Abstract
A large body of evidence consistently indicates a relationship between the apolipoprotein E (APOE) ε4 allele and memory decline in later life; however, the influence of the APOE ε4 allele on memory performance during the early stages of life remains poorly understood. Therefore, we explored whether the APOE ε4 allele is associated with cognitive advantages or disadvantages early in life from the perspective of memory function, specifically working memory and short-term memory. Based on a study of 516 university students aged 17–26 who completed short-term memory tasks and 156 students in the same age range who completed working memory tasks, our findings reveal that individuals carrying the APOE ε4 allele exhibited poorer performance in working memory, with no significant impact on short-term memory. Subsequently, employing a connectome-based predictive modeling approach in resting-state functional magnetic resonance imaging data, we defined a functional network model-dominated by default-sensorimotor network interactions that was capable of forecasting fluctuations in working memory among the held-out individuals (i.e., cross-brain prediction). Furthermore, the functional connectivity serves as a mediator in the association between APOE genotypes and working memory performance. Collectively, these findings provide novel insights into the early-life relationship between the APOE ε4 allele and memory performance, as well as its neural underpinnings.
Similar content being viewed by others
Introduction
Apolipoprotein E (APOE) is a polymorphic protein involved in neurogenesis, plasticity, and repair mechanisms1. Research has shown that the APOE genotype influences amyloid-β (Aβ) metabolism, cholesterol homeostasis, neurovascular function, and neuroinflammation, which are considered to play significant roles in the pathology of Alzheimer’s disease (AD)2,3,4. Therefore, the ε4 allele is recognized as a significant genetic risk factor for AD5,6. APOE ε4 carriers having 1 and 2 copies of the allele are 3 and 15 times more likely to develop AD respectively7. In addition, there were confirmed associations between ε4 allele and cognitive ability. Both cross-sectional and longitudinal studies of healthy people have shown that ε4-carriers demonstrated worse performance or accelerated decline in several cognitive domains (e.g. memory, executive functioning and overall global cognitive ability) compared to non-carriers8,9,10,11. During middle age, the ε4 allele may slightly impair cognition12, while after age 60, this impact becomes more pronounced13.
In recent years, there has been a growing research interest in investigating the impact of the APOE ε4 allele on cognition across the lifespan, extending beyond old age. However, the studies of young adults have yielded inconsistent results14,15,16,17,18. Notably, the impact of the APOE ε4 allele on cognitive function in young individuals remains a subject of considerable debate. Among the most contentious issues is whether APOE ε4 allele acts as an antagonist of pleiotropic genes. Many researchers suggested that the APOE ε4 allele may be an antagonistic pleiotropy gene, conferring a beneficial effect on cognition in early life and a detrimental impact on cognition during later years15,16,19,20. However, some evidence didn’t support this view14,21. Interestingly, one study has shown superior visual working memory in APOE ε4 carriers, indicating that some benefits of this genotype are demonstrable in older age22, which appears to be inconsistent with the notion that APOE ε4 acts as an antagonist of pleiotropic genes. A recent meta-analytic study across seven cognitive domains(intelligence/achievement, attention/working memory, executive functioning, memory, language, processing speed and visuospatial abilities) in younger individuals (participants’ ages ranged from 2 to 40) also did not find statistically significant differences in cognitive performance between APOE ε4 carriers and non-carriers23. Overall, the impact of the APOE ε4 allele on cognitive abilities in young populations remains inconclusive. The inconsistent results across studies may be attributed to differences in age, education level, and other demographic factors, as well as the varying cognitive domains examined.
Given the prominent role of memory impairment as a recognized symptom of dementia, investigating the onset and specific impact of the AD risk gene APOE ε4 on memory function in young adults is particularly compelling. Memory functions include short-term memory, working memory, and long-term memory. Individuals with AD and those in its prodromal stages show more pronounced declines in short-term memory and working memory24,25; these two cognitive domains are also fundamental for assessing learning abilities in young adult students26.To date, there have been relatively few studies exploring the relationship between these two types of memory performance and APOE genotypes15,21,27, as well as the structural basis underlying this association in young adults14,28,29. Cognitive functions require coordination among widely networked brain areas30. A growing body of neuroscience research highlights the importance of functional interactions within and between multiple brain networks for APOE ε4 and memory skills31,32,33,34. Among them, the default-mode network (DMN) has been extensively studied in cognitive neuroscience due to its close relationship with memory processing, particularly working memory. The DMN is typically active during rest and shows reduced activation during externally focused cognitive tasks, a pattern thought to reflect shifts in attention and memory resources35,36. Notably, the functional integrity and connectivity of the DMN, especially in core regions like the posterior cingulate cortex (PCC) and medial prefrontal cortex (mPFC), have been shown to correlate with performance in memory and working memory tasks37. Additionally, evidence suggests that the functional interaction between the DMN and task-positive networks, including the executive control network and salience network, is crucial for maintaining optimal cognitive function38. Unlike working memory networks, short-term memory relies more on primary sensory cortices and the hippocampus-parietal circuits for the temporary storage and processing of information39. However, the precise mechanisms of these large-scale network interactions, especially in the context of APOE genotype differences in young adults, remain poorly understood.
Therefore, we hypothesized that APOE genotypes may influence memory performance in young adults, potentially manifestations in the functional connectivity of a wide range of brain networks including the executive control network, the salience network, and the DMN. To test this hypothesis, we selected a homogeneous sample with matched age and educational background, focusing on short-term and working memory as core memory domains, aiming to elucidate both the relationship between APOE genotypes and memory performance and the associated functional connectivity patterns in young adults, thereby providing new evidence to address ongoing research debates.
