Abstract
There is a growing interest in identifying dietary strategies to prevent osteoporosis. Black tea has emerged as a potential candidate due to its demonstrated effects on bone metabolism. However, the presence of caffeine in black tea may have an adverse impact on bone health. In our study, we have formulated a compound black tea beverage (CBT) with reduced caffeine content and supplemented with food additives such as Polygonatum polysaccharide. This study aims to investigate alternative mechanisms underlying the anti-osteoporotic effects of this composite black tea preparation. Mice were randomly assigned to sham (S) group, OVX group, and CBT group. After surgery, statistical differences (P < 0.05) became evident when the thermal pain threshold was reached at 5 weeks, while the grip and mechanical pain thresholds were achieved at 6 weeks, persisting until 10 weeks. Following CBT intervention, IL-17 A, TNF-α, TGF-β, β-CTX and PINP levels displayed improvements (P < 0.05). Compared to the OVX mice, CBT mice exhibited varying degrees of improvement in BV/TV, Tb.Th, Tb.N, Tb.Sp (P < 0.05) and a significant decrease in RANKL and TNF-α protein expression in the bone tissue (P < 0.001), along with a significant increase in OPG and TGF-β1 protein expression (P < 0.001). Those treated with CBT exhibited various degrees of improvement in Th17 cells, Treg cells, and the Treg/Th17 cell ratio (P < 0.05) and displayed higher expressions of FOXP3 and lower expressions of ROR-γt in the spleen tissue (P < 0.05). CBT treatment was found to decrease the relative abundance of norank_f_Maurbaculaceae and Lactobacillus, while increasing the relative abundance of Lachnospiraceae NK4A136_group and Dubosella. CBT has been shown to functional behavioral assessments and bone microstructure, thereby effectively retarding the progression of osteoporosis. This impact is postulated to stem from certain bioactive constituents within CBT that act via immune regulation and gut microbiota modulation.
Similar content being viewed by others
Introduction
Postmenopausal osteoporosis (PMOP) is a systemic skeletal metabolic disorder resulting from reduced estrogen levels in postmenopausal women. It is typified by diminished bone density, degradation of bone microstructure, and heightened bone fragility, leading to an elevated risk of fractures that can contribute to disability and mortality in the elderly1. Prolonged use of anti-osteoporosis medications often entails notable side effects and low patient adherence. Hence, there is an urgent need to explore safe and effective novel therapies for the prevention and treatment of PMOP2. One current research focus involves seeking breakthroughs in diet, particularly natural plant-based interventions.
Tea is the world’s second most consumed beverage after water, with China being its birthplace and the cradle of tea culture3. China classifies tea into six main categories: green tea, oolong tea, white tea, black tea, yellow tea, and dark tea, among which black tea is the most widely produced and consumed worldwide4. With a history of medicinal use dating back to ancient times, tea, especially black tea, is valued for its unique medicinal properties attributed to active compounds such as theaflavins, thearubigins, catechins, and flavonoids5. Literature suggests that elderly women who consume black tea have higher bone density compared to non-consumers, and it may also offer potential benefits in mitigating osteoporosis6. However, the therapeutic effects of tea in combating osteoporosis may be affected by substances like caffeine, and relying solely on tea consumption as a daily drink may not suffice for optimal outcomes7.
Polygonatum polysaccharide is a polysaccharide derived from Polygonatum sibiricum Delar. ex Redoute, a traditional Chinese medicinal and edible plant. Its aqueous extract has demonstrated inhibitory effects on NFATc1 and c-Fos expression in vitro by suppressing the phosphorylation of p65 and p38 proteins. Furthermore, it reduces the activity of tartrate-resistant acid phosphatase (TRACP), cathepsin K (CTSK), and matrix metalloproteinase-9 (MMP-9) induced by receptor activator of nuclear factor-κ B ligand (RANKL), thereby impeding the differentiation of bone marrow monocytes (BMMs) into mature osteoclasts8,9. Despite its promising potential in osteoporosis treatment, there is currently no composite anti-osteoporosis formulation involving Polygonatum polysaccharides. To address the potential negative effects of caffeine in black tea on bone metabolism and to enhance its bone protection efficacy, the present study combined Polygonatum polysaccharide with decaffeinated black tea extract to form a composite formulation. The formulation was based on the principle of complementary mechanisms: theaflavins, catechins and other polyphenols in black tea can reduce osteoclast differentiation by inhibiting the NFATc1 pathway4,11, but the caffeine component may counteract some of the effects6, so the caffeine content was reduced to less than 5% by process optimization, and at the same time, Polygonatum polysaccharide were introduced, which can reduce RANKL-induced osteoclast differentiation by inhibiting NF-κB/p38 signaling pathway. RANKL-induced osteoclast activity by inhibiting the NF-κB/p38 signaling pathway7,8, which synergizes with black tea polyphenols to form a dual anti-inflammatory and anti-resorptive effect.Regarding the 1:2 ratio of Rhizoma Polygonati and black tea, the rationale came from the optimization of bone metabolism indexes in the pre-experiment: this ratio of the composite preparation had the best effect on the enhancement of bone volume fraction (BV/TV) in ovariectomized (OVX) mice. At the compositional level in Table 1, CBT retained active polyphenols such as epigallocatechin gallate and theaflavin-3,3’-dibisgallate in black tea, and introduced polysaccharides such as Polygonatum polysaccharides A-E, the latter possibly through macromolecular adsorption. The latter may form nano-complexes with polyphenols through polymer adsorption, prolonging their intestinal residence time and enhancing their bioavailability.
In the initial phase of this research, a daily composite beverage formulation was developed by optimizing the process to minimize caffeine content while enriching it with Polygonatum polysaccharides and black tea extracts. Given the emerging roles of gut microbiota and T-cell immunity in bone homeostasis, the present study further investigated whether CBT exerts its anti-osteoporotic effects through the dual modulation of gut microbiota and Treg/Th17 cell balance. Specifically, we hypothesized that the bioactive components of CBT could restore gut microbial diversity, enrich osteoprotective flora, rebalance the Treg/Th17 cell ratio by regulating FOXP3/RORγt protein expression in splenic tissues, and reduce systemic inflammation to inhibit bone resorption. In the present study, we evaluated the above mechanisms by microbial sequencing, flow cytometry, and protein expression analysis to reveal the multi-target therapeutic potential of CBT.
Materials and methods
The materials of CBT liquid
Black tea (Jiuqu-Hongmei, Food Production Number: SC11433010600069, Hangzhou Pinzhu Tea Co., Ltd., Zhejiang, China).
Huangjing (Polygonatum sibiricum Delar. ex Redoute, production batch number: 210303, Changshan Tiandao Traditional Chinese Medicine Decoction Co., Ltd., Zhejiang, China).
Polygonatum sibiricum Delar. ex Redoute polysaccharide extract and black tea extract are both samples extracted with deionized water and freeze-dried; The ratio of Huangjing (Polygonatum sibiricum) to black tea in the water extract is 1:2. For comparison, the chemical composition of a single black tea extract without Polygonatum polysaccharide was also analyzed. The extract was prepared under identical conditions with key components listed in Table 2. After identification, the main active ingredients are shown in Table 1.
