Abstract
Spinal stenosis frequently involves multiple regions, particularly the cervical and lumbar spine. Therefore, it is essential to identify biomarkers that can distinguish between central and peripheral neuropathy. Lysophosphatidylcholine (LPC), a biomarker involved in neuropathic pain, has been proposed as a potential diagnostic biomarker for distinguishing these conditions. This study aimed to evaluate the diagnostic utility of LPC levels in the cerebrospinal fluid (CSF) for differentiating peripheral neuropathy from central neuropathy. This study included 198 patients: 77 with cauda equina syndrome (CES), 34 with myelopathy, and 87 controls. CSF samples were collected by lumbar puncture. Using liquid chromatography-tandem mass spectrometry, six LPC species, including (16:0), (18:0), (18:1), (18:2), (20:4), and (22:6), were measured in the CSF. These LPC levels were compared among the groups, and the cutoff levels that could efficiently discriminate between the groups with high accuracy were determined. All levels of LPC species were significantly higher in the CES and myelopathy groups than in the controls, with CES showing the highest levels. LPC (18:1) and LPC (22:6) demonstrated high diagnostic accuracy in distinguishing CES from myelopathy. LPC (18:1), LPC (18:2), LPC (20:4), and LPC (22:6) demonstrated high diagnostic accuracy in distinguishing myelopathy from controls. The LPC species levels in CSF offer a reliable biomarker for differentiating CES from myelopathy. Measuring LPC species can enhance diagnostic accuracy, enabling precise treatment strategies.
Similar content being viewed by others
Introduction
Characteristic symptoms and signs of myelopathy include loss of hand dexterity, weakness, stiffness, spastic extremities, and gait dysfunction, such as a rigid or spastic gait1. The estimated rate of hospitalizations related to cervical spondylotic myelopathy is 4.04 per 100,000 person-years2. Conservative therapy is often applied to mild myelopathy, whereas operative treatment is needed for moderate-to-severe myelopathy1.
Characteristic symptoms and signs of cauda equina syndrome (CES) include bilateral neurogenic sciatica, reduced perineal sensation, altered bladder function leading to painless urinary retention, and anal tone loss3. CES affects 0.3–7 per 100,000 individuals4. Early diagnoses and treatment were found to result in satisfactory patient outcomes5.
Spinal stenosis can affect more than one level. Tandem spinal stenosis (TSS) is characterized by distinct concomitant stenosis in at least two regions of the cervical, thoracic, and lumbar spine. Patients may present with both upper and lower motor neuron symptoms and neurogenic claudication 6,7. Previous studies have reported that radiological TSS occurs in 7–60% of the population with spinal stenosis; however, symptomatic TSS affects 5–28% 6,8,9. Diagnosing TSS can be challenging because its signs and symptoms often present as a combination of those of both CES and myelopathy 6,8. Furthermore, asymptomatic cervical cord compression was reported in approximately one-third of patients with lumbar spinal stenosis10. Patients with TSS who are not well managed during conservative therapy require surgical intervention. The optimal surgical strategy for TSS is still debatable, including region selection, surgical patterns, and order8. The differentiation between CES and myelopathy is based on physical and imaging findings; however, a few objective indicators can be employed. Therefore, in TSS, a simple tool to differentiate between central and peripheral neuropathy is highly desirable. However, no biomarker utilized in clinical practice can reliably differentiate central from peripheral neuropathy or distinguish these conditions from healthy controls. Lysophosphatidylcholine (LPC) in the cerebrospinal fluid (CSF) has been reported to be useful in the diagnosis of neuropathic pain caused by spinal canal stenosis (SCS) 11,12. Based on these previous findings, this study aimed to evaluate the clinical utility of LPC species in the CSF to distinguish patients with CES from those with myelopathy.
Materials and methods
The Ethics Committee of Tokyo Medical and Dental University Hospital approved this study (M2021-323) and all participants provided written informed consent. All the methods were conducted in accordance with the relevant guidelines and regulations.