Methods
Participants and grouping
All data were obtained at the Southwest University (Chongqing, China), and all participants provided written informed consent and received payment for their time and task participation. The research protocol was approved by the ethics committee of the review committee of the Brain Imaging Center of Southwest University. The data used in this study were extracted from an ongoing research project, the Gene-Brain-Behavior (GBB) project, which has been referred to in several previous studies40,41. GBB is a large sample database that measures multiple behavioral variables, such as creativity, emotion, personality, growth experience, and health, and its recruitment program and exclusion criteria have been detailed in previous publications42. All participants underwent genotype testing for APOE ε4/ε3/ε2, and we divided all participants into three groups according to their genotype: group APOE 2 (ε2/ε2 and ε2/ε3), group APOE 3 (ε2/ε4 and ε3/ε3) and group APOE 4 (ε3/ε4 and ε4/ε4).
Initially, 1069 university students underwent APOE genotyping for this study. However, the subsequent experiments and magnetic resonance imaging (MRI) scanning required additional time, and not all students could complete these procedures. Ultimately, complete data, including MRI scans, were obtained from 516 students who completed the short-term memory experiment and 156 students who completed the working memory experiment. For the working memory analysis, 156 participants were included (3 APOE ε2/ε2, 20 APOE ε2/ε3, 1 APOE ε2/ε4, 102 APOE ε3/ε3, 29 APOE ε3/ε4, and 1 APOE ε4/ε4 carriers). Considering the protective role of APOE ε2 in AD and the dose effect of APOE ε4, 1 APOE ε2/ε4 participant was excluded from our analysis (n = 155). For the short-term memory analysis, 516 participants were included (8 APOE ε2/ε2, 75 APOE ε2/ε3, 1 APOE ε2/ε4, 346 APOE ε3/ε3, 82 APOE ε3/ε4, and 5 APOE ε4/ε4 carriers).
Assessment of working memory
Working memory was measured using the n-back task. We used only one condition (3-back) for the letter n-back task. Participants were asked to identify whether the current item had flashed three items earlier in the sequence. Participants were instructed to press “F” if they thought the current item matched two items earlier and to press “J” otherwise. Participants completed 90 trials. Each trial lasted 3000 ms and the letter for each trial was presented for 750 ms. The Psychophysics Toolbox (http://psychtoolbox.org/) for MATLAB was used to display the stimuli.
The mean reaction time (RT) was calculated after excluding the trials with no response and incorrect response. Accuracy (ACC) was also calculated after excluding the trials with no response. The ACC of n-back task was used as an indicator of of working memory ability.
Assessment of short-term memory
Short-term memory was measured using digit Span assessment43, which is a subscale of the Wechsler Adult Intelligent Scale (WAIS). The WAIS-IV was used in this study, which has a high degree of reliability (reliability coefficient, 0.92) and validity44.
There are two parts to digit span assessment: forward and digits Backward. Each tap represents distinct but interdependent cognitive functions. Digits forward primarily taps short-term auditory memory while digits backward measures the participants’ ability to manipulate verbal information while it is still in temporary storage. In digits forward, the participants listened to and repeated a sequence of numbers spoken aloud by the interviewer. In digits backward, the participants listened to a sequence of numbers and repeated them in reverse order. In both parts, the length of each sequence of numbers increases as the participants responds correctly.
The converted standard score of digit span assessment was used as an indicator of short-term memory ability.
Analyses
ANCOVA was used to compare the differences in working memory and short-term memory among individuals with different genotypes (group APOE 2, group APOE3 and group APOE4). Before the analysis, Levene’s test and the Shapiro–Wilk test were conducted to verify the assumptions of homogeneity of variances and normality required for ANCOVA, which indicated that the data analysis met the prerequisites for ANCOVA. Working memory (ACC in the n-back task) and short-term memory (digit span task) were included as dependent variables. Sex was included as a covariate in all analyses, and RT in the n-back task was additionally controlled when comparing working memory accuracy to account for potential speed–accuracy trade-offs. Furthermore, given the imbalance in group sizes, a non-parametric resampling (bootstrap) method was implemented to ensure robust comparisons. In simply terms, we conducted a random sampling with the replacement of group APOE3 10,000 times. In each sampling process of working memory, 30 participants belonging to group APOE ε3 were randomly selected (due to group APOE4 having a sample size of 30), and their average ACC in the n-back task was calculated and compared with that of group APOE4. In each sampling process of short-term memory, 87 participants belonging to the group APOE3 were randomly selected (due to the group APOE4 having a sample size of 87), and their average score was calculated and compared with those of the APOE4 group. Consequently, bootstrap-based p-values and 95% confidence intervals were obtained. All bootstrap analyses were conducted using the “boot” package in R.
Image acquisition and preprocessing.
All the functional and structural data were obtained using a 3 T SIEMENS PRISMA scanner (Erlangen, Germany) at the Brain Imaging Center of Southwest University. For resting-state fMRI, the functional imaging data were obtained using a multiband T2*-sensitive gradient-recalled single-shot echo-planar imaging pulse sequence: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°, field of view (FOV) = 220 × 220 mm2, slices = 32, thickness = 3.0 mm, and voxel size = 3.4 × 3.4 × 4.0 mm3. High-resolution, three-dimensional T1-weighted structural images were obtained using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequence: TR = 1900 ms, TE = 2.52 ms, FA = 9°, slices = 176, FOV = 256 × 256 mm2, thickness = 1.0 mm, and voxel size = 1.0 × 1.0 × 1.0 mm3.
