Abstract
Mercury exposure may increase the risk of depression. This study examined associations between blood mercury, depressive symptoms (PHQ-9), and metabolomic pathways in Chinese young adults. Fasting venous blood samples and questionnaire data (N = 477) were collected from the Chinese undergraduate cohort study in 2019 and 2021. Patient Health Questionnaire-9 was used to estimate depressive symptoms. Blood mercury and serum metabolomic were measured using inductively coupled plasma mass spectrometry (ICP-MS) and liquid chromatography-mass spectrometry (LC-MS). The association between whole-blood mercury concentrations and depressive symptoms were assessed by linear mixed-effects models. Linear mixed-effects models and ANOVAs were used to examine metabolomic changes associated with blood mercury based on baseline blood samples (N = 468). We found that for every 2-fold increase in blood mercury levels, the depression scale score increased by 0.50 [95% confidence interval (CI): 0.14, 0.86]. This effect was stronger in male, increasing their depression scores by 0.87 (95% CI: 0.12–1.61), while no significant association was observed in females. Individuals consuming fish ≥ once a month show negative correlation. Metabolomic analysis identified 44 altered metabolites, with 7 of these were enriched in 4 pathways (P < 0.05). These pathways are amino acid metabolism. Mercury exposure may affect depressive symptoms through neurotransmitter, energy and inflammation-related pathways.
Similar content being viewed by others
Introduction
Depression is one of the most prevalent mental disorders and is ranked 13th in the disease burden in the world and 11th in China in 20191,2. It is estimated that 5.00% of adults suffered from depression in 2019, with the highest incidence among young adults3,4. In China, the lifetime prevalence of depressive disorder among adults is 6.80%5. Multidimensional factors interact to make young adults vulnerable to depression6.
Heavy metal has been identified as one of the environmental risk factors for depression7. As a ubiquitous neurotoxin, mercury has drawn great concern8. China is the greatest emitter of anthropogenic mercury9. China’s anthropogenic mercury emissions to the atmosphere alone were estimated at 444 tons in 201710. Humans are exposed to mercury through air pollution, seafood consumption, dental amalgams, and occupational settings11. Mercury concentrations in whole blood reveal recent (1 to 2 months) exposure to methylmercury and inorganic mercury12.
A cross-sectional study of 15,140 Americans (age 31–63) indicated that greater levels of mercury were inversely associated with depressive symptoms [adjusted prevalence ratios = 0.62, 95% confidence interval (CI) = 0.50, 0.78]13. Another cross-sectional study of 11,754 Korean participants aged 43 to 49 years showed a positive correlation between higher blood mercury levels and the risk of depression symptoms in females (multivariate odds ratio = 2.05; 95% CI = 1.20, 3.48)14. However, a cross-sectional study of 6,911 adults aged 32 to 57 years from 2005 to 2008 NHANES in the United States has demonstrated that high blood mercury levels(>23.8ng/mL)were not related to increased odds of depressive symptoms15. Current studies on mercury and depressive symptoms have inconsistent results. At the same time, studies have shown that heavy metal exposure can have an effect on human metabolism16. For example, in an analysis examining the association between 106 serum exogenous chemical residues and five chronic diseases, a positive correlation was identified between serum perfluoroalkyl compounds (PFASs), hyperuricemia, and lipid metabolism17. An in-vivo study found exposure to the heavy metal arsenic may induce reproductive toxicity in male mice18. Another in-vivo study indicated that mixed heavy metal pollutants (MHMP) exposure may lead to significant changes in several metabolites in rat liver, kidney, and serum, especially purine metabolism and aminoacyl-tRNA biosynthesis pathway19. In addition, the available epidemiologic evidence is mainly conducted in developed countries such as South Korea and the United States. There is a shortage of studies examining the association between blood mercury levels and depressive symptoms among Chinese young adults. Meanwhile, the potential metabolomic pathways associated with mercury exposure remain unclear.
The objectives of this study were: (1) to assess the degree of depressive symptoms and blood mercury levels in Chinese young adults. (2) to explore the different impact of blood mercury levels on depressive symptoms among Chinese young adults of different genders and with different frequencies of fish consumption. (3) to estimate changes in serum metabolites associated with mercury exposure in Chinese young adults.