Laboratory animals and subgroups
36 eight-week-old female nulliparous C57BL/6 mice of SPF grade, weighing (20 ± 2) g, were selected for the study. The mice were procured from Zhejiang Weitong Lihua Experimental Animal Technology Co., Ltd., which possesses the necessary animal production license with batch number SYXK (Zhejiang) 2021-0012. The animals were housed under controlled conditions (25 ± 3 ℃, 50%~60% humidity, 12-h light-dark cycle), and were provided with standard nutrient pellet feed and distilled water ad libitum. All procedures strictly adhered to the guidelines and regulations set forth by the Animal Ethics Committee of the Experimental Animal Center of Zhejiang Chinese Medical University. The experiments were conducted under the approved protocol number IACUC-202402-16.
After a one-week acclimation period to the new environment, a total of 36 mice were randomly assigned to three groups with 12 mice per group: sham surgery (S) group, ovariectomy (OVX) group, and CBT group. The sham surgery group served as the blank control group, the OVX group was considered the postmenopausal osteoporosis (PMOP) model group, and the CBT group received a CBT intervention. Ovariectomy was performed on mice in the CBT and OVX groups, while mice in the Sham group underwent a sham procedure where fat of equivalent size near the ovaries was removed.
Following the bilateral oophorectomy, starting from the 3rd day, mice in the CBT group were orally administered CBT for a duration of 10 weeks. The clinical dose conversion was based on the body surface area method (mouse/human = 0.018), and the recommended dose for adults was 300 mg/kg/d (in terms of Polygonatum polysaccharide), which is equivalent to a mouse dose of 5.4 mg/kg/d. The gavage dose of CBT in the present study was 6 mg/kg/d (4 mg/kg black tea extract + 2 mg/kg Polygonatum polysaccharide), which is slightly higher than the clinically equivalent dose to ensure that effective concentrations were achieved in the ovariectomy model. Both the Sham and OVX groups of mice received the same volume of conventional distilled water. The body weight of the mice was recorded once every week, and the gavage dose was adjusted accordingly based on the body surface area. Throughout the study period, all groups of mice had unrestricted access to food and water. At the end of the 10-week treatment period, the mice were fasted for 12 h and then weighed. Subsequently, they were injected with 2% sodium pentobarbital solution (40 mg/kg). Femur, spleen, serum, and fecal samples were collected from all mice for further analysis.
Blood indicators determination by enzyme-linked immunoassay
Serum samples were analyzed to detect bone metabolism markers using commercially available ELISA kits (β-CTX (CAT: MM-44858M2), PINP (CAT: MM-44814M2), RANKL (CAT: MM-0504M2), TNF-α (CAT: MM-0132M2), TNF-γ (CAT: MM-0679M2), TGF-β (CAT: MM-46344M2), IL-10 (CAT: MM-0176M1), and IL-17 A (CAT: MM-0759M2)) obtained from Enzyme Immunoassay Biotechnology Co., Ltd., China. The serum samples were thawed at room temperature and the instructions provided in the ELISA kit were strictly followed to perform the measurements of β-CTX and PINP, RANKL, and inflammatory markers TNF-α, TNF-γ, TGF-β, IL-10, and IL-17 A levels in each group of mice.
The specific steps were as follows: Firstly, the corresponding test plate was removed from the ELISA kit and allowed to reach room temperature for 40 min. Samples were then added in a predetermined order according to the established standards and sample wells used in the pre-experiment. Following the instructions, the standard samples were added in sequence, followed by the addition of the test samples and their respective diluents. HRP-labeled antibodies were slowly added to each well, and the plate was sealed and incubated at 37 °C for 1 h. Subsequently, the test plate was removed, the liquid was poured out, and the plate was washed four times. After that, the substrate was added, the plate was wrapped in tin foil, and incubated at 37 °C for 15 min. After incubation, the plate was removed, and the reaction was terminated by adding the termination solution. The plate was immediately transferred to an ELISA reader to measure the optical density (OD) values of the samples at a wavelength of 450 nm. The OD values of each sample and the concentrations of each group were calculated based on the obtained data.
Behavioral testing related to osteoporosis
Forepaw grip force detection: The YLS-13A mouse grip tester (Shandong Academy of Medical Sciences Equipment Station, China) was utilized for measuring the forepaw grip of mice. Each mouse underwent three grip measurements, and the average value was recorded. The measurements were conducted once a week to monitor any changes in the grip strength over time.
Mechanical pain threshold: Vonfrey fibers (Danmic Global, USA) were applied to stimulate the soles of the hind paws of mice, gradually increasing pressure from 0. The mechanical pain threshold (PWT) was determined by observing the mice’s response of lifting their hind feet when experiencing pain. Each mouse underwent three plantar tests, and the average of these results was taken as the final measurement. To avoid adaptation, there was a 1-minute interval between every two tests. These measurements were conducted once a week to track any changes in the pain threshold over time.
Thermal pain threshold: An animal hot plate tester (Shanghai Xinruan, China) was employed, with the hot plate temperature set at 55 ℃. The thermal pain threshold was assessed by observing the mouse’s response of lifting its hind foot when feeling pain, and recording the paw withdrawal latency (PWL). Each mouse underwent three plantar tests with a 1-minute interval between each test, and the average of these three results was calculated as the final outcome. Measurements were conducted weekly to monitor any changes in the pain threshold over time.
Pathological and morphological analysis of bone tissue
Following drug intervention, the mice were fasted and provided only water for 24 h. The bilateral femurs were then collected and the dissected right femur was fixed in 4% paraformaldehyde at 4 ℃ for 24 h. Subsequently, decalcification was carried out in EDTA solution (pH 7.25) at 25 ℃ for a period of 28 days. The samples were dehydrated using a series of ethanol concentrations ranging from 70 to 100%, followed by embedding in paraffin for sectioning into 2–4 μm slices along the sagittal plane. These sections were stained with Alcian Blue-Eosin (ABH) to visualize morphological changes in the bone tissue under a microscope. Immunohistochemical technique was used to detect the expression of RANKL (CAT: ab239607) and OPG (CAT: ab255723) proteins in femoral tissue in paraffin sections.
Micro-CT analysis of bone tissue
Morphometric analysis of the left femur in an osteoporosis model mouse was conducted using Micro-CT (µCT 100, SCANCO Medical AG, Switzerland). The collection parameters were set as follows: resolution of 16 μm, exposure time of 300 ms, voltage of 50 kV, and current of 200 µA. The purpose of this analysis was to assess the bone trabecular structure and various parameters including bone trabecular volume fraction (BV/TV), bone trabecular density (Tb. N), bone trabecular thickness (Tb. Th), and bone trabecular separation (Tb. Sp). Following the scanning process, a 1.5-mm area at the distal femur was selected as the region of interest for further analysis. Three-dimensional image reconstruction of the bone trabeculae was performed, and subsequent analysis was carried out using Data Viewer software.
Sequencing analysis of gut microbiota
Fresh fecal samples were collected from each group and stored in liquid nitrogen-sealed tubes. The extraction of DNA from the fecal samples was performed using the HiPure fecal DNA kit (Ma River, China) following the manufacturer’s instructions. Gel electrophoresis was utilized to identify bacterial genomic DNA. The composition of gut microbiota was determined through research on the 16S rDNA gene. To enrich the 16S V3-V4 rRNA region of bacteria, barcode specific primers were used during sample DNA amplification. Each sample was tested twice in parallel for accuracy. Data clustering was conducted using the unweighted pair-group method with arithmetic mean (UPGMA) method, and a sample clustering tree was generated to assess the similarity among different samples. The PICRUSt 2 program was employed to predict the community function, utilizing KEGG pathway data to estimate the pathway status of the entire community.