FMRIPrep45,46 based on Nipype47 was used to preprocess the task-fMRI functional imaging data with the following parameters. First, a reference volume and its skull-stripped version were generated using a custom methodology of fMRIPrep. Head-motion parameters with respect to the BOLD reference (transformation matrices, and six corresponding rotation and translation parameters) were estimated before any spatiotemporal filtering using MCFLIRT48. BOLD runs were slice-time corrected to 0.962 s (0.5 of slice acquisition range 0–1.93 s) using 3dTshift from AFNI49. The BOLD time-series (including slice-timing correction when applied) were resampled onto their original, native space by applying the transforms to correct for head-motion. These resampled BOLD time-series are referred to as preprocessed BOLD in original space, or just preprocessed BOLD. The BOLD reference was then co-registered to the T1w reference using mri_coreg (FreeSurfer) followed by flirt50 with the boundary-based registration51 cost-function. Co-registration was configured with six degrees of freedom. Several confounding time-series were calculated based on the preprocessed BOLD: frame wise displacement (FD), DVARS and three region-wise global signals. FD was computed using two formulations following Power47 and Jenkinson48. FD and DVARS were calculated for each functional run, both using their implementations in Nipype47. The three global signals were extracted within the the cerebrospinal fluid (CSF), the white matter (WM), and the whole-brain masks. Additionally, a set of physiological regressors were extracted to allow for component-based noise correction52. Principal components were estimated after high-pass filtering the preprocessed BOLD time-series (using a discrete cosine filter with 128 s cut-off) for the two CompCor variants: temporal (tCompCor) and anatomical (aCompCor). tCompCor components were then calculated from the top 2% variable voxels within the brain mask. For aCompCor, three probabilistic masks (CSF, WM and combined CSF + WM) were generated in anatomical space. The implementation differs from that of Behzadi et al. in that instead of eroding the masks by 2 pixels on BOLD space, the aCompCor masks were subtracted a mask of pixels that likely contain a volume fraction of GM. This mask is obtained by thresholding the corresponding partial volume map at 0.05, and it ensures components were not extracted from voxels containing a minimal fraction of GM. Finally, these masks were resampled into BOLD space and binarized by thresholding at 0.99 (as in the original implementation). Components were also calculated separately within the WM and CSF masks. For each CompCor decomposition, the k components with the largest singular values were retained, such that the retained components’ time series were sufficient to explain 50 percent of variance across the nuisance mask (CSF, WM, combined, or temporal). The remaining components were dropped from consideration. The head-motion estimates calculated in the correction step were also placed within the corresponding confounds file. The confound time series derived from head motion estimates and global signals were expanded with the inclusion of temporal derivatives and quadratic terms for each53. Frames that exceeded a threshold of 0.5 mm FD or 1.5 standardized DVARS were annotated as motion outliers. The BOLD time-series were resampled into standard space, generating a preprocessed BOLD run in MNI152NLin2009cAsym space. First, a reference volume and its skull-stripped version were generated using a custom methodology of fMRIPrep. All resamplings were performed with a single interpolation step by composing all the pertinent transformations (i.e. head-motion transform matrices, susceptibility distortion correction when available, and co-registrations to anatomical and output spaces). Gridded (volumetric) resamplings were performed using antsApplyTransforms (ANTs), configured with Lanczos interpolation to minimize the smoothing effects of other kernels54. Non-gridded (surface) resamplings were performed using mri_vol2surf (FreeSurfer).
Functional connectivity feature extraction.
We used the MATLAB R2016b-based functional connectivity toolbox “Conn toolbox” version 19c55 to extract functional connectivity feature (i.e., 34 participants × 11 trials) on the basis of previously described intrinsic functional network atlases in the MNI space56. First, we performed fMRI denoising based on the linear regression of the following parameters from each node: (a) five noise components each from minimally-eroded WM and CSF (one-node binary erosion of nodes with values above 50% in posterior probability maps), respectively, on the basis of aCompCor procedures52,57; (b) linear BOLD signal trend within session. In a separate step after nuisance regression58, data were then temporally filtered with a bandpass of 0.008–0.09 Hz. Then, we extracted the denoised BOLD time series from the mean across all voxels within each node. We computed a matrix of functional connectivity values between all region pairs on the basis of the Fisher z-transformed Pearson correlation coefficient of time series.
Predictive modeling analysis of working memory
We adapted the connectome-based predictive modeling (CPM) method59 to identify functional connectivity patterns that predicted the working memory.
For each of the 155 participants, we generated model-based working memory scores (ACC of n-back task) prediction based on the independent data form all other participants (i.e., leave-one-participant-out). For each cross-validation fold, after controlling for sex, the mean frame-wise head motion and RT of n-Back task, we calculated the partial correlations between each edge (node pair) in the functional connectivity matrix (derived from the Schaefer400 atlas) and within-participant scores. Then, we ‘masked’ the brain-behavior correlations such that only the edges positively correlated with novelty and appropriateness scores at the suprathreshold level of P ≤ 0.01 (two-tailed) were retained, resulting in positive edge masks. In the held-out-participant, we performed a Pearson correlation between model-predicted working memory scores and observed, within-participant working memory scores.
To determine whether predicted versus observed correlations were statistically significant at the group level, we generated a distribution of null values. To this end, we repeated all of the same CPM procedures, as previously described, except the assignments of functional connectivity matrix were randomly permuted (1,000 iterations) to obtain null correlation values to assess the significance (permutation test, Ppt ≤ 0.05, two-tailed). The code for CPM analyses was adapted from publicly available scripts (https://github.com/YaleMRRC/CPM).
Finally, we retained the edges that positively correlated with working memory scores at every cross-validation fold to constitute the CPM mask of working memory (here after referred to as “WM-CPM”).
Analysis of functional neuroanatomical patterns contributing to the CPM of working memory
We visualized the edges comprising the WM-CPM (see Fig. 2C) using the BioImage Suite Connectivity Visualization Tool (https://bioimagesuiteweb.github.io/webapp). To observe the neuroanatomical patterns that contributed to the WM-CPM, we assigned each node to one of the 7 canonical Yeo-Krienen60 intrinsic functional networks. For these analyses, we used the Schaefer atlas of 400 cortical regions, which includes a Yeo-Krienen network label for each node56.
Based on the Schaefer atlas, the WM-CPM included 208 edges in the positive masks. We assigned each of these edges to one of 28 within- or between-network Yeo-Krienan pairs.
Mediation analysis
To explore whether the relationship between genotype and working memory is mediated by the WM-CPM, we performed a mediation analysis. For each participant, the edges of WM-CPM were summed as mediating variable. Three general linear regression models were defined to test (1) the total effect of genotype on working memory, (2) the effect of genotype on WM-CPM, and (3) the direct effect of genotype on working memory, controlling for WM-CPM. The significance of the indirect effect of WM-CPM on the relationship between genotype and working memory was tested via the quasi-Bayesian Monte Carlo simulation as implemented in the “mediation” package in R. Specifically, 1000 simulations were performed to compute the 95% confidence interval of the average causal mediation effects.