Materials and methods
Participants and study design
The data was from a follow-up study of college students at Binzhou Medical University in Yantai, Shandong Province20,21. The first wave of data was collected between August 23 and September 23, 2019. Baseline information including questionnaires and blood samples22. The questionnaire contained the following:
-
Demographic data (age, sex, BMI).
-
Lifestyle information (smoking, drinking).
-
Frequency of food consumption (fish consumption).
-
Mental health information (depressive symptoms).
The follow-up information collection was conducted when they were set to leave school for internships. The follow-up was conducted from May 25, 2021 to May 28, 2021 for nursing majors, and from December 19, 2021 to December 21, 2021 for clinical medicine majors. The collection of follow-up data comprised a self-reported questionnaire and blood samples. Inclusion criteria: high school and residence locations in Shandong Province; age of 18 or older; admission to Binzhou Medical University was in 2019. Exclusion Criteria: have chronic medical conditions; surgery within the past month; have hearing or speech impairments. There were 857 participants recruited at baseline and 477 at follow-up, with 380 participants lost to follow-up. Finally, we included 477 subjects with total results of blood mercury and questionnaire data from both 2019 and 2021 in the analyses. Fig. 1 shows the distribution map of the study subjects. This study was reviewed and approved by the Ethics Committee of Binzhou Medical University, and all study subjects signed the informed consent form (NO.2019075).
Collection and measurement of mercury
Venous blood samples were collected by an experienced nurse using a tube containing EDTA. Whole blood samples and centrifuged upper serum layers were stored at -80 °C until analysis. The quantitative analysis was conducted using inductively coupled plasma mass spectrometry (ICP-MS) at the Central Lab of Biological Elements in Peking University Health Science Center with the analytical protocol certified by China metrology accreditation (CMA). The 0.35 mL blood sample was transferred into a quartz tube, followed by the addition of 0.40 mL nitric acid. The quartz tube was pre-digested at room temperature for two hours and then placed in an Ultra WAVE microwave digestion system (Ultra WAVE, Milestone, Italy) for 50 min. Subsequently, 0.10 mL of indium internal standard (2 ng/mL) was added to the sample, which was then diluted to a final volume of 8 mL with ultra-pure water. Finally, the heavy metal content in the whole blood was determined using ICP-MS. The methodological details of laboratory analysis have been described in previous studies22. The total mercury concentration of the whole blood was reported in nanograms per milliliter (ng/mL). Quality assurance and quality control (QA/QC) measures are as follows: Reagent blanks and procedural blanks were prepared along with each analysis batch to eliminate potential background contamination. The limit of detection (LOD) for mercury was calculated as three times the standard deviation (SD) of the replicate measurements (11 times) of the procedural blank samples. The LOD of mercury was 0.002 ng/mL. The concentration of mercury used standard blood samples (Clinchek®- Whole Blood Control:8841) for quality control and its values (mean ± standard deviation) were 5.63 ± 0.12 ng/mL (n = 6), which was consistent with the certified values range 5.57 (4.18, 6.96) ng/mL. Besides, to investigate the antagonistic effect of selenium on mercury, selenium concentrations were measured simultaneously with mercury concentrations using the same analytical method, achieving a LOD of 0.05 ng/mL.
Measurement of depressive symptoms
The 9-item Patient Health Questionnaire (PHQ-9) was used to assess participants’ depressive symptoms. Each of the nine items in the questionnaire is rated on a scale from 0 (not at all) to 3 (almost every day), for a total score of 0–2723. Based on the diagnostic criteria for depression in the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV), the PHQ-9 is widely used in screening for depression and is considered reliable and effective24.
Liquid chromatography and mass spectrometry
We employed a liquid-liquid extraction method to extract metabolites and lipids from serum samples25,26. Subsequently, the resulting dried samples were frozen at − 80 °C until subsequent LC-MS analysis. The Ultimate 3000 UHPLC system with Q-Exactive HF MS (Thermo Fisher Scientific, Waltham, MA, USA) was used for metabolomics and lipidomics analysis. Specifically, an Xbridge amide column (100 × 2.1 mm i.d., 3.5 μm; Waters, USA) was utilized at 30 °C for the metabolomics analysis, while a reversed-phase BEH C18 column (2.1 mm×100 mm, 2.5 μm, Waters, USA) was employed at 40 °C for the lipidomic analysis. Further details are provided in the Supplementary Methods section.