Diagnosis
CES and myelopathy were diagnosed based on medical history; typical clinical symptoms, such as intermittent claudication, upper or lower extremity pain, numbness, fine motor dysfunction, and spastic gait; and physical findings, including muscle weakness, sensory deficits, abnormal tendon reflexes, pathological reflexes, and clonus. Additionally, diagnoses were supported by magnetic resonance imaging (MRI) showing spinal nerve compression or cauda equina. The myelopathy group was defined as patients presenting with clinical myelopathy who demonstrated consolidation at the spinal cord level, as evaluated by T2-weighted sagittal and axial MRI. The CES group was defined as patients presenting with clinical CES who demonstrated consolidation at the cauda equina level, as evaluated by corresponding T2-weighted MRI. Patients were eligible for inclusion if they presented with a chief complaint of nerve pain of spinal origin, if the severity of this pain warranted consideration for surgical intervention, and if they had undergone myelography. Cases with co-occurrence of CES and myelopathy based on imaging findings and cases in which distinguishing between the two during physical examination was difficult, and patients with myelopathy and CES whose chief complaint was not of spinal origin were excluded. These diagnoses were made by medical advisors from the Japanese Society for Spine Surgery and Related Research and specialists from the Japanese Orthopedic Association. The control group, which included patients with prostate hypertrophy or bladder cancer did not exhibit neuropathic pain; this absence of neuropathic pain was confirmed by anesthesiologists. The control group with neuropathic pain was excluded. CSF was collected during lumbar anesthesia. This study included 198 patients: 77 with CES, 34 with myelopathy, and 87 controls. The patients with myelopathy included 28 with cervical myelopathy and 6 with thoracic myelopathy. All patients with cervical myelopathy had pain and numbness in the upper extremities, with 79% of this group also experiencing numbness in the lower extremities; all patients with thoracic myelopathy presented with numbness in the lower extremities; and all those with CES exhibited pain and numbness in the lower extremities. To investigate the association between LPC levels and patient-reported outcomes, we assessed health-related quality of life (HRQoL) using the EuroQol 5-Dimension 3-Level questionnaire (EQ-5D-3 L)13 and pain characteristics using the Brief Pain Inventory (BPI)14 in CES and myelopathy groups. We utilized the average pain score obtained from the Brief Pain Inventory (BPI), which was rated on a 10-point numerical rating scale (NRS).
CSF measurement
All participants provided CSF samples collected through lumbar puncture. Preliminary MRI scans were assessed, and the stenosis-free intervertebral space was selected for puncture. The CSF samples were sonicated and centrifuged, and the supernatants were collected and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). A reliable and validated method for measuring LPC in CSF using LC-MS/MS had already been established11. Briefly, LC-MS/MS was performed using a Nexera HPLC System (Shimadzu Co., Kyoto, Japan) and LCMS-8060 (Shimadzu Co., Kyoto, Japan). The LC-MS/MS system was controlled using the LabSolutions software (Shimadzu Co., Kyoto, Japan), and data were collected using the same software. The LPC types were determined by the type of acyl chain. In this study, six LPC types—LPC [16:0], [18:0], [18:1], [18:2], [20:4], and [22:6]—were monitored and analyzed. Both within-run and between-run coefficient of variation (CV) (%) were nearly < 10%, suggesting a sufficient performance for clinical introduction. We further evaluated LPC levels among the three groups by matching their age and sex (CES: 18 males, 9 females; Myelopathy: 18 males, 9 females; Control: 18 males, 9 females).
Statistical analysis
Spearman’s rank correlation coefficient analysis was performed to determine the correlation between LPC species levels and the EQ-5D-3 L index value and BPI Score. To examine the differences among the groups, the significance of the differences was evaluated using the Kruskal–Wallis test, followed by Benjamini–Krieger–Yekutieli two-stage false discovery rate correction15 as a post hoc test. The effect size for significant Kruskal–Wallis tests was calculated using eta-squared (η2). The false discovery rate (FDR) was calculated to address type I error inflation due to multiple testing. To investigate the ability of LPC species to discriminate among the pain groups, a receiver operating characteristic (ROC) curve analysis was performed. The cutoff values were determined by the Youden index. Clinical outcomes including EQ-5D-3 L and BPI were compared between patients with CES and myelopathy using the Mann–Whitney U test. JMP version 12 (SAS Institute, Cary, North Carolina, USA) was used for statistical analyses, and the graphic figures were prepared using Graphpad Prism 10 (GraphPad Software). A p-value and adjusted p-value (q) of < 0.05 were considered to indicate significance.