Results
In our study, we assessed the impact of APOE genotypes on memory performance in young adults. Specifically, we evaluated working memory using the n-back task and short-term memory using the digit span test. For the working memory assessment, a total of 155 participants (43 males, mean age = 19.2 years, SD = 1.14 years) were included in the analysis. For the short-term memory assessment, 516 participants (153 males, mean age = 19.7 years, SD = 1.54 years) were included in the analysis. The demographic and performance data for each APOE group are summarized in Table 1.
Main effects of APOE genotypes on working memory
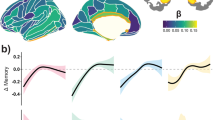
To explore the effect of APOE ε4 on memory, two ANCOVAs were performed on working memory (n-back task) and short-term memory (digit span assessment) separately. Significant effects were noted on working memory (F(2152) = 3.08, P < 0.05, Fig. 1A). The Tukey Post-Hoc tests indicated that group APOE 4 performed worse than group APOE3 in the n-back task (Fig. 1A). However, there was no significant effect on short-term memory (F(2513) = 2.13, p > 0.05, Fig. 1B).
Considering the large differences in sample sizes between participant groups, we employed non-parametric resampling (10,000 times) procedures61 to prove the robustness of our results. The results showed that in 10,000 comparisons, group APOE4 performed better in fewer than 10 instances in working memory performance (P < 0.001, Fig. 1C). However, there is no significant difference between group APOE 4 and group APOE3 in short-term memory performance (p > 0.05, Fig. 1D).
A connectome-based predictive model predicts working memory across brains
To further investigate the differences in the functional neuroanatomical basis between different genotype groups, we used CPM59 to construct functional connectivity patterns from the whole-brain functional connections that could successfully predict working memory (n = 155, 43 males, mean age = 19.2 years, SD = 1.14 years). For each participant, we calculated the functional connectivity matrix within resting-state fMRI based on the whole-brain functional map of 400 nodes56. Within each cross-validation fold, we identified all node pairs (edges) exhibiting suprathreshold-level (P < 0.01) positive partial correlations with ACC of working memory (RT of working memory and mean frame-wise head motion were controlled). Based on the positive edge sum scores for each trial, we constructed a linear model to predict ACC of working memory based on all participants within a given cross-validation fold. For the held-out participant, we applied this linear model to compute predicted ACC of working memory. Then, we then correlated the predicted value with the observed ACC of working memory. Finally, we randomly shuffled the functional connectivity matrix 1,000 times and ran the above prediction pipeline for each time to obtain a null distribution of correlation coefficients between the predicted and observed scores to assess the significance (permutation test, Ppt ≤ 0.05, two-tailed) (Fig. 2B). We conducted the same analysis to explore edges exhibiting suprathreshold-level negative partial correlations with working memory.
Functional connectivity-based predictive modeling of working memory. (A) A linear model, based on summary scores, was used to correlate 155 predicted versus observed novelty ACC scores of working memory. (B) Correlation value (indicated with red line) between predicted and observed scores was compared with a null distribution of r values derived from 1000 permutations of shuffled functional connectivity matrix. (C) The perspectives of the top ten nodes contributing to the predictive functional connectivity pattern of working memory. (D) The number of edges, among those within the WM-CPM positive mask, assigned to each within- or between-network pair based on the Schaefer400 and Yeo-Krienen 7-network atlases. Nodes: IFG = inferior frontal gyrus; dmpfc = dorsomedial prefrontal cortex; dlpfc = dorsolateral prefrontal cortex; STG = superior temporal gyrus; MTG = middle temporal gyrus. Networks: VIS = visual; SMN = somatomotor; DAN = dorsal attention; SAL = salience; LIM = limbic; FPCN = frontoparietal control; DMN = default mode.
For positive functional connectivity, the permutation results showed that CPM of working memory (r = 0.171, Ppt = 0.046) was effective (Fig. 2A). The edges contributing to the models included 208 edges positively associated with working memory. These edges of the WM-CPM were distributed widely throughout the brain, with high-degree nodes (i.e., nodes involved in multiple contributing edges) situated in parietal, temporal and prefrontal (Fig. 2C). For negative functional connectivity, the permutation results showed that CPM of working memory was not effective.
To better explain the functional neuroanatomical basis of patterns contributing to the WM-CPM, we examined the relationships with the functional networks previously associated with working memory. According to the Schaefer400 atlas, with each node assigned to 1 of 7 standard Yeo-Krienen networks56,60, we quantified the number of WM-CPM mask edges belonging to each intra- or inter-network pair.
The highest number of edges positively correlated with working memory (Fig. 2D) derived from Default mode network (DMN) between-network connections. The few network pairs that contributed the most to positive edges were the DMN-sensorimotor network (SMN), DMN-salience network (SAL), DMN-frontoparietal control network (FPCN) and the SMN-visual network (VIS). The DMN-SMN connections contributed the most positive edges (29 total pairs in total).
Mediation analysis
In previous analyses, we found a relationship between genotype and working memory and furthermore, we used CPM to construct whole-brain functional connectivity of working memory. In the final step, we analyzed whether the relationship between genotype and working memory is mediated by the WM-CPM.
To this end, using mediation analysis, we used genotype as the independent variable, working memory as the dependent variable and the sum of edges of the WM-CPM as the mediator variable to establish mediation model. We observed a significant indirect impact of the genotype on working memory through the mediation of the WM-CPM (average causal mediation effects = 0.014, p = 0.042, 95% CI [0.000, 0.030], Fig. 3).
Discussion
By examining the differences in working memory and short-term memory among individuals with different APOE genotypes, the current study found that APOE ε4 carriers performed worse than the individuals with APOE ε3/ε3 genotype in terms of working memory, while no significant differences in short-term memory were found between them. Meanwhile, the resting-state brain functional connections that are closely related to working memory were identified using the CPM method. Subsequently, a mediation analysis was performed to verify the mediating role of these functional connections in the relationship between APOE genotypes and working memory performance. This study extends the knowledge on the relationship between APOE genotypes and cognitive functions in early adulthood and uncovers the underlying neural basis. These findings contribute to a better understanding of the role of APOE genotypes in influencing individuals’ lifelong cognitive development.