Covariates
The covariates including age, sex, body mass index (BMI), smoking, passive smoking, drinking, household income, fish consumption frequency, and physical activity frequency were collected by the questionnaire. The covariate selection process is visually represented in the Directed Acyclic Graph (DAG) presented in Fig. 2. Annual household income exceeding 100,000RMB (≈ US$15,000) is defined as high household income, and the rest is defined as low household income27. Fish consumption was separated into two categories (fish consumption less than once a month versus fish consumption at least once a month)15. The following lifestyle behaviors were dichotomously defined: smoking, at least one cigarette per day for 6 months; passive smoking, breathing in other people’s smoke at least one cigarette per day for 6 months; drinking, at least once per month for the past 3 months; physical activity, physical activity at least once a week28.
Statistical analysis
Blood mercury levels were analyzed as a continuous measure and log2-transformed to meet normality. We used the linear mixed-effects model with adjustment for all covariates to examine the association between blood mercury levels and depressive symptoms scores. We conducted a follow-up study, with blood mercury concentrations and depression scores measured as repeated data. Linear mixed-effects model is ideal for data that exhibit an internal hierarchical structure, making it suited for repeated measurement data, such as the repeated PHQ9 scores in this study. Additionally, by accounting for random effects that may vary between individuals, the linear mixed-effects model can effectively incorporate both fixed and random effects within the data. We used restricted maximum likelihood estimation (REML) to estimate regression coefficients and standard deviations. The linear mixed-effects model adjusted potential confounders based on the association between depressive symptom scores and blood mercury levels, including gender, age, BMI, income, smoking status, alcohol consumption, fish consumption, physical activity, and passive smoking status. We used the same model to conduct stratification analyses by sex and fish consumption. We also conducted several sensitivity analyses. First, separate analyses were performed in non-smoking or non-drinking populations to assess the robustness of our results. Second, selenium (Se) acts antagonistically with mercury in vivo. The toxicity of mercury is reduced by complexes such as Se - Hg precipitation, or by antioxidant effects29. Therefore, we used a linear mixed-effects model, incorporating mercury and selenium, to construct a two-pollutant model to investigate the stability of the model. We also stratified participants into groups based on PHQ-9 scores and then used the ordered logistic mixed model (cumulative link model, CLMM) to analyze the association between mercury concentrations and the severity of depression.
To investigate the effect of blood mercury exposure on metabolites, we conducted a metabolomic analysis on samples stratified by quartile blood mercury levels. Firstly, single-factor analysis of variance and post-hoc Duncan analysis were utilized to compare statistical metabolic changes associated with mercury exposure, with significance determined at a corrected p-value less than 0.05. Secondly, a linear mixed - effect model was applied to evaluate the associations between identified metabolites and blood mercury level. Here, the mercury exposure level was set as the independent variable and the relative content of metabolites was set as the dependent variable. Covariates including age, BMI, gender, fish consumption, and socioeconomic status were incorporated as fixed effects in the model to control for their potential confounding effects. P-values less than 0.05 were considered significant. All analyses were conducted in R (version 4.2.1) software.
Metabolite identification
Metabolites and lipids were identified based on primary mass spectrometry (MS1) and secondary mass spectrometry (MS2) spectra using MSDIAL software. MS/MS refers to a tandem mass spectrometry technique that consists of MS1 and MS2. During MS1, sample molecules can be ionized and then separated based on the mass-to-charge ratio (m/z). Precursor ions are guided to the impact chamber and then break to form product ions. MS2 is the second stage of mass spectrometry, which is mainly responsible for further fragmentation and analysis of specific ions selected by MS1, providing detailed information about the molecular structure information30. The MassBank database was searched to identify metabolites. Also, a Lipid Blast-based silica spectral database (version: LipidDBs-VS23-FiehnO) was used to identify lipids. MS1 and MS/MS search tolerances were set to 0.01 Da and 0.05 Da, respectively, with an identification score cutoff of 70%. Other parameters used in MS-DIAL are set to default values.