Results
Table 1 shows the demographic characteristics of the patients. Patients with myelopathy were younger than the other groups. Table 2 presents the correlations between LPC species levels and clinical outcomes. All levels of LPC species showed significant positive correlations with BPI scores, and nearly all LPC species levels, except for LPC (18:0), showed significant negative correlations with EQ-5D-3 L scores. BPI was higher and EQ-5D-3 L was lower in patients with CES than in those with myelopathy (Table S1). Figure 1 presents the CSF LPC species levels in the CES, myelopathy, and control groups. All levels of LPC species were higher in the CES group than in the myelopathy and control groups, and all LPC species levels were higher in the myelopathy group than in the control group. Figure 2 presents the CSF LPC species levels in the CES, myelopathy, and control groups after matching of age and sex. The results were, except for LPC (18:0), similar to those prior to age and sex matching.
Concentrations of LPC species in the CSF of the CES, myelopathy, and control groups. The differences were evaluated using the Kruskal–Wallis test, followed by Benjamini–Krieger–Yekutieli two-stage false discovery rate correction as a post hoc test. Adjusted P value (q) of < 0.05 was considered to indicate significance.
Concentrations of LPC species in the CSF of the CES, myelopathy, and control groups after matching of age and sex. The differences were evaluated using the Kruskal–Wallis test, followed by Benjamini–Krieger–Yekutieli two-stage false discovery rate correction as a post hoc test. Adjusted P value (q) of < 0.05 was considered to indicate significance.
Table 3 shows the ROC curve analyses for the LPC species in the CSF for discriminating CES from myelopathy. LPC (18:1) and (22:6), which demonstrated high diagnostic accuracy, showed significant discriminating ability for the differential diagnosis of CES and myelopathy. The areas under the curve in the ROC analyses of LPC (18:1) and (22:6) were 0.829 and 0.831, respectively. The ROC curves for LPC (18:1) and (22:6) are shown in Fig. 3.
Table 4 presents the ROC curve analyses for the CSF LPC species for differentiating myelopathy from control. LPC (18:1), (18:2), (20:4), and (22:6), which demonstrated high diagnostic accuracy, and LPC (16:0) with moderate diagnostic accuracy showed significant discriminating ability for the differential diagnosis of myelopathy and control. The areas under the curve in the ROC analyses of LPC (18:1), (18:2), (20:4), and (22:6) were 0.930, 0.846, 0.860, and 0.806, respectively. The ROC curves for LPC (18:1), (18:2), and (20:4) are presented in Fig. 4.
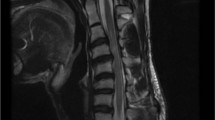
(A-1) Sagittal T2-weighted MRI showing nerve compression at L4/5. (A-2) Axial T2-weighted MRI at the L4/5 level showing central stenosis. (B-1) Sagittal T2-weighted MRI showing spinal cord compression at C3/4, C5/6, and C6/7. (B-2) Axial T2-weighted MRI at the C3/4 level, showing severe spinal cord compression. (B-3) Axial T2-weighted MRI at the C5/6 level, showing spinal cord compression. (B-4) Axial T2-weighted MRI at the C6/7 level, showing mild spinal cord compression.
Case presentation
A 53-year-old female presented with chief complaints of bilateral lower extremity pain, numbness, and intermittent claudication. MRI of the lumbar spine revealed nerve compression and central stenosis at the L4/5 level (Fig. 5A-1,A-2). Based on these clinical and radiological findings, she was diagnosed with CES.
A 75-year-old female presented with bilateral upper extremity numbness and impaired manual dexterity. MRI of the cervical spine demonstrated multi-level spinal cord compression. Specifically, sagittal views showed compression at C3/4, C5/6, and C6/7 (Fig. 5B-1). Axial images further detailed severe spinal cord compression at the C3/4 level (Fig. 5B-2) and the C5/6 level (Fig. 5B-3), with mild compression noted at the C6/7 level (Fig. 5B-4). She was subsequently diagnosed with cervical myelopathy.