Western blotting
The tibia and spleen were extracted from each group of mice, and the bone tissue was processed using the Minute™ Bone Tissue Total Protein Extraction Kit (Invent, USA). Spleen tissue was extracted using the T-PER™ Tissue Total Protein Extraction Reagent (Thermo Pierce, USA). Following extraction, the supernatant was centrifuged as per the BCA reagent kit instructions (Beyotime, China) to determine protein concentration. The denatured protein samples were then packaged and stored at −20 ℃. For SDS-PAGE electrophoresis analysis, 8–12% separation gel and 5% concentrated gel were prepared. Protein samples were separated, transferred to a polyvinylidene fluoride membrane, and the membrane was subsequently sealed. The total protein samples extracted from spleen tissue were incubated with primary and secondary antibodies for FOXP3 (Abcam, USA, CAT: ab20034) and RORγt(Thermo Fisher, USA, CAT: ab187657). Total protein samples extracted from femoral tissue were incubated with primary and secondary antibodies for TNF-α (Abcam, USA, CAT: ab183218), RANKL (Proteintech, USA, CAT: ab239607), TGF-β1 (Abcam, USA, CAT: ab142139), and OPG(Abcam, USA, CAT: ab255723). Subsequently, color exposure was performed and ImageJ software was used to analyze the developed bands, including the β-actin grayscale value as an internal reference, followed by the calculation of the ratio of actin grayscale values with respect to the other groups.
Flow cytometry detection
Treg cell labeling and detection: A portion of spleen and lymph node cell suspensions were taken and placed in flow cytometry tubes at a density of 1 × 106 cells per tube. These cells were then treated with a membrane-breaking fixation kit (Lianke Biotechnology Co., Ltd., China). Subsequently, FITC-CD4 antibody (Lianke Biotechnology Co., Ltd., China) was added at a volume of 5 µL per tube, PerCP-CD25 antibody (Lianke Biotechnology Co., Ltd., China) was added at a volume of 0.5 µL per tube, and APC FOXP3 antibody (Lianke Biotechnology Co., Ltd., China) was added at a volume of 2 µL per tube. The tubes were incubated at 4 ℃ for 30 min, followed by the addition of 2 mL of PBS per tube. After incubation, the cells were washed with 1× Permeabilization Buffer at 1200 r/min, centrifuged for 8 min, the supernatant was discarded, and the cells were resuspended in 300 µL of PBS for detection using flow cytometry.
Th17 cell labeling and detection: Spleen and lymph node cell suspensions were resuspended in RPMI 1640 cell culture medium supplemented with 10% FBS. The cell concentration was adjusted to 1 × 106 cells/mL, and 1 mL of this cell suspension was seeded in each well of a 24-well plate. To stimulate T cell activation, PMA (final concentration 25 ng/mL) and ionomycin (final concentration 1 µg/mL) were added to each well. After incubating for 4 h at 37 ℃ in a 5% CO2 incubator, Brefeldin A (final concentration 10 µg/mL) was added to block cytokines from being secreted by the cells. The cells were then collected and treated with a membrane-breaking and fixing kit (Lianke Biotechnology Co., Ltd., China). FITC-CD4 antibody (Lianke Biotechnology Co., Ltd., China) was added to each tube at a concentration of 0.5 µL, while PE-IL-17 A (Th17) antibody (Lianke Biotechnology Co., Ltd., China) was added at a concentration of 1 µL. The tubes were incubated at 4 ℃ for 30 min. Following the incubation period, 1 mL of Permeabilization Buffer wash solution was added to each tube for washing. The cells were washed once and then resuspended in 300 µL of PBS for detection using flow cytometry.
Statistical methods
The data analysis was performed utilizing SPSS 25.0 software. Quantitative data were presented as mean ± standard deviation (SD). Inter-group comparisons were carried out using one-way analysis of variance (ANOVA). LSD-t test or Dunnett’s test was selected based on the assumptions of normality and homogeneity of variance. Correlation analysis was conducted using Spearman’s rank correlation coefficient. Statistical significance was defined as *P < 0.05.
Results
The impact of CBT on behavioral changes and serum cytokines in OVX mice
Osteoporosis patients often experience a decline in their quality of daily life due to pain and reduced mobility10,11. To assess and analyze the grip strength and pain levels of three groups of mice before and 10 weeks after intervention (Figures 1A-C). The results indicated no significant differences in grip strength, thermal pain threshold, and mechanical pain threshold among the three groups prior to surgery. However, 3 days post-surgery, both the OVX group and CBT group exhibited a significant decrease in various indicators compared to the sham surgery group mice (*P < 0.05), suggesting that abdominal ovarian removal surgery might have led to reduced pain sensitivity and impairment in daily function. Subsequently, the grip strength, thermal pain threshold, and mechanical pain threshold of the OVX and CBT groups gradually improved over 1 to 4 weeks post-surgery, approaching levels similar to the sham surgery group. Notably, the CBT group mice displayed a more pronounced recovery trend in various indicators. Statistical differences (*P < 0.05) became evident when the thermal pain threshold was reached at 5 weeks, while the grip and mechanical pain thresholds were achieved at 6 weeks, persisting until 10 weeks. However, the grip and pain thresholds of mice in the OVX and CBT groups began to decline again at 6–10 weeks, possibly due to reduced pain and activity associated with active bone metabolism. However, in contrast to the OVX group, the CBT group exhibited a less pronounced decline in mice, suggesting that CBT intervention in osteoporosis may have a protective effect on pain and functional deterioration associated with active bone remodeling.
To elucidate the changes in certain cytokines among the different mouse groups, we measured the serum levels of IFN-γ, IL-10, RANKL, IL-17 A, TNF-α, and TGF-β after 10 weeks of behavioral monitoring (Fig. 1D-I). Our findings revealed that, relative to the sham surgery group, mice in the OVX group exhibited decreased levels of IFN-γ, TGF-β, and IL-10 but increased levels of RANKL, IL-17 A, and TNF-α (*P < 0.05). Following CBT intervention, these parameters showed improvements compared to the OVX group. Notably, IL-17 A, TNF-α, and TGF-β demonstrated the most significant enhancements (**P < 0.01). Furthermore, the serum levels of IFN-γ, IL-10, and RANKL in the CBT group also displayed improvements compared to those in the OVX group (*P < 0.05).
(A-C) Changes in terms of grip strength, thermal pain threshold and mechanical pain threshold in three groups of mice before and after 10 weeks of intervention (D-I) Levels of serum IFN-γ, IL-10, RANKL, IL-17 A, TNF-α, and TGF-β after 10 weeks of behavioural monitoring. n = 12, *P < 0.05, **P < 0.01, ***P < 0.001.