Pathway analysis
Subsequently, the metabolites exhibiting statistically significant differences were cross-referenced with the HMDB and KEGG databases. The Human Metabolome Database or HMDB (http://www.hmdb.ca) is a web-enabled metabolomic database containing comprehensive information about human metabolites along with their biological roles, physiological concentrations, disease associations, chemical reactions, metabolic pathways, and reference spectra31. Kyoto Encyclopedia of Genes and Genomes or KEGG (www.genome.jp/kegg/) is a database resource for understanding high-level functions and utilities of the biological system, such as the cell, the organism, and the ecosystem, from molecular-level information, especially large-scale molecular datasets generated by genome sequencing and other high-throughput experimental technologies32,33,34. Lastly, to elucidate the major metabolic pathways involving the identified metabolites, we conducted a pathway analysis using MetaboAnalyst 5.0. We mapped all significantly altered metabolites onto relevant metabolic pathways.
Results
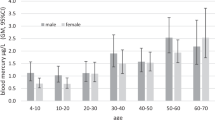
Table 1 shows the demographic characteristics, lifestyle characteristics, depressive symptoms, and blood mercury levels in 2019 and 2021. There were 477 participants in this cohort (32.08% male). The mean age of the cohort at baseline was 18.44 ± 0.58 years (Table 1). There were 37.74% of participants consumed fish less than once a month. The subjects of high household income accounted for 21.80%. The mean (SD) PHQ-9 score was 2.81 (± 3.65) at baseline, and slightly decreased to 2.79 (± 4.10) in 2021. The median blood mercury levels were 0.77 ng/mL (IQR 0.56–1.04) at baseline and 0.76 ng/mL (IQR 0.59–1.00) in 2021. PHQ-9 scores at different blood mercury levels are shown in Supplementary Material Table S1.
Table 2 shows the blood mercury levels and PHQ-9 scores in different groups. Males (n = 153) had higher depressive symptoms (P = 0.21) and higher blood mercury levels (P = 0.01) than females (n = 324) in 2019 and 2021. During follow-up, the mean blood mercury levels increased in the groups of smoking, drinking, and fish consumption at least once a month. However, blood mercury concentrations had no change in passive smoking exposure and high household income group. We also found that the depressive symptoms score increased in low household income, non-exercising, and fish consumption at least once a month groups in the follow-up.
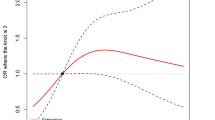
Fig. 3 shows the association between young adults’ blood mercury levels and PHQ-9 score and results stratified by sex and fish consumption frequency. Detailed results of the association can be found in Table S2. The results showed that blood mercury levels were positively associated with depressive symptom scores in the fully adjusted model. For a 2-fold increase in blood mercury levels, depressive symptoms score increased by 0.50 (95% CI: 0.14, 0.86; P < 0.01). We found that blood mercury levels were positively associated with the deterioration of depressive symptoms in males. For each 2-fold increase in blood mercury levels, the depressive symptoms score increased by 0.87 (95% CI: 0.12, 1.61; P = 0.02) in males, while such association was not observed in females. We also found that blood mercury levels were positively associated with depressive symptom scores in the groups of fish consumption at least once a month. For each 2-fold increase in blood mercury levels, the depressive symptoms score increased by 0.56 (95% CI:0.13, 0.99; P = 0.02). After dividing the subjects into groups based on PHQ-9 scores, the results also showed that the blood mercury concentration was positively correlated with PHQ-9 scores (β = 0.61, P = 0.009).
The result of the linear mixed effects model of the association between PHQ-9 scores and blood mercury levels and stratified analysis. The total model included the subject as a random effect and adjusted for sex, age, BMI, lifestyle factors (smoking status, passive smoking, physical activity), and fish consumption.
The results of the sensitivity analyses are presented in Table S3. The population of no smoking (n = 472) or no drinking (n = 458) were analyzed. For every 2-fold increase in blood mercury levels, depressive symptom score increased by 0.51 (95% CI: 0.15, 0.88; P < 0.01) in the non-smoking model and 0.50 (95% CI: 0.14, 0.87; P < 0.01) in the non-drinking model. The two-metal model of mercury and selenium was also constructed, and the results were still robust. Depressive symptom score increased by 0.47 (0.10, 0.85; P < 0.01) for each 2-fold of blood mercury in the two-metal model.