Discussion
This study was conducted to differentiate CES from myelopathy by evaluating LPC in the CSF. All LPC species levels were higher in the CES group than in the myelopathy and control groups, and all LPC species levels were higher in the myelopathy group than in the control group. These results were similar after age/sex matching, except for LPC (18:0). All levels of LPC species showed significant positive correlations with pain scores. The ROC curve analyses revealed that LPC (18:1) and (22:6) were particularly useful in differentiating CES from myelopathy and LPC (18:1), (18:2), (20:4), and (22:6) in differentiating myelopathy from controls.
LPC is mainly generated from phosphatidylcholine through the enzymatic action of phospholipase A216. Extracellularly released LPC is converted into lysophosphatidic acid (LPA) by autotaxin (ATX) present in the CSF17and LPA is involved in neuropathic pain through LPA1 and LPA318,19. Indeed, the triad of LPC–ATX–LPA has been identified as a significant candidate in the development and maintenance of neuropathic pain20. LPC, locally produced due to cauda equina compression, is converted by ATX to LPA, which subsequently enters the CSF21. Regarding neuropathic pain of the spine, previous studies have shown a positive correlation between the CSF levels of lysophospholipids and the clinical severity of lumbar spinal stenosis12. Measuring CSF LPC levels is also considered useful for the differential diagnosis of SCS-related neuropathic pain11,22 and persistent spinal pain syndrome22. Even in SCS, neuropathic pains originating from CES and myelopathy are different. The measurement of central motor conduction time (CMCT), derived from motor evoked potentials (MEP) and motor conduction studies, is widely used as an objective and highly sensitive method for evaluating myelopathy 23,24. MEP is recorded by transcranial magnetic stimulation. However, CMCT and MEP measurements are technically complex and require specialization, making them not universally accessible. While MRI can independently confirm the presence of CES and myelopathy, it offers restricted insight into the underlying pathology. In this study, LPC can be measured by simply collecting CSF samples. LPC measurement allowed for the differentiation of CES from myelopathy and patients with pathologies from healthy controls. In cases where determining whether the pathology is CES or myelopathy is difficult through imaging or physical examination, LPC measurement can help in identifying the pathology.
In the treatment of TSS, patients with TSS who are not well managed conservatively require surgical intervention. A study recommended performing simultaneous surgery due to advantages, such as operating time, blood loss, and position-related complications6. Conversely, other reports recommend staged surgery because of its advantages, including long-term outcomes, invasiveness, and ability to avoid unnecessary procedures by considering the recovery time25,26,27. Regarding surgical order, cervical procedures not only resolve typical cervical spinal stenosis symptoms but can also improve lumbar symptoms, including radiculopathy and low back pain, which decreases the need for subsequent lumbar surgery28,29. Conversely, in patients with cervicolumbar TSS who predominantly present with lumbar symptoms, lumbar surgery alone provides better outcomes than cervical surgery or both procedures30. Therefore, the final decision on where to perform the surgery first should be made by evaluating the clinical manifestations and results of radiological and electrophysiological examinations8. However, such evaluations are often difficult. In this study, evaluation was performed by measuring LPC levels in the CSF. CES is suspected if LPC (18:1) is ≥ 39.3 nM and LPC (22:6) is ≥ 2.37 nM. If these criteria are not met, but LPC (18:1) is ≥ 5.45 nM, LPC (18:2) is ≥ 0.529 nM, LPC (20:4) is ≥ 0.546 nM, and LPC (22:6) is ≥ 0.291 nM, myelopathy is suspected. If none of the above criteria are met, both CES and myelopathy are considered negative as potential pathophysiological causes (Tables 3 and 4). These observed differences may be attributed to the distinct nature of pain presentation in CES and myelopathy. No studies have specifically reported on differences in pain profiles between CES and myelopathy. Most CES cases are characterized by low back pain and severe leg pain31. In contrast, myelopathy presentations are more varied, typically ranging from chronic symptoms to acute exacerbations, with numbness frequently being the predominant symptom32. Consequently, this divergence in primary clinical features—CES being largely pain-driven versus myelopathy often being numbness-driven—could plausibly explain or contribute to the variations observed in LPC levels, particularly when considering LPC as a potential indicator of pain. This can be attributed to the correlations identified between LPC and HRQoL, and between LPC and pain scores, and BPI was higher in patients with CES than in those with myelopathy (Table 2 and Table S1). Even spine specialists may find it difficult to determine whether the pathology is caused by CES or myelopathy. However, simply measuring LPC levels in the CSF and confirming the values can enable a non-spine specialist to make an accurate diagnosis. Previous reports have shown high LPC levels in patients with neuropathic pain caused by conditions other than SCS, such as postherpetic neuralgia and chemotherapy-induced peripheral neuropathy 11,33. Although LPC measurements can distinguish SCS from neuropathic pain caused by other pathologies11, physical examination and imaging findings should also be considered. Conversely, among the LPC species, LPC (18:1) is the best indicator in distinguishing SCS from non-SCS neuropathic pain11. When considering collectively the results of previous studies 11,22 and the present study, LPC (18:1) may appear to be the most reliable indicator among LPC species for distinguishing among the four groups: CES, myelopathy, neuropathic pain caused by conditions other than SCS, and healthy groups.