The impact of CBT on bone metabolism biomarkers, bone microstructure, and associated protein expression in the bone tissue of OVX mice
CBT was found to ameliorate the pain phenotype and enhance mobility in OVX mice during the active phase of bone transformation. Following intervention, bone metabolism biomarkers, bone microstructure, and related protein expression were assessed in each group of mice. In terms of bone metabolism markers (Fig. 2A-B), OVX mice exhibited elevated serum β-CTX levels and decreased PINP levels compared to sham-operated mice (*P < 0.05). However, when compared to the OVX mice, the CBT group displayed improvements in both β-CTX and PINP levels (*P < 0.05), with the most significant enhancement observed in β-CTX (**P < 0.01). These findings suggest that CBT may exert a more pronounced impact on osteoporosis by influencing the process of bone resorption.
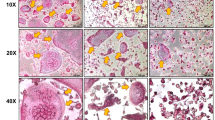
In terms of bone microstructure, ABH staining (Fig. 2C) revealed a significant decrease in the number of trabeculae beneath the growth plate of the distal femur in OVX mice when observed under a 40x light microscope, with the trabeculae appearing finer. Conversely, the CBT group demonstrated an amelioration of bone loss in OVX mice post-castration, with the number and thickness of trabeculae falling between those of the other two groups. Upon closer examination under a 100x light microscope following ABH staining (Fig. 2D), a higher presence of inflammatory cells (bone marrow-derived macrophages) was noted infiltrating the distal femur growth plate of OVX mice compared to the other two groups. Additionally, an increased occurrence of larger fat vacuoles was observed in this group, whereas the CBT group exhibited improvements in these characteristics. These changes were further confirmed using micro CT scanning. The reconstructed 3D model (Fig. 2E) demonstrated that mice in the OVX group had sparser, smaller trabeculae in the growth plate of the distal femur compared to the sham group. However, mice in the CBT group showed enhancement in these aspects. Immunohistochemistry (Fig. 2F-G) suggested that the bone tissue RANKL in the model group was significantly increased and OPG was reduced compared to the Sham group, while CBT inhibits RANKL activity in the femur of OVX mice.Upon analyzing the CT scan data (Fig. 2H-K), it was observed that both the OVX mice, compared to sham-operated mice, displayed reduced BV/TV (bone volume fraction), Tb.Th (trabecular thickness), and Tb.N (trabecular number), while showing an increase in Tb.Sp (trabecular separation) (*P < 0.05). In contrast, the CBT mice exhibited varying degrees of improvement in BV/TV, Tb.Th, Tb.N, and Tb.Sp compared to the OVX mice (*P < 0.05), with the improvements in BV/TV, Tb.Th, and Tb.Sp being particularly significant (**P < 0.01).
Based on the aforementioned indicators suggesting that CBT may inhibit the osteoclastogenesis process in OVX mice and influence certain cytokines, we proceeded to conduct Western Blot analysis on the mice’s bone tissue, targeting proteins related to the RANKL/OPG pathway and inflammatory factors (Fig. 2L). The results revealed that the expression of RANKL and TNF-α proteins in the bone tissue of OVX mice was elevated compared to the sham surgery group, whereas the expression of OPG and TGF-β1 proteins decreased (*P < 0.05). In contrast, CBT-treated mice exhibited a significant decrease in RANKL and TNF-α protein expression in the bone tissue compared to the OVX group (***P < 0.001), along with a significant increase in OPG and TGF-β1 protein expression (***P < 0.001) (Fig. 2M-P).
(A-B) Changes in bone metabolism biomarkers, bone microstructure and related protein expression in three groups of mice after CBT intervention. (C-D) Representative images of ABH staining (40× scale bar: 1000 μm, 100× scale bar: 400 μm) (E) Analysis of the distal femoral epiphyseal region of mice using micro-computed tomography (CT). (F-G) Representative images of Immunohistochemistry of RANKL and OPG. (H-K) Comparison of BV/TV, Tb.Th, Tb.N, Tb.Sp. (L) Protein blot analysis of bone tissue targeting proteins associated with the RANKL/OPGpathway and inflammatory factors. (M-P) Expression of RANKL, TNF-α, OPG, and TGF-β1 proteins in bone tissues. n = 12, *P < 0.05, **P < 0.01, ***P < 0.001.
The impact of CBT on the ratio of Treg/Th17 cells and the expression of associated transcription proteins in the spleen tissue of OVX mice
Based on the effects of CBT on serum cytokines in OVX mice, particularly on the expression of cytokines in Th17 and Treg cells, we investigated the proportion of Treg/Th17 cells and the expression of related transcription proteins in the spleen tissues of each mouse group (Fig. 3A-H). Our results demonstrated that the proportion of Th17 cells in the spleen tissue of OVX group mice increased compared to the sham surgery group, while the proportion of Treg cells decreased. Consequently, the Treg/Th17 cell ratio decreased significantly (*P < 0.05). In comparison to OVX mice, those treated with CBT exhibited various degrees of improvement in Th17 cells, Treg cells, and Treg/Th17 cell ratio (*P < 0.05).
Furthermore, further examination of Th17 and Treg cell-related transcription proteins in spleen tissue revealed a reduction in FOXP3 expression and an increase in ROR-γt expression in the OVX group mice as opposed to the sham surgery group (*P < 0.05). In contrast, CBT-treated mice displayed higher expressions of FOXP3 and lower expressions of ROR-γt in the spleen tissue compared to OVX mice (*P < 0.05). These findings suggest that CBT may regulate the differentiation of naive CD4 + cells into Treg cells within OVX mouse spleen cells while inhibiting the process of Th17 cell differentiation.
(A) Flow cytometry quantification of cell numbers in three groups with CD25 + FOXP3+/CD4 + staining. (B) Flow cytometry quantitative analysis of cell numbers in three groups, IL-17 A+/CD4 + staining. (C-E) Expression ratios of Th17, Treg, and Treg/Th17 in the spleen. (F-H) Blotting analysis of Th17 and Treg cell-associated transcriptional proteins in splenic tissues, changes in the expression ratios of FOXP3/β-catin and ROR-γt/β-catin among the three groups. n = 12, *P < 0.05, ***P < 0.001.
The impact of CBT on the gut microbiota of OVX mice
CBT alters gut microbiota diversity in OVX mice
Further investigation into the impact of CBT on gut microbiota was conducted using 16 S rRNA sequencing to analyze the fecal microbiota of 18 samples from 3 groups. α Diversity, as indicated by Sobs, ACE, and Chao indices, reflects community species richness. Among these indices, the CBT group exhibited higher Sobs, ACE, and Chao index values compared to the sham group, suggesting that CBT treatment augmented the abundance and diversity of gut microbiota and enhanced community richness (Fig. 4A-C). Shannon and Simpson indices represent community diversity, with the CBT group demonstrating elevated Shannon and Simpson index values compared to the OVX group, signifying an increase in community diversity due to CBT treatment (Fig. 4D-E). β Diversity analysis utilized PCoA analysis to elucidate alterations in gut microbiota. PCoA analysis (Fig. 4F) based on the Bray-Curtis distance algorithm revealed distinct separation between the OVX and Sham groups, while the CBT and Sham groups were closely positioned and overlapped, indicating substantial differences in microbial communities among OVX group mice and the Sham and CBT groups. CBT treatment mitigated these differences, supported by NMDS analysis (Fig. 4G) at the same distance confirming altered aggregation patterns. The stress value measurement analysis yielded a value of less than 0.2, affirming the reliability of the NMDS analysis. Irrespective of statistical significance, partial least squares discriminant analysis (PLS-DA) (Fig. 4H-I) was employed to visually illustrate the separation between groups at the OTU and genus levels, showing significant differentiation between groups.