Fig. 4 shows the results of the KEGG metabolic pathway enrichment analysis32,35,36. Based on analysis of variance and linear mixed model analysis, 213 differential metabolites were screened out with a significance threshold of P < 0.05. The identified metabolites were then put into the HMDB database and KEGG database for cross-matching, and finally, 44 differential metabolites were identified. Further metabolic pathways analysis showed that the metabolic dysregulation associated with mercury exposure was concentrated in amino acid metabolism. There were 7 dysregulated metabolites enriched in four differential metabolic pathways, specifically arginine biosynthesis, proline metabolism, aminyl-tRNA biosynthesis, and phenylalanine, tyrosine, and tryptophan biosynthesis.
Enrichment results for metabolic pathway. The vertical coordinate is the metabolic pathway name and the horizontal coordinate is -log(p-value), the color from green to red indicates that the p-value decreases in order, the larger the point, the more the number of metabolites enriched to that pathway.
Fig. 5 shows the dysregulation of metabolites after mercury exposure. For example, it has been observed that levels of phenylalanine, pyroglutamic acid, arginine, and oxaloacetate decrease with increasing blood mercury levels, while levels of L-leucine, taurine, arachidonic acid, and leukotriene decreased with increasing blood mercury levels. Among them, arginine, Phenylalanine, and L-leucine were significantly enriched in metabolic pathways of arginine biosynthesis, proline metabolism, aminyl-tRNA biosynthesis, and phenylalanine, tyrosine, and tryptophan biosynthesis shown in Fig. 4.
Discussion
As far as we know, this is the first longitudinal cohort study to explore the association between blood mercury levels and depressive symptoms in Chinese young adults. We found that the increased blood mercury levels among Chinese undergraduates were positively associated with depressive symptoms scores. This association was sex-specific and affected males more than females. In addition, we found a more significant association between blood mercury levels and depressive symptoms in those who ate fish at least once a month. In the metabolomic analysis, high blood mercury level was associated with 44 altered metabolites, mainly enriched in amino acid metabolism pathways.
This study revealed a positive association between blood mercury levels and depressive symptoms. In line with our findings, a cross-sectional study of 11,754 Korean adults indicated a positive association between greater blood mercury concentration and the incidence of depression in Korean females14. According to a case-control study with 3,517 participants from Japan, those in the Minamata region who were severely exposed to methylmercury were more likely to experience psychiatric symptoms37. However, the cross-sectional 2005–2008 NHANES study of 6,911 US adults age ≥ 20 years revealed that total blood mercury levels were not associated with depression after adjusting for sociodemographic variables (income-poverty ratio, education, marital status). However, in the elderly, an inverse effect of total blood mercury on depression was found15. The authors attributed this finding to residual factors such as socioeconomic factors and seafood consumption. Another cross-sectional study of 15,140 US adults aged ≥ 18 years from the 2005–2010 NHANES found that higher concentration of mercury was negatively associated with depression (adjusted Prevalence Ratios (PR) = 0.62, 95% CI = 0.50, 0.78)13. Differences in study design could partly explain heterogeneity in the above-mentioned results and our results. Additionally, although depression was assessed in both studies using the PHQ-9 scale, the 2005–2008 NHANES study employed a cut-off point for the diagnosis of depression was 4/5, and the 2005–2010 NHANES was 9/10. Inconsistency may be attributable to research populations, which may differ according to the individual’s physical condition, lifestyle, dietary habits, education, and mercury exposure levels. Toxicological studies have revealed that mercury metabolism and toxicity can be affected by sex, diet, or co-exposure to other pollutants38. Nevertheless, knowledge regarding the role of potential modifying factors in the association between mercury exposure and depressive symptoms is currently limited. Dietary intake and environmental characteristics are considered to increase blood mercury concentrations in human beings.