Notably, as mentioned numerous genetics studies62, the role of genotype in influencing the onset and development of individuals’ psychological behavior is complex and influenced by extensive environmental factors. In the present study analyzed the working memory performance of different gene carriers using an ANOVA and found differences among carriers. However, the existence of such differences cannot be taken as proof of the genotype’s determining role on working memory. Indeed, we tend to attribute this discrepancy to the influence role of genotype. That is to say, as in the case of executive function63 and attentional modulation64, genotypes also an influential factor on working memory, and this influence is clearly crucial. The decline in late-life cognitive function among APOE ε4 carriers is associated with structural brain changes65, and were more likely to develop AD66. A meta-analysis revealed that APOE ε4 carriers were at a disadvantage compared to non-carriers in terms of overall cognitive function, situational memory and executive function67. Therefore, in line with previous studies68, the present study found that APOE ε4 carriers performed worse in terms of working memory, which may be because APOE ε4 is associated with lower cognitive performance and higher risk of cognitive deficits. In addition, APOE ε2 was associated with a better short-term memory performance than APOE ε4 in our results, but the effect was not significant (Fig. 1B). However, this trend approaching a significant difference also seems to illustrate the protective effect of APOE ε2 on short-term memory.
CPM results show that resting-state brain functional connectivity, which is closely related to working memory, comes primarily from connections between the DMN and other networks. The next highest active network is the SMN. The DMN has been widely proven to be associated with working memory29,36,69,70,71. A study found that participants with reduced default network reactivity performed worse on a multilevel working memory task72. In addition, the DMN is also involved in a various of higher mental processes, including working memory, through flexible interactions with other networks73. Baddeley’s model of working memory identifies the phonological loop as a component of working memory74, a process that involves encoding verbal information and maintaining auditory information, which are both related to the SMN75. Besides, verbal working memory is reportedly related to the frontotemporal sensorimotor circuits76 and cerebellum77. Furthermore, the embodied cognition perspective suggests that many higher cognitive processes are closely related to perceptual and motor processes78 Therefore, the SMN may be involved in working memory by participating in information representation and manipulation79,80,81.
The results of the mediation analyses suggest that the WM-CPM mediates the relationship between the genotype and working memory scores. As found in previous studies, the genotype can influence specific mental behavioral performance by triggering certain changes in the brain82,83. Therefore, APOE ε4 may affect individuals’ working memory performance by altering the basis of brain functional connectivity that is closely related to working memory, including the DMN and SMN.
Our study has some limitations. First, the effects of a genotype on individuals are complex and may act in various ways; the present study revealed one possibility on how genes affect mental behavioral scores by influencing functional brain connectivity. However, this cannot be understood as a determinative causal relationship. It is not possible to enumerate all the possibilities of gene action on cognitive outcomes, or to include all environmental variables in the analyses. Second, a limitation of our study is the relatively small sample size, particularly among APOE ε4 carriers, with only 30 participants in our working memory analysis. This geographic and genetic limitation may affect the generalizability of our findings. Future research should expand the sample size and include participants from diverse regions, especially by recruiting more APOE ε4 carriers to provide a more accurate understanding of how APOE genotypes influence memory performance in young adults. Thirdly, while our study focused on short-term and working memory as key areas of interest, it did not cover the long-term memory domain, including episodic memory, which is notably affected in older APOE ε4 carriers. Future studies should extend their scope to include these additional memory domains to explore the full spectrum of memory functions influenced by APOE genotypes in young adults. Lastly, our study did not differentiate between APOE ε4 heterozygotes and homozygotes. Prior research indicates that the APOE ε4 allele’s impact may be dose-dependent, with ε4/ε4 carriers facing a higher Alzheimer’s disease (AD) risk and more significant cognitive and neural changes than ε3/ε4 carriers. A recent study by Fortea et al. identified ε4 homozygosity as a distinct genetic profile associated with elevated AD risk and altered biomarkers even in preclinical stages84. Future research should separately examine ε4 homozygotes and heterozygotes to clarify the effects of APOE ε4 dosage on cognitive function and its neural basis.
Data availability
The data for this study belong to the Faculty of Psychology at Southwest University, and is currently not publicly available. If anyone wishes to request access to parts of the data from this study, please contact me( My name is Li Ling) via email(llmedpsy@tmmu.edu.cn).
References
Mahley, R. W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 240, 622–630. https://doi.org/10.1126/science.3283935 (1988).
Jackson, R. J., Hyman, B. T. & Serrano-Pozo, A. Multifaceted roles of APOE in Alzheimer disease. Nat. Rev. Neurol. 20, 457–474. https://doi.org/10.1038/s41582-024-00988-2 (2024).
Kloske, C. M. et al. Advancements in APOE and dementia research: Highlights from the 2023 AAIC Advancements: APOE conference. Alzheimers Dement 20, 6590–6605. https://doi.org/10.1002/alz.13877 (2024).
Fernández-Calle, R. et al. APOE in the bullseye of neurodegenerative diseases: Impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol. Neurodegener. 17, 62. https://doi.org/10.1186/s13024-022-00566-4 (2022).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51, 414–430. https://doi.org/10.1038/s41588-019-0358-2 (2019).
Palmer, J. M., Huentelman, M. & Ryan, L. More than just risk for Alzheimer’s disease: APOE ε4’s impact on the aging brain. Trends Neurosci. 46, 750–763. https://doi.org/10.1016/j.tins.2023.06.003 (2023).
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C.-C. & Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518. https://doi.org/10.1038/s41582-019-0228-7 (2019).
Caselli, R. J. et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N. Engl. J. Med. 361, 255–263. https://doi.org/10.1056/NEJMoa0809437 (2009).