(A-E) Comparison of Sobs, ACE, Chao, Shannon, and Simpson index values in α-diversity analysis (F) PCoA analysis based on the Bray-Curtis distance algorithm (G) NMDS analysis (Fig. 4H-I) Partial Least Squares Discriminant Analysis (PLS-DA) at the OTU and Genus levels. n = 6, *P < 0.05.
CBT alters the composition of gut microbiota at the multispecies level in OVX mice
In order to investigate specific changes in bacterial communities, a clustering histogram was utilized to depict the phylum, family, and genus levels in each sample. At the phylum level (Fig. 5A), Bacteroidetes and Firmicutes were found to be predominant, and their respective percentages were analyzed. The findings revealed an increase in the relative abundance of Bacteroidetes in the OVX group compared to the sham group, while the relative abundance of Firmicutes decreased. Conversely, CBT treatment led to a reduction in the relative abundance of Bacteroidetes and an increase in the relative abundance of Firmicutes.
At the family level (Fig. 5B), Muribaculaceae, Lachnospiraceae, Erysipelotrichaceae, Lactobacillaceae, and Prevotellaceae were identified as the principal species, and their percentages were analyzed. The results indicated an increase in the relative abundance of Muribaculaceae, Lachnospiraceae, and Prevotellaceae in the OVX group compared to the sham group, along with a decrease in the relative abundance of Erysipelotrichaceae and Lactobacillaceae. Following CBT treatment, the relative abundance of Muribaculaceae and Prevotellaceae was reduced, while there was a notable increase in the relative abundance of Erysipelotrichaceae and Lachnospiraceae, with minimal impact on the relative abundance of Lactobacillaceae.
At the genus level (Fig. 5C), the dominant species were norank_f_muribaculaceae, Lactobacillus, Lachnospiriaceae NK4A136_group, Dubosella, and Alloprevotella, with their respective percentages analyzed. Results indicated an increase in the relative abundance of norank_f_Muribaculaceae and Lachnospiriaceae NK4A136_group in the OVX group compared to the sham group, while the relative abundance of Lactobacillus and Dubosella decreased, having minimal impact on Alloprevotella. Following CBT treatment, the relative abundance of norank_f_muribaculaceae and Lachnospiriaceae NK4A136_group was reduced. The relative abundance of rank_f_ Maurbaculaceae and Lactobacillus increased, affecting the relative abundance of Lachnospiraceae NK4A136_group and Dubosella, with little effect on Alloprevotella.
A Venn diagram (Fig. 5D) was constructed to illustrate the overlaps between the Sham group, OVX group, and CBT group. The total number of overlaps among the three groups was 496, with 834 in the Sham group, 838 in the OVX group, and 1022 in the CBT group. To further explore differences in gut microbiota among the groups, specific bacterial phenotypic changes were identified at each phylogenetic level using LEfSe (Fig. 5E), revealing biomarkers with *P < 0.05 and LDA > 3.0 indicative of significant differences in rank sum tests. A total of 51 specific bacteria were categorized into 3 groups, including 30 in the sham surgery group, 16 in the OVX group, and 5 in the CBT group (Fig. 5F). The Sham group with LDA > 4.0 exhibited Helicobacter, Turicibacter, and Lactobacillus; the OVX group showed norank_f_ Maurbaculaceae; and the CBT group featured Dubosella (Fig. 5G).
(A-C) Relative abundance analyses of bacterial communities at the phylum, family and genus levels. (D) Venn diagrams were constructed for the Sham, OVX and CBT groups (E) LEfSe identified specific bacterial phenotypic variations (LDA > 3.0) (F) LDA score plots (LDA > 3.0) (G) Intestinal flora species (LDA > 4). n = 6, *P < 0.05, **P < 0.01, ***P < 0.001.
Correlation between gut microbiota and serum cytokine indicators in OVX mice
In Spearman correlation analysis, we examined the relationship between various metabolites and the top 20 abundant phyla, families, and genera to assess the impact of CBT on the gut microbiota and metabolic interactions in OVX mice (Fig. 6A-C). Our data revealed that at the phylum level, Bacteroidota exhibited a positive correlation with IL-17 A and IFN-γ, while showing a negative correlation with IL-10. Firmicutes demonstrated a positive correlation with IFN-γ and IL-10, and a negative correlation with IL-17 A. Campilobacterota was positively correlated with IFN-γ, TGF-β, and IL-10, but significantly negatively correlated with IL-17 A and RANKL. At the family level, Helicobacteraceae, Marinifilaceae, Clostridaceae, Peptostreptococcaceae showed positive correlations with IFN-γ, TGF-β, and IL-10, while displaying negative correlations with IL-17 A and RANKL. Moving down to the genus level, norank_f_Muribaculaceae, Muribaculum were found to have negative correlations with IFN-γ, TGF-β, and IL-10, but positive correlations with IL-17 A and RANKL. The correlation with TNF-α and gut microbiota appeared relatively weak.
Furthermore, PICRUSt2 analysis (Fig. 6D) identified the top 10 KEGG pathways, including Biosynthesis of secondary metabolites, Microbial metabolism in diverse environments, Biosynthesis of amino acids, Ribosome, Purine metabolism, Pyrimidine metabolism, Glycolysis/Gluconeogenesis, Aminoacyl-tRNA biosynthesis.
Discussion
Black tea is a popular beverage consumed worldwide, known for its ability to effectively protect and repair monocyte oxidative stress and bone loss resulting from ovariectomy4,12. While increasing estrogen levels in OVX mice can help prevent osteoporosis, the high caffeine content in black tea limits its product development. To address these limitations, we have developed a CBT preparation that removes most of the caffeine, a product was reviewed by the China National Intellectual Property Administration. Following the principles outlined in the “Evaluation Method for Increasing Bone Density Function of Health Food,” it is recommended to utilize a bilateral ovariectomy-induced osteoporosis model in rodents, as it simulates bone loss due to estrogen deficiency in postmenopausal women13,14. Micro-CT technology, known for its high-resolution histological detection and non-invasive imaging capabilities, allows for clear scanning and reconstruction of the three-dimensional bone trabecular structure. This technology enables the evaluation of both overall bone mass parameters and provides morphological and quantitative assessments of bone microstructure15,16. In this study, Micro CT was employed to assess changes in bone microstructure in mice with osteoporosis induced by bilateral ovariectomy and treated with CBT. The results demonstrated that mice treated with CBT exhibited denser bone trabeculae distribution, improved morphological structure, and reduced fracture incidences compared to the model group. The enhanced bone density and trabecular thickness led to an increase in trabecular volume fraction and a decrease in trabecular separation17. These findings provide experimental support for the synergistic effects of CBT in the treatment of osteoporosis (Fig. 7A). Although the present study did not address bone mechanical strength, Micro-CT parameters (e.g., BV/TV, Tb.Th) are highly correlated with mechanical properties, and the increase in BV/TV in the CBT group suggests a potential mechanical improvement.