In subgroup analysis, our results indicated sex-specific associations between blood mercury levels and depressive symptoms. Males seem to be more sensitive to mercury exposure in terms of depressive symptoms. The finding of a cross-sectional study of 408 individuals in Zhoushan City, China, supported our results that prenatal methylmercury exposure from fish intake caused a neurodevelopmental risk for males but not females39. We can consider several potential causes for this difference: metabolic levels and the protective effects of estrogen. In the study on mercury-induced immunotoxicity effects on mice, males had more tissue mercury retention than females, particularly in the kidneys40. Additionally, estrogen may perform a protective function in the association between blood mercury and depressive symptoms. Estrogen has antioxidant properties that can provide an additional defense against oxidative stress by working as scavengers or activating estrogen receptors to stimulate the synthesis of protective molecules41. This may indicate a protective role of estrogen in psychiatric disorders. We also found sex-specific blood mercury level differences in our study. During follow-up, median blood mercury levels were significantly higher in males (0.82 ng/mL, IQR 0.59–1.11) than in females (0.76 ng/mL, IQR 0.58–0.97). In a 2010–2011 cross-sectional study including 4,000 Korean participants aged 0 to 83, the geometric mean concentration of total mercury in whole blood of all subjects was 2.92 µg/L, which was also significantly higher in males (3.11 µg/L) than in females (2.77 µg/L)42. Males have higher blood mercury levels than females, which may induce more severe mental health problems. Given that the mechanisms of distribution and susceptibility in mercury remain obscure, further study is essential to clarify it.
Our stratified analysis revealed that the effect of blood mercury levels on depressive symptoms was more significant among those who consumed fish at least once a month. The Japan Public Health Center-based Prospective Study (JPHC) demonstrated that moderate fish consumption (an average of 111 g of fish per day) could reduce depression risk43. However, consuming contaminated fish may increase neurotoxicity, as fish may be the primary source of human mercury exposure44. Mercury concentrations were higher in Koreans from coastal regions than in Korean from inland areas due to their higher intake of seafood45. A study in Hong Kong, China, analyzed 151 plasma samples and found a positive association between fish consumption rates and plasma methylmercury concentrations46. It is clear from all of the above studies that a high intake of fish could affect blood mercury levels. This supports our finding that people who eat fish at least once a month are more likely to be exposed to mercury, which affects depressive symptoms. Although we excluded the antagonistic effect of the heavy metal selenium on mercury. There might be other synergized effect pollutants that need to be further explored.
A Korean study found that exposure to multiple heavy metals such as cadmium, lead, mercury, and arsenic caused activation of glycolytic pathways, alterations in amino acid metabolism, disturbances in energy metabolism, dysregulation of lipid metabolism, and changes in polar metabolites associated with antioxidant47. Our study found that high levels of mercury exposure disrupted amino acid metabolism, mainly involving arginine biosynthesis, arginine and proline metabolism, and the biosynthesis of phenylalanine, tyrosine, and tryptophan. In arginine biosynthesis, L-citrulline and L-arginine expression were downregulated. L-citrulline is a key intermediate in the synthesis of nitric oxide. L-arginine reacts with oxygen to produce NO and L-citrulline48,49. NO is an important regulator of neuronal function50. The administration of L-arginine can mitigate cognitive decline and behavioral depression by counteracting the oxidative stress response in the brain and reducing the excitotoxic cell stress of neurons51,52. A study investigating serum L-arginine and L-citrulline levels in patients with major depressive disorder also found that serum L-arginine and L-citrulline levels were decreased53. High level mercury exposure may affect depressive symptoms by reducing L-arginine and L-citrulline levels.
In the biosynthesis of phenylalanine, tyrosine, tryptophan and aminoacyl-tRNA biosynthesis, the expression of L-phenylalanine is downregulated. Phenylalanine serves as a precursor for dopamine54, a monoamine transmitter in the brain. Impairment of dopamine signaling could lead to depression55. Previous studies have also found that patients with a history of depression exhibit significantly lower levels of phenylalanine in the brain parenchyma compared to those without depression56.