Davies, G. et al. A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol. Psychiatry 19, 76–87. https://doi.org/10.1038/mp.2012.159 (2014).
Daly, J. et al. The effect of apolipoprotein E genotype on spatial processing in humans: A meta-analysis and systematic review. Cortex 177, 268–284. https://doi.org/10.1016/j.cortex.2024.05.006 (2024).
Solomon, A. et al. Effect of the apolipoprotein e genotype on cognitive change during a multidomain lifestyle intervention: A subgroup analysis of a randomized clinical trial. JAMA Neurol. 75, 462–470. https://doi.org/10.1001/jamaneurol.2017.4365 (2018).
Lancaster, C., Tabet, N. & Rusted, J. The elusive nature of APOE ε4 in mid-adulthood: Understanding the cognitive profile. J. Int. Neuropsychol. Soc. JINS 23, 239–253. https://doi.org/10.1017/s1355617716000990 (2017).
Marioni, R. E. et al. Differential effects of the APOE e4 allele on different domains of cognitive ability across the life-course. Eur. J. Hum. Genet. EJHG 24, 919–923. https://doi.org/10.1038/ejhg.2015.210 (2016).
Taylor, W. D. et al. APOE ε4 associated with preserved executive function performance and maintenance of temporal and cingulate brain volumes in younger adults. Brain Imaging Behav. 11, 194–204. https://doi.org/10.1007/s11682-016-9522-9 (2017).
Li, X. et al. Cognitive performance in young APOE ε4 carriers: A latent variable approach for assessing the genotype-phenotype relationship. Behav. Genet. 49, 455–468. https://doi.org/10.1007/s10519-019-09961-y (2019).
Takeuchi, H. et al. Sex-dependent effects of the APOE ɛ4 allele on behavioral traits and white matter structures in young adults. Cereb. Cortex N. Y. 1991 31, 672–680. https://doi.org/10.1093/cercor/bhaa251 (2021).
Koelewijn, L. et al. APOEOscillatory hyperactivity and hyperconnectivity in young -ɛ4 carriers and hypoconnectivity in Alzheimer’s disease. Elife https://doi.org/10.7554/eLife.36011 (2019).
Muñoz-Neira, C. et al. Differences in grey matter concentrations and functional connectivity between young carriers and non-carriers of the APOE ε4 genotype. J. Clin. Med. https://doi.org/10.3390/jcm13175228 (2024).
Shibata, K., Chen, C., Tai, X. Y., Manohar, S. G. & Husain, M. Impact of APOE, Klotho, and sex on cognitive decline with aging. Proc. Natl. Acad. Sci. U. S. A. 122, e2416042122. https://doi.org/10.1073/pnas.2416042122 (2025).
Reynolds, C. A. et al. APOE effects on cognition from childhood to adolescence. Neurobiol. Aging 84(239), e231-239. https://doi.org/10.1016/j.neurobiolaging.2019.04.011 (2019).
Sinclair, L. I., Button, K. S., Munafò, M. R., Day, I. N. & Lewis, G. Possible association of APOE genotype with working memory in young adults. PLoS ONE 10, e0135894. https://doi.org/10.1371/journal.pone.0135894 (2015).
Lu, K. et al. APOEDissociable effects of -ε4 and β-amyloid pathology on visual working memory. Nat. Aging 1, 1002–1009. https://doi.org/10.1038/s43587-021-00117-4 (2021).
Weissberger, G. H., Nation, D. A., Nguyen, C. P., Bondi, M. W. & Han, S. D. Meta-analysis of cognitive ability differences by apolipoprotein e genotype in young humans. Neurosci. Biobehav. Rev. 94, 49–58. https://doi.org/10.1016/j.neubiorev.2018.08.009 (2018).
Akhtar, A., Singh, S., Kaushik, R., Awasthi, R. & Behl, T. Types of memory, dementia, Alzheimer’s disease, and their various pathological cascades as targets for potential pharmacological drugs. Ageing Res. Rev. 96, 102289. https://doi.org/10.1016/j.arr.2024.102289 (2024).
Gilardone, G., Longo, C. & Papagno, C. The role of working memory and short-term memory in sentence comprehension: A systematic review and meta-analysis in probable Alzheimer’s disease. Neuropsychol. Rev. 34, 530–547. https://doi.org/10.1007/s11065-023-09595-2 (2024).
Wagner, A. D. Working memory contributions to human learning and remembering. Neuron 22, 19–22. https://doi.org/10.1016/s0896-6273(00)80674-1 (1999).
Atkinson, R., Gaysina, D. & Rusted, J. M. Responses to executive demand in young adulthood differ by APOE genotype. Behav. Brain Res. 360, 158–168. https://doi.org/10.1016/j.bbr.2018.11.033 (2019).
Nao, J. et al. Adverse effects of the apolipoprotein E ε4 allele on episodic memory, task switching and gray matter volume in healthy young adults. Front. Hum. Neurosci. 11, 346. https://doi.org/10.3389/fnhum.2017.00346 (2017).
Mentink, L. et al. Functional co-activation of the default mode network in APOE ε4-carriers: A replication study. Neuroimage 240, 118304. https://doi.org/10.1016/j.neuroimage.2021.118304 (2021).
Zheng, L. J. et al. Different posterior hippocampus and default mode network modulation in young APOE epsilon4 carriers: A functional connectome-informed phenotype longitudinal study. Mol. Neurobiol. 58, 2757–2769. https://doi.org/10.1007/s12035-021-02292-2 (2021).
Carnevale, L. et al. Brain functional magnetic resonance imaging highlights altered connections and functional networks in patients with hypertension. Hypertension 76, 1480–1490. https://doi.org/10.1161/HYPERTENSIONAHA.120.15296 (2020).
Li, X. et al. Disrupted frontoparietal network mediates white matter structure dysfunction associated with cognitive decline in hypertension patients. J. Hypertens. 35, 10015–10024. https://doi.org/10.1523/JNEUROSCI.5113-14.2015 (2015).