Normal bone homeostasis in the human body is intricately regulated by the delicate balance between osteoblasts and osteoclasts. Any disruption in this balance, leading to either excessive bone resorption by osteoclasts or inadequate bone formation by osteoblasts, can contribute to the development of osteoporosis. Therefore, it is of paramount importance to investigate drugs or health products that can effectively treat or prevent osteoporosis by inhibiting osteoclast differentiation and/or promoting osteoblast proliferation. The postmenopausal osteoporosis model induced by bilateral ovariectomy in mice utilized in this experiment represents a type of osteoporosis characterized by increased osteoclast proliferation. However, there has been limited research conducted on models that specifically promote osteoblast proliferation. Therefore, future studies should focus on developing such models to comprehensively elucidate the role of CBT in mitigating osteoporosis. Furthermore, since this study employed a compound extract, further analysis of its active ingredients will contribute to establishing quality control standards for future product development. This study confirms the efficacy of CBT in improving bone microstructure. Previous research has indicated that various signaling pathways are involved in osteoclast differentiation, including but not limited to OPG/RANKL/RANK18, NF-κB19,20, c-Src, PI3K-AKT21,22, and MAPKs23,24,25. Similarly, common signaling pathways associated with osteoblast proliferation include Wnt/β-Catenin26,27, BMP/Smads28, Notch29, and Hedgehog30. Our investigation suggests that the active ingredients present in CBT may exert their effects through the OPG/RANKL/RANK signaling pathway. However, further research is warranted, particularly through cell biology and molecular biology experiments, to gain a more comprehensive understanding of the underlying mechanisms31.
The immune system and the skeletal muscle system are intricately linked. In general, B lymphocytes play a role in reversing bone destruction to promote bone protection, while T lymphocytes are more involved in mediating bone loss induced by estrogen deficiency. Postmenopausal osteoporosis is essentially a chronic inflammatory orthopedic condition. In the presence of inflammation, T cells are activated in postmenopausal osteoporosis patients, leading to a hindered differentiation of bone marrow mesenchymal stem cells (BMMSCs) into osteoblasts32. Consequently, researchers have developed T cell reduction nanoparticles (TDNs) in recent years, which have shown efficacy in mitigating the osteopenia phenotype and correcting the osteogenic differentiation defect in castrated mice. The proportion of T lymphocytes in human bone marrow cells is approximately 5%. Under the influence of cytokines such as TGF-β, T lymphocytes can differentiate into CD4 + T cells, which further differentiate into two “functionally opposite” yet complementary cell types: Th17 and Treg. Th17 and Treg play opposing roles in the immune system, with Th17 mediating inflammatory responses and autoimmune diseases, while Treg cells work to suppress these processes and maintain homeostasis. Subgroup regulatory T cells (iTregs) within the Treg population can be activated by cytokines like interleukin-2, leading to the production of IL-10 and TGF-β33. These iTreg cells can secrete TGF-β and use intracellular effectors like mitogen-activated protein kinase (MAPK) to induce BMMSC differentiation into osteoblasts, thereby promoting their proliferation and differentiation. Research indicates that interleukin-10 (IL-10) plays a crucial role in promoting osteoblast formation by enhancing osteoblast (OB) synthesis and the secretion of osteoprotegerin (OPG). Numerous studies have demonstrated that a deficiency in IL-10 can result in decreased bone mass in experimental mice34,35. Conversely, the upregulation of IL-10 has been shown to effectively inhibit the progression of osteoporosis in postmenopausal mice. In patients with postmenopausal osteoporosis (PMOP), serum IL-10 levels are notably reduced, but the expression of IL-10 is significantly upregulated following osteoporosis treatment. Experimental findings suggest that IL-10 produced by inducible regulatory T cells (iTregs) can modulate osteoblast proliferation and differentiation, thereby influencing the course of osteoporosis.
Th17 and Treg cells have the capacity to release specific cytokines that regulate the balance between osteoclasts and osteoblasts. FOXP3, a surface marker and specific transcription factor of Treg cells, is involved in the regulation of cytokines such as TGF-β and IFN-γ, as well as the activation of signaling molecules like MAPK and Smad, which induce the differentiation of bone marrow mesenchymal stem cells into osteoblasts. On the other hand, RORγt, a surface marker of Th17 cells and a specific transcription factor, controls the secretion of cytokines like IL-17 and IL-6. Additionally, a study by Talaat RM et al. examined the effects of bisphosphonates on PMOP patients before and after treatment, measuring relevant serum indicators. The levels of Th17-regulated cytokines, such as IL-6 and IL-23, were significantly reduced after treatment, while the levels of Treg-regulated cytokines, such as IL-10 and TGF-β, also demonstrated significant decreases post-treatment36. These findings suggest that bisphosphonates may be employed in OP treatment by modulating the Th17/Treg balance. Previous research has indicated that estrogen deficiency can lead to aberrant expression of FOXP3 and RORγt, thereby affecting the Th17/Treg equilibrium37. FOXP3 and RORγt are specific proteins associated with Treg and Th17 cells, and their expression levels partly reflect the quantity of Treg and Th17 cells. In the context of estrogen deficiency, Treg cells can synthesize and secrete IL-10, which promotes an increase in osteoprotegerin (OPG) content in osteoblasts.
In this study, we examined the protein expression levels of FOXP3 and RORγt, detected IL-10 protein expression levels, assessed the quantity and proportion of Th17 and Treg cells in mouse spleen tissue using flow cytometry, and evaluated the expression levels of FOXP3 and RORγt in spleen tissue using western blotting. The experimental results demonstrated that traditional Chinese medicine Compound Baitouweng (CBT) can increase the number of Treg cells and decrease the number of Th17 cells, consequently elevating the Treg/Th17 ratio and restoring the Th17/Treg balance. These findings suggest that CBT can effectively regulate the Th17/Treg equilibrium and thereby achieve its anti-osteoporotic effects. Furthermore, both western blotting and flow cytometry revealed that CBT upregulates the expression of FOXP3 and downregulates the expression of RORγt in spleen tissue, highlighting its ability to modulate the Treg/Th17 balance. The consistent trends observed between the flow cytometry and western blotting results further support CBT’s corrective effect on the Treg/Th17 imbalance in bone tissue. This mechanism could potentially serve as a therapeutic approach for PMOP.
In recent years, numerous studies have demonstrated the crucial role of gut microbiota in regulating bone metabolism38,39. Alpha diversity analysis is capable of reflecting the richness and diversity of microbial communities40. The results of our study revealed a decrease in the alpha diversity of intestinal microbiota in mice from the OVX group, which is consistent with previous findings. Beta diversity analysis, on the other hand, allows for the evaluation of differences in the community structure of gut microbiota. Samples with high similarity in community structure tend to cluster together, while those with significant differences in community structure tend to be noticeably separated. Our research identified substantial differences in the microbial communities of mice from the OVX group compared to the Sham and CBT groups. Moreover, CBT treatment was found to ameliorate these differences, indicating that CBT intervention can be beneficial in improving the gut microbiota of OVX mice. At the phylum level, the relative abundance of Bacteroidetes was observed to increase in the OVX group compared to the Sham group, whereas the relative abundance of Firmicutes decreased. However, CBT treatment led to a reduction in the relative abundance of Bacteroidetes and an increase in the relative abundance of Firmicutes. The phylum Bacteroidetes consists of various Gram-negative bacteria present in the gastrointestinal tract. Lipopolysaccharides (LPS), which are components of the outer membrane of Gram-negative bacteria, can stimulate the production of pro-inflammatory cytokines41. These LPS-induced pro-inflammatory cytokines actively participate in the process of osteoclast formation and bone destruction. At the genus level, CBT treatment was found to decrease the relative abundance of norank_f_ Maurbaculaceae and Lactobacillus, while increasing the relative abundance of Lachnospiraceae NK4A136_group and Dubosella. However, it had little impact on the relative abundance of Alloprevotella, indicating that this particular microbial community may play a crucial role in the preventive and therapeutic effects of CBT on postmenopausal osteoporosis (PMOP). In Spearman correlation analysis, at the genus level, there were correlations observed between norank_f_ Maurbaculaceae, Muribaculum, IFN-γ, TGF-β, IL-10, IL-17 A, RANKL, and TNF-α. Notably, the correlations between these inflammatory factors and gut microbiota were relatively weak. Zhang et al. also reported that regulating the abundance of norank_f_ Maurbaculaceae microbiota in the intestines can effectively prevent the development of PMO. Additionally, Irene Maier found a significant correlation between the abundance of Muribaculum and the bone microstructure in osteoporotic mouse models, which is consistent with our study’s findings. Furthermore, this correlation was found to be significantly associated with the expression levels of IL-17 A (Fig. 7B-C).