High level mercury exposure disturbed the arginine and proline metabolism, leading to the downregulation of guanidinoacetate, 4-acetylaminobutyrate, and L-arginine. Guanidinoacetate is a precursor to creatine57, which is essential for the energy supply of brain cells58. Patients with depression have impaired brain energy metabolism. The downregulation of guanidinoacetic acid levels may lead to insufficient cellular energy supply, thereby exacerbating depressive symptoms. 4-Acetamidobutyric acid ester is a derivative of γ-aminobutyric acid (GABA)59, an important inhibitory neurotransmitter in the central nervous system. Dysfunction of GABA is associated with neurological disorders such as anxiety60. Mercury exposure could lead to depressive symptoms through disrupting the expression of guanidinoacetate and 4-acetylaminobutyrate.
The primary merit of the present study is that it presents the first report on the association between blood mercury and depressive symptoms among Chinese young adults. At the same time, the association between mercury exposure and serum metabolomics was preliminarily discussed. Potential biomarkers and metabolic pathways for mercury exposure to affect depressive symptoms were identified. Moreover, given the cohort study design, we could infer causality between blood mercury concentration and depressive symptoms. However, this study had some limitations. First, a recall bias on the food frequency questionnaire might have occurred owing to the subjects’ seasonal or daily dietary habits. Additionally, depression symptoms were evaluated by a self-assessment questionnaire. Second, we did not validate the metabolites in this study. Last, we did not collect information on exposure to other contaminants in fish. In the further study, we will collect more detailed data on pollutants. The potential confounding effects of these pollutants should be excluded, and the specific effects of mercury on depressive symptoms should be further analyzed. In addition, previous in vivo studies have found that mercury exposure disrupts lipid metabolism and amino acid metabolism. Epidemiological studies have also found that combined exposure to multiple heavy metals alters amino acid metabolic pathways and causes dysregulation of lipid metabolism. We will validate them in future studies. Although the study has some inevitable limitations, our findings still give fresh insights into the neurotoxicity of mercury exposure among young adults.
Conclusion
Blood mercury levels were positively associated with depressive severity in this population-based cohort study. Males and those who consume more than one serving of fish per month are more susceptible to the effects of mercury, which can exacerbate depressive symptoms. Mercury exposure may affect depressive symptoms through neurotransmitter, energy, inflammation, and oxidative stress-related metabolic pathways. Stricter mercury pollution legislations are warranted to protect human health.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Collaborators, G. M. D. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry. 9(2), 137–150 (2022).
IHME. GBD Compare Data Visualization. https://ghdx.healthdata.org/gbd-2019 (2022).
Organization, W. H. & Depression. https://www.who.int/news-room/fact-sheets/detail/depression (2022).
Herrman, H. et al. Time for united action on depression: a Lancet–World psychiatric association commission. Lancet 399(10328), 957–1022 (2022).
Huang, Y. et al. Prevalence of mental disorders in china: a cross-sectional epidemiological study. Erratum In: Lancet Psychiatry. 6(3), 211–224 (2019).
Varma, P. et al. Younger people are more vulnerable to stress, anxiety and depression during COVID-19 pandemic: A global cross-sectional survey. Prog Neuropsychopharmacol. Biol. Psychiatry. 109, 110236 (2021).
Ceccatelli, S. et al. Long-lasting neurotoxic effects of exposure to methylmercury during development. J. Intern. Med. 273(5), 490–497 (2013).
Sharma, B. M. et al. An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects. Environ. Int. 125, 300–319 (2019).
Zhang, L. et al. Updated emission inventories for speciated atmospheric mercury from anthropogenic sources in China. Environ. Sci. Technol. 49(5), 3185–3194 (2015).
Liu, K. et al. Measure-specific effectiveness of air pollution control on China’s atmospheric mercury concentration and deposition during 2013–2017. Environ. Sci. Technol. 53(15), 8938–8946 (2019).
Maqbool, F. et al. Immunotoxicity of mercury: pathological and toxicological effects. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 35(1), 29–46 (2017).
Clarkson, T. W. & Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 36(8), 609–662 (2006).
Berk, M. et al. Pop, heavy metal and the blues: secondary analysis of persistent organic pollutants (POP), heavy metals and depressive symptoms in the NHANES National epidemiological survey. BMJ Open. 4(7), e005142 (2014).