This study has some limitations. First, the myelopathy group was relatively small and younger. However, most of the important comparisons reached a significant difference and the results were similar after age/sex matching, except for LPC (18:0). Second, if both CES and myelopathy are present, the interpretation of LPC levels may be difficult. Third, a limitation of this study is the exclusion of radiological assessments, electrophysiological testing. It should be noted, however, that within CES and myelopathy groups, myelography was generally performed on patients whose pain and numbness were sufficiently severe to meet indications for surgical intervention. This suggests that these groups likely presented with a significant symptom burden. Furthermore, considering that LPC levels have been previously reported to correlate with neuropathic pain12and given that imaging findings in spinal diseases often demonstrate a poor correlation with clinical symptoms 34,35,36, we believe that the absence of dedicated imaging evaluations in this study imposes a relatively minor constraint on the interpretation of our findings. Fourth, this study included patients primarily presenting with neuropathic pain attributed to CES or myelopathy, and patients without neuropathic pain. However, potential confounding by other diseases, nutritional factors, disease duration, or standard CSF parameters that might affect LPC levels was not systematically evaluated. Ideally, control patients with spinal stenosis but without neurological symptoms should have been included to more precisely differentiate the biochemical changes attributable solely to myelopathy or CES from those potentially related to spinal stenosis itself. Future studies may be needed to evaluate cases of CES and myelopathy. For the future clinical application of LC-MS/MS analysis, continuous improvements in accuracy and more rigorous diagnostic criteria will be essential. Moreover, realizing its widespread clinical utility will also require addressing practical challenges, including the standardization of LC-MS/MS analytical protocols across facilities and the overall cost associated with this specialized methodology, to ensure broader accessibility.
In conclusion, LPC levels were higher in the CES and myelopathy groups than in the control group, with CES showing the highest levels. LPC (18:1) and LPC (22:6) effectively differentiated CES from myelopathy, and LPC (18:1), LPC (18:2), LPC (20:4), and LPC (22:6) effectively differentiated myelopathy from the controls. The present findings indicate that CSF LPC levels are useful for the differential diagnosis of neuropathic pain caused by CES and myelopathy and evaluation of surgical application.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bakhsheshian, J., Mehta, V. A. & Liu, J. C. Current diagnosis and management of cervical spondylotic myelopathy. Glob. Spine J. 7, 572–586. https://doi.org/10.1177/2192568217699208 (2017).
Wu, J. C. et al. Epidemiology of cervical spondylotic myelopathy and its risk of causing spinal cord injury: a National cohort study. Neurosurg. Focus. 35, E10. https://doi.org/10.3171/2013.4.Focus13122 (2013).
Greenhalgh, S., Finucane, L., Mercer, C. & Selfe, J. Assessment and management of cauda equina syndrome. Musculoskelet. Sci. Pract. 37, 69–74. https://doi.org/10.1016/j.msksp.2018.06.002 (2018).
Gavin, L., Curran, M. G. & McCabe, J. P. A comparison of available guidelines for the detection of cauda equina syndrome and assessing the need for further clinical guidance in Ireland. Ir. J. Med. Sci. 193, 1865–1872. https://doi.org/10.1007/s11845-024-03633-5 (2024).
Gardner, A., Gardner, E. & Morley, T. Cauda equina syndrome: a review of the current clinical and medico-legal position. Eur. Spine J. 20, 690–697. https://doi.org/10.1007/s00586-010-1668-3 (2011).