In exploring the mechanisms underlying these effects in CBT, beyond the established role of black tea in modulating inflammation and gut microbiota, our understanding suggests that Polygonatum polysaccharides within the compound may also exert a certain influence. Black tea is rich in catechins and theaflavins, which are the core active ingredients. Theaflavins reduce the expression of RANKL-induced osteoclast differentiation markers by inhibiting the NFATc1 signaling pathway, and the expression of RANKL protein in bone tissues of the CBT group was lower than that of the OVX group in the present study, which is consistent with the antioxidant effect of theaflavins, whereas the catechins (e.g., EGCG) inhibit the damage to osteocytes caused by oxidative stress by scavenging free radicals [[11]]..Catechins (e.g. EGCG) inhibit the damage to osteoblasts by scavenging free radicals, and the level of serum antioxidant factor TGF-β1 in the CBT group was higher than that in the OVX group, indirectly reflecting its protective effect on the microenvironment of osteogenesis. Polygonatum polysaccharide, classified as a traditional Chinese medicine polysaccharide, distinguishes itself from conventional medicinal polysaccharides by its frequent application as a food additive due to its favorable compatibility with the human body and minimal adverse effects. Simultaneously, the source material for this extract currently serves a dual function in both Chinese medicine and culinary applications. It contains a variety of glycan components, which promotes the differentiation of Treg cells through the up-regulation of the expression of FOXP3 protein in the spleen, and reduces the proportion of Th17 cells by inhibiting the expression of RORγt to restore Treg/Th17 ratio to a value close to that of the sham-operated group. It restored the Treg/Th17 ratio to a level close to that of the sham group, accompanied by an increase in serum IL-10 and a decrease in IL-17 A, while increasing the relative abundance of the beneficial bacteria Lachnospiraceae NK4A136_group and Dubosella, and decreasing that of the harmful bacteria norank_f_. Muribaculaceae and Lactobacillus, in which the enrichment of Dubosella was positively correlated with the serum TGF-β1 level, and the decrease of Muribaculaceae was significantly correlated with the decrease of IL-17 A, suggesting that the metabolites of the bacterial flora may regulate bone metabolism through the intestinal-osteobone axis.
Reports indicate that Polygonatum polysaccharides and black tea could potentially impact metabolic processes within the intestines, involving short-chain fatty acids (SCFAs) and tryptophan. SCFAs, as gut microbiota metabolites encompassing propionate, butyrate, and acetate, play crucial roles in bone metabolism42. Studies have demonstrated that supplementing SCFAs to osteoporotic mice not only impedes osteoclast differentiation and downregulates the expression of osteoclast-associated genes such as TRAF6 and NFATc1 but also stimulates osteogenic differentiation and formation, thereby enhancing the microstructure of mouse bones43. (Fig. 7B-C) Nevertheless, the current scope of our research is constrained by limited resources and materials, hindering an in-depth exploration of this aspect of the effect. Subsequently, further investigations will delve into this domain in future endeavors.
(A) Schematic diagram of the effect of CBT intervention in ovariectomized osteoporotic mice; (B) Main and possible mechanism of CBT extract of improving bone metabolism through Treg/Th17 cell balance; (C) Main and possible mechanisms of improving bone metabolism through intestinal microecological balance.
Conclusion
CBT has been shown to enhance pain and functional behavioral assessments, as well as improve bone microstructure and markers of bone metabolism in OVX mice, thereby effectively retarding the progression of osteoporosis. This regulatory impact is postulated to stem from certain bioactive constituents within CBT that act via modulation of gut microbiota and the regulation of Treg/Th17 cell equilibrium in the spleen.
Data availability
Data are provided in manuscripts information documents. Due to the ongoing nature of related research and potential privacy concerns of the samples, the datasets generated and/or analyzed during the current study are not publicly available, but can be obtained from the corresponding author upon reasonable request. The corresponding author can be contacted at 1010002697@qq.com.
References
Li, Z. et al. Dual targeting of bile acid receptor-1 (TGR5) and farnesoid X receptor (FXR) prevents Estrogen-Dependent bone loss in mice. J. Bone Min. Res. 34 (4), 765–776 (2019).
Al-Saleh, Y. et al. Diagnosis and management of osteoporosis in Saudi arabia: 2023 key updates from the Saudi osteoporosis society. Arch. Osteoporos. 18 (1), 75 (2023).
Pan, H., Gao, Y. & Tu, Y. Mechanisms of body weight reduction by black tea polyphenols. Molecules 21 (12), 1659 (2016).
Pan, S. Y. et al. Tea and tea drinking: china’s outstanding contributions to the mankind. Chin. Med. 17 (1), 27 (2022).
Gao, Y., Rankin, G. O., Tu, Y. & Chen, Y. C. Inhibitory effects of the four main Theaflavin derivatives found in black tea on ovarian Cancer cells. Anticancer Res. 36 (2), 643–651 (2016).
Walallawita, U. S., Wolber, F. M., Ziv-Gal, A., Kruger, M. C. & Heyes, J. A. Potential role of lycopene in the prevention of postmenopausal bone loss: evidence from molecular to clinical studies. Int. J. Mol. Sci. 21 (19), 7119 (2020).
Yang, C. S., Zhang, J., Zhang, L., Huang, J. & Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 60 (1), 160–174 (2016).
Zhang, C. et al. Morinda officinalis polysaccharides ameliorates bone growth by attenuating oxidative stress and regulating the gut microbiota in Thiram-Induced tibial dyschondroplasia chickens. Metabolites 12 (10), 958 (2022).
Pingping, W. Study on the Mechanism of polygonatum Polysaccharide on Osteoclast Differentiation Based on miR: 1224 (Guangxi Medical University) (2018).
Luo, L., Lin, J., Ma, W. & Fan, J. Effectiveness of teriparatide in in improving healing rates and bone-turnover markers of osteoporotic hip fracture: a meta-analysis. J. Pak Med. Assoc. 74 (4), 741–751 (2024).
Montgomery, G. et al. Daily pain severity but not vertebral fractures is associated with lower physical activity in postmenopausal women with back pain. J. Aging Phys. Act. 32 (3), 428–437 (2024).
