could affect the results. Lastly, the sample of this study comes from the U.S. population, and the generalizability of the study results may be somewhat limited. Future studies can further explore the long-term impact of RFM on infertility through longitudinal cohort studies and assess whether intervening in RFM can reduce the risk of infertility. In addition, multi-level studies combining genetic factors, lifestyle, and environmental factors will help fully understand the role of RFM in reproductive health.
Conclusion
This study demonstrates that Relative Fat Mass (RFM) is significantly associated with female infertility, with higher RFM associated with increased infertility risk. Even after adjusting for all covariates, this association remains significant. RFM can serve as a potential indicator for infertility screening, and future studies should further explore its clinical application value.
Data availability
The data is publicly available, and all information can be accessed through the Centers for Disease Control and Prevention (CDC) website (https://www.cdc.gov/nchs/nhanes).
References
Boivin, J. et al. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum. Reprod. 22 (6), 1506–1512 (2007).
Evers, J. L. Female subfertility. Lancet 360 (9327), 151–159 (2002).
Infertility Workup for the Women’s Health Specialist. ACOG committee opinion, number 781. Obstet. Gynecol. 133 (6), e377–e384 (2019).
Bourrion, B. et al. The economic burden of infertility treatment and distribution of expenditures over time in france: a self-controlled pre-post study. BMC Health Serv. Res. 22 (1), 512 (2022).
Lei, A. et al. The associations between infertility-related stress, family adaptability and family cohesion in infertile couples. Sci. Rep. 11 (1), 24220 (2021).
Rooney, K. L. & Domar, A. D. The relationship between stress and infertility. Dialogues Clin. Neurosci. 20 (1), 41–47 (2018).
Roomaney, R. et al. A scoping review of the psychosocial aspects of infertility in African countries. Reprod. Health. 21 (1), 123 (2024).
Chen, J. et al. Role of m6A modification in female infertility and reproductive system diseases. Int. J. Biol. Sci. 18 (9), 3592–3604 (2022).
Poppe, K. & MANAGEMENT OF ENDOCRINE DISEASE. Thyroid and female infertility: more questions than answers?! Eur. J. Endocrinol. 184 (4), R123–R135 (2021).
Sen, A. et al. Endocrine autoimmune diseases and female infertility. Nat. Rev. Endocrinol. 10 (1), 37–50 (2014).
Chen, J. et al. Microbiology and immune mechanisms associated with male infertility. Front. Immunol. 14, 1139450 (2023).
Palomba, S. et al. Lifestyle and fertility: the influence of stress and quality of life on female fertility. Reprod. Biol. Endocrinol. 16 (1), 113 (2018).
Braverman, A. M. et al. Depression, anxiety, quality of life, and infertility: a global lens on the last decade of research. Fertil. Steril. 121 (3), 379–383 (2024).
Ennaab, F. & Atiomo, W. Obesity and female infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 89, 102336 (2023).
Thong, E. P. et al. Diabetes: a metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol. 8 (2), 134–149 (2020).
Carson, S. A. & Kallen, A. N. Diagnosis and management of infertility: A review. JAMA 326 (1), 65–76 (2021).
Bonavina, G. & Taylor, H. S. Endometriosis-associated infertility: from pathophysiology to tailored treatment. Front. Endocrinol. (Lausanne). 13, 1020827 (2022).
Hanson, B. et al. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J. Assist. Reprod. Genet. 34 (2), 167–177 (2017).
Broughton, D. E. & Moley, K. H. Obesity and female infertility: potential mediators of obesity’s impact. Fertil. Steril. 107 (4), 840–847 (2017).
Glueck, C. J. & Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism 92, 108–120 (2019).
Stener-Victorin, E. et al. Polycystic ovary syndrome. Nat. Rev. Dis. Primers. 10 (1), 27 (2024).
Silvestris, E. et al. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 16 (1), 22 (2018).
Tang, J. et al. Association between metabolic healthy obesity and female infertility: the National health and nutrition examination survey, 2013–2020. BMC Public. Health. 23 (1), 1524 (2023).
Fedorcsák, P. et al. Obesity is a risk factor for early pregnancy loss after IVF or ICSI. Acta Obstet. Gynecol. Scand. 79 (1), 43–48 (2000).
Wen, Z. & Li, X. Association between weight-adjusted-waist index and female infertility: a population-based study. Front. Endocrinol. (Lausanne). 14, 1175394 (2023).
Mintziori, G. et al. The effect of excess body fat on female and male reproduction. Metabolism 107, 154193 (2020).
Barati, E., Nikzad, H. & Karimian, M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 77 (1), 93–113 (2020).
Snider, A. P. & Wood, J. R. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction 158 (3), R79–R90 (2019).
Xia, W. et al. Association between different insulin resistance surrogates and infertility in reproductive-aged females. BMC Public. Health. 23 (1), 1985 (2023).
Dube, L. et al. Efficacy of psychological interventions for mental health and pregnancy rates among individuals with infertility: a systematic review and meta-analysis. Hum. Reprod. Update. 29 (1), 71–94 (2023).
Dong, M. et al. Impact of infertility duration on male sexualfunction and mental health. J. Assist. Reprod. Genet. 39 (8), 1861–1872 (2022).
Drosdzol, A. & Skrzypulec, V. Depression and anxiety among Polish infertile couples–an evaluative prevalence study. J. Psychosom. Obstet. Gynaecol. 30 (1), 11–20 (2009).
Greil, A. L., Slauson-Blevins, K. & McQuillan, J. The experience of infertility: a review of recent literature. Sociol. Health Illn. 32 (1), 140–162 (2010).