Overley, S. C., Kim, J. S., Gogel, B. A., Merrill, R. K. & Hecht, A. C. Tandem spinal stenosis: A systematic review. JBJS Rev. 5, e2. https://doi.org/10.2106/jbjs.Rvw.17.00007 (2017).
Dowlati, E. et al. Should asymptomatic cervical stenosis be treated in the setting of progressive thoracic myelopathy? A systematic review of the literature. Eur. Spine J. 31, 275–287. https://doi.org/10.1007/s00586-021-07046-1 (2022).
Bai, Q. et al. Current Understanding of tandem spinal stenosis: epidemiology, diagnosis, and surgical strategy. EFORT Open. Rev. 7, 587–598. https://doi.org/10.1530/eor-22-0016 (2022).
Ahorukomeye, P. et al. Outcomes in surgical treatment for tandem spinal stenosis: systematic literature review. Spine J. 22, 1788–1800. https://doi.org/10.1016/j.spinee.2022.07.088 (2022).
Farahbakhsh, F. et al. The prevalence of asymptomatic cervical spinal cord compression in individuals presenting with symptomatic lumbar spinal stenosis: A meta-analysis. Glob. Spine J. 14, 1052–1060. https://doi.org/10.1177/21925682231202776 (2024).
Kurano, M. et al. Usefulness of lysophosphatidylcholine measurement in the cerebrospinal fluid for differential diagnosis of neuropathic pain: possible introduction into clinical laboratory testing. Clin. Chim. Acta. 541, 117249. https://doi.org/10.1016/j.cca.2023.117249 (2023).
Hayakawa, K. et al. Lysophosphatidic acids and their substrate lysophospholipids in cerebrospinal fluid as objective biomarkers for evaluating the severity of lumbar spinal stenosis. Sci. Rep. 9, 9144. https://doi.org/10.1038/s41598-019-45742-7 (2019).
EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 16, 199–208. https://doi.org/10.1016/0168-8510(90)90421-9 (1990).
Uki, J., Mendoza, T., Cleeland, C. S., Nakamura, Y. & Takeda, F. A brief cancer pain assessment tool in japanese: the utility of the Japanese brief pain Inventory–BPI-J. J. Pain Symptom Manag. 16, 364–373. https://doi.org/10.1016/s0885-3924(98)00098-0 (1998).
Benjamini, Y., Krieger, A. M. & Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93, 491–507. https://doi.org/10.1093/biomet/93.3.491 (2006).
Ma, L. et al. Evidence for de Novo synthesis of lysophosphatidic acid in the spinal cord through phospholipase A2 and autotaxin in nerve injury-induced neuropathic pain. J. Pharmacol. Exp. Ther. 333, 540–546. https://doi.org/10.1124/jpet.109.164830 (2010).
Yatomi, Y. et al. Lysophospholipids in laboratory medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 94, 373–389. https://doi.org/10.2183/pjab.94.025 (2018).
Inoue, M. et al. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 10, 712–718. https://doi.org/10.1038/nm1060 (2004).
Ueda, H. LPA receptor signaling as a therapeutic target for radical treatment of neuropathic pain and fibromyalgia. Pain Manag. 10, 43–53. https://doi.org/10.2217/pmt-2019-0036 (2020).
Ueda, H. Lysophosphatidic acid signaling is the definitive mechanism underlying neuropathic pain. Pain. 158 (Suppl 1), S55–s65. https://doi.org/10.1097/j.pain.0000000000000813 (2017).
Uranbileg, B. et al. Alteration of the lysophosphatidic acid and its precursor lysophosphatidylcholine levels in spinal cord stenosis: A study using a rat cauda equina compression model. Sci. Rep. 9, 16578. https://doi.org/10.1038/s41598-019-52999-5 (2019).
Sumitani, M. et al. Cerebrospinal fluid lysophosphatidylcholine species for distinguishing narrowing of the lumbar spine. World Neurosurg. 183, e571–e575. https://doi.org/10.1016/j.wneu.2023.12.148 (2024).
Ofuji, A. et al. New method to measure central motor conduction time using transcranial magnetic stimulation and T-response. J. Neurol. Sci. 160, 26–32. https://doi.org/10.1016/s0022-510x(98)00160-9 (1998).