Li, X. et al. Vulnerability of the frontal and parietal regions in hypertensive patients during working memory task. J. Hypertens. 35, 1044–1051. https://doi.org/10.1097/HJH.0000000000001250 (2017).
Lu, H. et al. Aberrant interhemispheric functional connectivity within default mode network and its relationships with neurocognitive features in cognitively normal APOE ε 4 elderly carriers. Int. Psychogeriatr. 29, 805–814. https://doi.org/10.1017/S1041610216002477 (2017).
Hu, M. L. et al. A review of the functional and anatomical default mode network in schizophrenia. Neurosci. Bull. 33, 73–84. https://doi.org/10.1007/s12264-016-0090-1 (2017).
Sambataro, F. et al. Age-related alterations in default mode network: Impact on working memory performance. Neurobiol. Aging 31, 839–852. https://doi.org/10.1016/j.neurobiolaging.2008.05.022 (2010).
Koshino, H., Minamoto, T., Yaoi, K., Osaka, M. & Osaka, N. Coactivation of the default mode network regions and working memory network regions during task preparation. Sci. Rep. 4, 5954. https://doi.org/10.1038/srep05954 (2014).
Liang, X., Zou, Q., He, Y. & Yang, Y. Topologically reorganized connectivity architecture of default-mode, executive-control, and salience networks across working memory task loads. Cereb. Cortex 26, 1501–1511. https://doi.org/10.1093/cercor/bhu316 (2016).
Emrich, S. M., Riggall, A. C., Larocque, J. J. & Postle, B. R. Distributed patterns of activity in sensory cortex reflect the precision of multiple items maintained in visual short-term memory. J. Neurosci. Off. J. Soc. Neurosci. 33, 6516–6523. https://doi.org/10.1523/jneurosci.5732-12.2013 (2013).
Wang, X., Zhuang, K., Li, Z. & Qiu, J. The functional connectivity basis of creative achievement linked with openness to experience and divergent thinking. Biol. Psychol. 168, 108260. https://doi.org/10.1016/j.biopsycho.2021.108260 (2022).
Zhuang, K. et al. Connectome-based evidence for creative thinking as an emergent property of ordinary cognitive operations. Neuroimage 227, 117632. https://doi.org/10.1016/j.neuroimage.2020.117632 (2021).
Chen, Q. et al. Brain hemispheric involvement in visuospatial and verbal divergent thinking. Neuroimage 202, 116065. https://doi.org/10.1016/j.neuroimage.2019.116065 (2019).
Woods, D. L. et al. Improving digit span assessment of short-term verbal memory. J. Clin. Exp. Neuropsychol. 33, 101–111. https://doi.org/10.1080/13803395.2010.493149 (2011).
Ryan, J. J. & Ward, L. C. Validity, reliability, and standard errors of measurement for two seven-subtest short forms of the Wechsler Adult Intelligence Scale—III. Psychol. Assess. 11, 207. https://doi.org/10.1037/1040-3590.11.2.207 (1999).
Esteban, O. et al. fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116. https://doi.org/10.1038/s41592-018-0235-4 (2019).
Gorgolewski, K. et al. Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform. 5, 13. https://doi.org/10.3389/fninf.2011.00013 (2011).
Power, J. D. et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341. https://doi.org/10.1016/j.neuroimage.2013.08.048 (2014).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. https://doi.org/10.1016/S1053-8119(02)91132-8 (2002).
Cox, R. W. & Hyde, J. S. Software tools for analysis and visualization of fMRI data. NMR Biomed. Int. J. Devot. Dev. Appl. Magn. Reson. In Vivo 10, 171–178. https://doi.org/10.1002/(SICI)1099-1492(199706/08)10:4/5%3c171::AID-NBM453%3e3.0.CO;2-L (1997).
Jenkinson, M. & Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156. https://doi.org/10.1016/S1361-8415(01)00036-6 (2001).
Greve, D. N. & Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72. https://doi.org/10.1016/j.neuroimage.2009.06.060 (2009).
Behzadi, Y., Restom, K., Liau, J. & Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. https://doi.org/10.1016/j.neuroimage.2007.04.042 (2007).
Satterthwaite, T. D. et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64, 240–256. https://doi.org/10.1016/j.neuroimage.2012.08.052 (2013).
Lanczos, C. Evaluation of noisy data. J. Soc. Ind. Appl. Math. Ser. B Numer. Anal. 1, 76–85. https://doi.org/10.1137/0701007 (1964).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. https://doi.org/10.1089/brain.2012.0073 (2012).
Schaefer, A. et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 28, 3095–3114. https://doi.org/10.1093/cercor/bhx179 (2018).
Chai, X. J., Castañón, A. N., Öngür, D. & Whitfield-Gabrieli, S. Anticorrelations in resting state networks without global signal regression. Neuroimage 59, 1420–1428. https://doi.org/10.1016/j.neuroimage.2011.08.048 (2012).
Hallquist, M. N., Hwang, K. & Luna, B. The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 82, 208–225. https://doi.org/10.1016/j.neuroimage.2013.05.116 (2013).
Shen, X. et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat. Protoc. 12, 506–518. https://doi.org/10.1038/nprot.2016.178 (2017).
Yeo, B. T. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. https://doi.org/10.1152/jn.00338.2011 (2011).
Efron, B. & Tibshirani, R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statist. Sci. https://doi.org/10.1214/ss/1177013815 (1986).
Serrano-Pozo, A., Das, S. & Hyman, B. T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 20, 68–80. https://doi.org/10.1016/S1474-4422(20)30412-9 (2021).
Titz, C. & Karbach, J. Working memory and executive functions: Effects of training on academic achievement. Psychol. Res. 78, 852–868. https://doi.org/10.1007/s00426-013-0537-1 (2014).
Gazzaley, A. Influence of early attentional modulation on working memory. Neuropsychologia 49, 1410–1424. https://doi.org/10.1016/j.neuropsychologia.2010.12.022 (2011).
Ma, Y. et al. APOE epsilon4 and late-life cognition: Mediation by structural brain imaging markers. Eur. J. Epidemiol. 37, 591–601. https://doi.org/10.1007/s10654-022-00864-7 (2022).