Cedars, M. I. Evaluation of female Fertility—AMH and ovarian reserve Testing. J. Clin. Endocrinol. Metabolism. 107 (6), 1510–1519 (2022).
Vollenhoven, B. & Hunt, S. Ovarian ageing and the impact on female fertility. F1000Research 7, 1835 (2018).
Harris, B. S. et al. Markers of ovarian reserve as predictors of future fertility. Fertil. Steril. 119 (1), 99–106 (2023).
Kuang, M., Yu, Y. & He, S. Association between the age-adjusted visceral adiposity index (AVAI) and female infertility status: a cross-sectional analysis of the NHANES 2013–2018. Lipids Health Dis. 23 (1), 314 (2024).
Woolcott, O. O. & Bergman, R. N. Relative fat mass (RFM) as a new estimator of whole-body fat percentage A cross-sectional study in American adult individuals. Sci. Rep. 8 (1), 10980 (2018).
Zhu, X. et al. The relationship between depression and relative fat mass (RFM): A population-based study. J. Affect. Disord. 356, 323–328 (2024).
Zwartkruis, V. W. et al. Relative fat mass and prediction of incident atrial fibrillation, heart failure and coronary artery disease in the general population. Int. J. Obes. (Lond). 47 (12), 1256–1262 (2023).
Leisegang, K. et al. Obesity and male infertility: mechanisms and management. Andrologia 53 (1), e13617 (2021).
Talmor, A. & Dunphy, B. Female obesity and infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 29 (4), 498–506 (2015).
Barbagallo, F. et al. Molecular mechanisms underlying the relationship between obesity and male infertility. Metabolites 11(12). 840 (2021).
Pasquali, R. Obesity, fat distribution and infertility. Maturitas 54 (4), 363–371 (2006).
Zhu, X. et al. The relationship between depression and relative fat mass (RFM): A population-based study. J. Affect. Disord. 356, 323–328 (2024).
Woolcott, O. O. & Bergman, R. N. Relative fat mass (RFM) as a new estimator of whole-body fat percentage—a cross-sectional study in American adult individuals. Sci. Rep. 8 (1), 10980 (2018).
Zwartkruis, V. W. et al. Relative fat mass and prediction of incident atrial fibrillation, heart failure and coronary artery disease in the general population. Int. J. Obes. (Lond). 47 (12), 1256–1262 (2023).
Zhao, L., Cao, R. & Zhang, S. Association between relative fat mass and periodontitis: results from NHANES 2009–2014. Sci. Rep. 14 (1), 18251 (2024).
Wang, W. et al. Dietary inflammatory index and female infertility: findings from NHANES survey. Front. Nutr. 11, 1391983 (2024).
Wang, X. et al. Body fat distribution and female infertility: a Cross-Sectional analysis among US women. Reprod. Sci. 30 (11), 3243–3252 (2023).
Xu, W. et al. Insights into modifiable risk factors of infertility: a Mendelian randomization study. Nutrients 14(19), 4042 (2022).
Zhuang, J. et al. Association between visceral adiposity index and infertility in reproductive-aged women in the united States. Sci. Rep. 14 (1), 14230 (2024).
Deng, C. et al. Association between indicators of visceral lipid accumulation and infertility: a cross-sectional study based on U.S. Women. Lipids Health Dis. 23 (1), 186 (2024).
Hosseini, S. A. et al. Assessment of the appropriate cutoff points for anthropometric indices and their relationship with cardio-metabolic indices to predict the risk of metabolic associated fatty liver disease. BMC Endocr. Disord. 24 (1), 79 (2024).
Suthahar, N. et al. Associations of relative fat mass, a new index of adiposity, with type-2 diabetes in the general population. Eur. J. Intern. Med. 109, 73–78 (2023).
Muoio, D. M. & Newgard, C. B. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell. Biol. 9 (3), 193–205 (2008).
He, F. F. & Li, Y. M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J. Ovarian Res. 13 (1), 73 (2020).
Lliberos, C. et al. Evaluation of inflammation and follicle depletion during ovarian ageing in mice. Sci. Rep. 11 (1), 278 (2021).
Tchernof, A. & Despres, J. P. Pathophysiology of human visceral obesity: an update. Physiol. Rev. 93 (1), 359–404 (2013).
Rich-Edwards, J. W. et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology 13 (2), 184–190 (2002).
Catalano, P. M. & Shankar, K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 356, j1 (2017).
Kuang, M., Yu, Y. & He, S. Association between the age-adjusted visceral adiposity index (AVAI) and female infertility status: a cross-sectional analysis of the NHANES 2013–2018. Lipids Health Dis. 23 (1), 314 (2024).
Acknowledgements
We wish to thank all the patients and the staff members of the medical staff for their cooperation.
Funding
Research Project of 940th Hospital of PLA Joint Logistic Support Force, 2023YXKY009; Military Logistics Research Project (2023HQZZ-04); Science and Technology Project of Gansu Province (2023-ZD-182).
Author information
Authors and Affiliations
Contributions
T Q: Writing - Original manuscript, methodology. Z Q : Writing - Data curation.X R, L Xt, D J: Reviewer. C Yp: Writing Review Editing, Data Curation. W L: Writing - Criticism and Editing, Methodology, Formal Analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’snote
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, Q., Zhang, Q., Xia, R. et al. Association of relative fat mass with female infertility: a cross-sectional study based on NHANES 2017–2020. Sci Rep 15, 22713 (2025). https://doi.org/10.1038/s41598-025-08595-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08595-x