Mazur, M. D., White, A., McEvoy, S. & Bisson, E. F. Transcranial magnetic stimulation of the motor cortex correlates with objective clinical measures in patients with cervical spondylotic myelopathy. Spine (Phila Pa. 1976). 39, 1113–1120. https://doi.org/10.1097/brs.0000000000000358 (2014).
Cao, J. et al. Simultaneous or staged operation for tandem spinal stenosis: surgical strategy and efficacy comparison. J. Orthop. Surg. Res. 16, 214. https://doi.org/10.1186/s13018-021-02357-x (2021).
Luo, C. A. et al. Staged surgery for tandem cervical and lumbar spinal stenosis: which should be treated first? Eur. Spine J. 28, 61–68. https://doi.org/10.1007/s00586-018-5795-6 (2019).
Krishnan, A., Dave, B. R., Kambar, A. K. & Ram, H. Coexisting lumbar and cervical stenosis (tandem spinal stenosis): an infrequent presentation. Retrospective analysis of single-stage surgery (53 cases). Eur. Spine J. 23, 64–73. https://doi.org/10.1007/s00586-013-2868-4 (2014).
Alvin, M. D., Alentado, V. J., Lubelski, D., Benzel, E. C. & Mroz, T. E. Cervical spine surgery for tandem spinal stenosis: the impact on low back pain. Clin. Neurol. Neurosurg. 166, 50–53. https://doi.org/10.1016/j.clineuro.2018.01.024 (2018).
Inoue, T. et al. Primary cervical decompression surgery May improve lumbar symptoms in patients with tandem spinal stenosis. Eur. Spine J. 30, 899–906. https://doi.org/10.1007/s00586-020-06693-0 (2021).
Pennington, Z. et al. Quality of life changes after lumbar decompression in patients with tandem spinal stenosis. Clin. Neurol. Neurosurg. 184, 105455. https://doi.org/10.1016/j.clineuro.2019.105455 (2019).
Karikaran, A. et al. Cauda Equina Syndrome: A review of classification, diagnosis, treatment, and best practices. JBJS Rev. 13, e24.00156. https://doi.org/10.2106/jbjs.Rvw.24.00156.
Milligan, J., Ryan, K., Fehlings, M. & Bauman, C. Degenerative cervical myelopathy: Diagnosis and management in primary care. Can. Fam. Physician. 65, 619–624 (2019).
Kuwajima, K. et al. Lysophosphatidic acid is associated with neuropathic pain intensity in humans: An exploratory study. PLoS One. 13, e0207310. https://doi.org/10.1371/journal.pone.0207310 (2018).
Hong, J. H., Lee, M. Y., Jung, S. W. & Lee, S. Y. Does spinal stenosis correlate with MRI findings and pain, psychologic factor and quality of life? Korean J. Anesthesiol. 68, 481–487. https://doi.org/10.4097/kjae.2015.68.5.481 (2015).
Splettstößer, A. et al. Correlation of lumbar lateral recess stenosis in magnetic resonance imaging and clinical symptoms. World J. Radiol. 9, 223–229. https://doi.org/10.4329/wjr.v9.i5.223 (2017).
Yukawa, Y. et al. Postoperative changes in spinal cord signal intensity in patients with cervical compression myelopathy: comparison between preoperative and postoperative magnetic resonance images. J. Neurosurg. Spine. 8, 524–528. https://doi.org/10.3171/spi/2008/8/6/524 (2008).
Acknowledgements
We would like to thank enago (https://www.enago.jp) for English language editing.
Funding
This work was supported by the Research Project on the Elucidation of Chronic Pain (22ek0610028h0001) and the Translational Research Network Program (19 lm0203084h001) of the Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Contributions
T.T. and T.H. conceived and designed the study. All authors collected the data. T.T. and T.H. prepared the manuscript, and all authors reviewed and edited the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Takahashi, T., Hirai, T., Sumitani, M. et al. Efficacy of measuring lysophosphatidylcholine levels in human cerebrospinal fluid to differentiate myelopathy from cauda equina syndrome. Sci Rep 15, 24524 (2025). https://doi.org/10.1038/s41598-025-08715-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08715-7