Gharbi-Meliani, A. et al. The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimer’s Res. 13, 1–11. https://doi.org/10.1186/s13195-020-00740-0 (2021).
Small, B. J., Rosnick, C. B., Fratiglioni, L. & Bäckman, L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychology 19, 592. https://doi.org/10.1037/0882-7974.19.4.592 (2004).
Rawle, M. J. et al. Apolipoprotein-E (Apoe) epsilon4 and cognitive decline over the adult life course. Transl. Psychiatry 8, 18. https://doi.org/10.1038/s41398-017-0064-8 (2018).
Kim, D. I. et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum. Brain Mapp. 30, 3795–3811. https://doi.org/10.1002/hbm.20807 (2009).
Mayer, J. S., Roebroeck, A., Maurer, K. & Linden, D. E. Specialization in the default mode: Task-induced brain deactivations dissociate between visual working memory and attention. Hum. Brain Mapp. 31, 126–139. https://doi.org/10.1002/hbm.20850 (2010).
Piccoli, T. et al. The default mode network and the working memory network are not anti-correlated during all phases of a working memory task. PLoS ONE 10, e0123354. https://doi.org/10.1371/journal.pone.0123354 (2015).
Čeko, M. et al. Is a responsive default mode network required for successful working memory task performance?. J. Neurosci. 35, 11595–11605. https://doi.org/10.1523/JNEUROSCI.0264-15.2015 (2015).
Vatansever, D. et al. Angular default mode network connectivity across working memory load. Hum. Brain Mapp. 38, 41–52. https://doi.org/10.1002/hbm.23341 (2017).
Baddeley, A. D. & Hitch, G. J. Working memory. Psychol. Learn. Motivat. 8, 47–89. https://doi.org/10.1016/S0079-7421(08)60452-1 (1974).
Schulze, K. & Koelsch, S. Working memory for speech and music. Ann. N. Y. Acad. Sci. 1252, 229–236. https://doi.org/10.1111/j.1749-6632.2012.06447.x (2012).
Buchsbaum, B. R. & D’Esposito, M. A sensorimotor view of verbal working memory. Cortex 112, 134–148. https://doi.org/10.1016/j.cortex.2018.11.010 (2019).
Ashida, R., Cerminara, N. L., Edwards, R. J., Apps, R. & Brooks, J. C. W. Sensorimotor, language, and working memory representation within the human cerebellum. Hum. Brain Mapp. 40, 4732–4747. https://doi.org/10.1002/hbm.24733 (2019).
Mahon, B. Z. What is embodied about cognition?. Lang. Cogn. Neurosci. 30, 420–429. https://doi.org/10.1080/23273798.2014.987791 (2015).
Marneweck, M., Barany, D. A., Santello, M. & Grafton, S. T. Neural representations of sensorimotor memory-and digit position-based load force adjustments before the onset of dexterous object manipulation. J. Neurosci. 38, 4724–4737. https://doi.org/10.1523/JNEUROSCI.2588-17.2018 (2018).
Meteyard, L. & Vigliocco, G. The role of sensory and motor information in semantic representation: A review. In Handbook of Cognitive Science 291–312. https://doi.org/10.1016/B978-0-08-046616-3.00015-3 (2008).
Wilson, M. Six views of embodied cognition. Psychon. Bull. Rev. 9, 625–636. https://doi.org/10.3758/bf03196322 (2002).
Brummelte, S., Mc Glanaghy, E., Bonnin, A. & Oberlander, T. F. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 342, 212–231. https://doi.org/10.1016/j.neuroscience.2016.02.037 (2017).
Witte, A. V. & Flöel, A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res. Bull. 88, 418–428. https://doi.org/10.1016/j.brainresbull.2011.11.012 (2012).
Fortea, J. et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease. Nat. Med. 30, 1284–1291. https://doi.org/10.1038/s41591-024-02931-w (2024).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31771231 and 32071070), the Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0520 and cstc2020jcyj-msxmX0299), 111 program (B21036), the Research Program Funds of the Collaborative Innovation Centre of Assessment toward Basic Education Quality at Beijing Normal University, the planned project of Chongqing humanities and Social Sciences (2018PY80, 2019PY51), Fundamental Research Funds for the Central Universities (SWU119007), the Chang Jiang Scholars Program, the National Outstanding Young People Plan and Chongqing Talent Program, and the Chongqing Postdoctoral Research Project Special Funding Project (2022CQBSHTB1013).
Funding
This research was supported by the National Natural Science Foundation of China (31771231 and 32071070), the Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0520 and cstc2020jcyj-msxmX0299), 111 program (B21036), the Research Program Funds of the Collaborative Innovation Centre of Assessment toward Basic Education Quality at Beijing Normal University, the planned project of Chongqing humanities and Social Sciences (2018PY80, 2019PY51), Fundamental Research Funds for the Central Universities (SWU119007), the Chang Jiang Scholars Program, the National Outstanding Young People Plan and Chongqing Talent Program, and the Chongqing Postdoctoral Research Project Special Funding Project (2022CQBSHTB1013).
Author information
Authors and Affiliations
Contributions
Ling Li completed the data collation, data analysis, and manuscript drafting. Wanning Wang contributed to manuscript writing, formatting, and submission. Wenjing Li and Yanli Chen participated in writing the methodology section, updating and verifying the references. Yu Mao was in charge of drawing the pictures in the paper. Yu Li and Jiang Qiu, as database managers, provided guidance on the study design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All participants had provided written informed consents prior experiments, and the experimental procedures approved by the ethical committee at the Southwest University (IRB NO. spy-2012–012) in accordance with the Declaration of Helsinkiwere.
Consent for publication
The manuscript containing detailed information pertaining to an individual have obtained written informed consent from all participants (or the parents or legal guardians of children under 18 years of age).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, L., Wang, W., Li, W. et al. The relationship between the APOE genotypes and memory performance of young adults and its neural basis. Sci Rep 15, 25150 (2025). https://doi.org/10.1038/s41598-025-08958-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08958-4