Chen, C. C. et al. Association of coffee and tea intake with bone mineral density and hip fracture: A Meta-Analysis. Med. (Kaunas). 59 (6), 1177 (2023).
Liang, J. et al. Neferine alleviates ovariectomy-induced osteoporosis by enhancing osteogenic differentiation of bone marrow mesenchymal stem cells via regulation of the p38MAPK pathway. Connect. Tissue Res. 65 (3), 253–264 (2024).
Park, O. J. et al. Muramyl dipeptide alleviates Estrogen deficiency-induced osteoporosis through canonical Wnt signaling. J. Pathol. 260 (2), 137–147 (2023).
Jeong, C. et al. Ulmus Macrocarpa hance trunk bark extracts inhibit RANKL-induced osteoclast differentiation and prevent ovariectomy-induced osteoporosis in mice. J. Ethnopharmacol. 319 (Pt 3), 117285 (2024).
Qi, H., Shen, E., Shu, X., Liu, D. & Wu, C. ERK-estrogen receptor α signaling plays a role in the process of bone marrow mesenchymal stem cell-derived exosomes protecting against ovariectomy-induced bone loss. J. Orthop. Surg. Res. 18 (1), 250 (2023).
Hossain, M., Sultana, T., Moon, J. E., Moon, G. S. & Jeong, J. H. Anti-osteoporotic potential of a probiotic mixture containing Limosilactobacillus reuteri and Weissella cibaria in ovariectomized rats. Sci. Rep. 15 (1), 18586 (2025).
Theill, L. E., Boyle, W. J. & Penninger, J. M. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 20, 795–823 (2002).
Galibert, L., Tometsko, M. E., Anderson, D. M., Cosman, D. & Dougall, W. C. The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-kappaB, a member of the TNFR superfamily. J. Biol. Chem. 273 (51), 34120–34127 (1998).
Lin, T. H. et al. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-κB as a therapeutic target. Acta Biomater. 10 (1), 1–10 (2014).
Ke, K. et al. Reactive oxygen species induce the association of SHP-1 with c-Src and the oxidation of both to enhance osteoclast survival. Am. J. Physiol. Endocrinol. Metab. 307 (1), E61–E70 (2014).
Chen, L. L. et al. PI3K/AKT pathway involvement in the osteogenic effects of osteoclast culture supernatants on preosteoblast cells. Tissue Eng. Part. A. 19 (19–20), 2226–2232 (2013).
Choi, S. W. et al. Anti-osteoclastogenic activity of Matairesinol via suppression of p38/ERK-NFATc1 signaling axis. BMC Complement. Altern. Med. 14, 35 (2014).
He, J. et al. p38 MAPK in myeloma cells regulates osteoclast and osteoblast activity and induces bone destruction. Cancer Res. 72 (24), 6393–6402 (2012).
Tai, T. W., Su, F. C., Chen, C. Y., Jou, I. M. & Lin, C. F. Activation of p38 MAPK-regulated Bcl-xL signaling increases survival against Zoledronic acid-induced apoptosis in osteoclast precursors. Bone 67, 166–174 (2014).
Shi, Y. C. et al. Effects of continuous activation of vitamin D and Wnt response pathways on osteoblastic proliferation and differentiation. Bone 41 (1), 87–96 (2007).
Monroe, D. G., McGee-Lawrence, M. E., Oursler, M. J. & Westendorf, J. J. Update on Wnt signaling in bone cell biology and bone disease. Gene 492 (1), 1–18 (2012).
Chen, G., Deng, C. & Li, Y. P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 8 (2), 272–288 (2012).
Zanotti, S. et al. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology 149 (8), 3890–3899 (2008).
Kim, W. K., Meliton, V., Bourquard, N., Hahn, T. J. & Parhami, F. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress. J. Cell. Biochem. 111 (5), 1199–1209 (2010).
Wu, C. S. et al. The alterations of molecular repertoire of the RANKL-induced osteoclastogenesis in the M1 macrophage-derived inflammatory milieu. Sci. Rep. 15 (1), 16137 (2025).
Yang, X. et al. T cell-depleting nanoparticles ameliorate bone loss by reducing activated T cells and regulating the Treg/Th17 balance. Bioact Mater. 6 (10), 3150–3163 (2021).
Fischer, L. et al. FOXP3 + Regulatory T cells in bone and hematopoietic homeostasis. Front. Endocrinol. (Lausanne). 10, 578 (2019).
Xiong, Y. et al. IL-10 induces MC3T3-E1 cells differentiation towards osteoblastic fate in murine model. J. Cell. Mol. Med. 24 (1), 1076–1086 (2020).
Sapra, L. et al. Regulatory B cells (Bregs) inhibit osteoclastogenesis and play a potential role in ameliorating Ovariectomy-Induced bone loss. Front. Immunol. 12, 691081 (2021).
Talaat, R. M., Sidek, A., Mosalem, A. & Kholief, A. Effect of bisphosphonates treatment on cytokine imbalance between TH17 and Treg in osteoporosis. Inflammopharmacology 23 (2–3), 119–125 (2015).
D’Amelio, P. et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 43 (1), 92–100 (2008).
Li, J. Y. et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Invest. 126 (6), 2049–2063 (2016).
He, J. et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY). 12 (9), 8583–8604 (2020).
Wang, J. et al. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 5, e3450 (2017).
Mörmann, M. et al. Lipopolysaccharides (LPS) induce the differentiation of human monocytes to osteoclasts in a tumour necrosis factor (TNF) alpha-dependent manner: a link between infection and pathological bone resorption. Mol. Immunol. 45 (12), 3330–3337 (2008).
Zaiss, M. M., Jones, R. M., Schett, G. & Pacifici, R. The gut-bone axis: how bacterial metabolites Bridge the distance. J. Clin. Invest. 129 (8), 3018–3028 (2019).
Hao, H., Liu, Q., Zheng, T. & Li, J. Oral Milk-Derived extracellular vesicles inhibit osteoclastogenesis and ameliorate bone loss in ovariectomized mice by improving gut microbiota. J. Agric. Food Chem. 72 (9), 4726–4736 (2024).
Funding
Zhejiang Province Traditional Chinese Medicine Science andTechnology Program Proiect(No:2023ZL429); General scientific researchprojects of Zhejiang Provincial Department of Education (No: Y202044448); Zhejiang Province College Student Science and Technology Innovation Project (No:2017R410007); 2023 Chinese college students Innovation and Entrepreneurship training program (No:202310344039).
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The animal experiments conducted in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Zhejiang Chinese Medical University, and were performed in accordance with relevant guidelines and regulations, including the ARRIVE guidelines. All procedures strictly adhered to the guidelines and regulations set forth by the Animal Ethics Committee of the Experimental Animal Center of Zhejiang Chinese Medical University. The experiments were conducted under the approved protocol number IACUC-202402-16.
Consent to participate
Not applicable.
Consent to publish
The authors agree to publication. This manuscript has not been published in any journals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, S., Yu, Y., shu, J. et al. Black tea rich in polygonatum sibiricum polysaccharides inhibits osteoporosis in ovariectomized mice through immune regulation and gut microbiota suppression. Sci Rep 15, 20701 (2025). https://doi.org/10.1038/s41598-025-08827-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08827-0