Kim, K. W. et al. Association of blood mercury level with the risk of depression according to fish intake level in the general Korean population: findings from the Korean National health and nutrition examination survey (KNHANES) 2008–2013. Nutrients 12(1), 189 (2020).
Ng, T. H., Mossey, J. M. & Lee, B. K. Total blood mercury levels and depression among adults in the united states: National health and nutrition examination survey 2005–2008. PLoS One. 8(11), e79339 (2013).
Haidar, Z. et al. Disease-associated metabolic pathways affected by heavy metals and metalloid. Toxicol. Rep. 10, 554–570 (2023).
You, L. et al. Metabolome-wide association study of serum exogenous chemical residues in a cohort with 5 major chronic diseases. Environ. Int. 158, 106919 (2022).
Peng, Z. et al. Multi-omics analyses reveal the mechanisms of arsenic-induced male reproductive toxicity in mice. J. Hazard. Mater. 424, 127548 (2022).
Zhou, J. et al. Metabolome analysis to investigate the effect of heavy metal exposure and chemoprevention agents on toxic injury caused by a multi-heavy metal mixture in rats. Sci. Total Environ. 906, 167513 (2024).
Miao, J. et al. Life-time summer heat exposure and lung function in young adults: A retrospective cohort study in Shandong China. Environ. Int. 160, 107058 (2022).
Young adults’ blood selenium and lung function in Shandong Province, China: A prospective cohort study. The Innovation Medicine. 1(1), 100013-27 (2023)
Miao, J. et al. Association between mercury exposure and lung function in young adults: A prospective cohort study in shandong, China. Sci. Total Environ. 878, 162759 (2023).
Spitzer, R. L. et al. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA 282, 1737–1744 (1999).
Martin, A. et al. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. Gen. Hosp. Psychiatry. 28(1), 71–77 (2006).
Chen, W. et al. Comprehensive metabolomic and lipidomic analysis reveals metabolic changes after mindfulness training. Mindfulness 11, 1390–1400 (2020).
Zhou, J. et al. Comprehensive metabolomic and proteomic analyses reveal candidate biomarkers and related metabolic networks in atrial fibrillation. Metabolomics 15, 1–13 (2019).
Statistics, N.B.o. http://www.stats.gov.cn/tjsj/sjjd/201901/t20190121_1645944.html.
Lu, P. et al. Mental health of new undergraduate students before and after COVID-19 in China. Sci. Rep. 11(1), 18783 (2021).
Tinggi, U. & Perkins, A. V. J. N. Selenium status: its interactions with dietary mercury exposure and implications in human health. Nutrients 14(24), 5308 (2022).
Thomas, S. N. et al. Liquid chromatography–tandem mass spectrometry for clinical diagnostics. Nat. Rev. Methods Primers. 2(1), 96 (2022).
Wishart, D. S. et al. HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 37(suppl_1), D603–D610 (2009).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1), 27–30 (2000).
Kanehisa, M. et al. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45(D1), D353–D361 (2017).
Muto, A. et al. Modular architecture of metabolic pathways revealed by conserved sequences of reactions. J. Chem. Inf. Model. 53(3), 613–622 (2013).
Kanehisa, M. et al. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53(D1), D672–D677 (2025).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28(11), 1947–1951 (2019).
Yorifuji, T. et al. Long-term exposure to methylmercury and psychiatric symptoms in residents of Minamata, Japan. Environ. Int. 37(5), 907–913 (2011).
Llop, S. et al. Prenatal exposure to mercury and infant neurodevelopment in a multicenter cohort in spain: study of potential modifiers. Am. J. Epidemiol. 175(5), 451–465 (2012).
Tchounwou, P. B. et al. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 18(3), 149–175 (2003).
Thomas, D. J. et al. Sexual differences in the distribution and retention of organic and inorganic mercury in methyl mercury-treated rats. Environ. Res. 41(1), 219–234 (1986).
Brann, D. W. et al. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids 72(5), 381–405 (2007).
Eom, S. Y. et al. Lead, mercury, and cadmium exposure in the Korean general population. J. Korean Med. Sci. 33(2), e9 (2018).
Matsuoka, Y. et al. Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in japan: a population-based prospective cohort study. Transl Psychiatry. 7(9), e1242–e1242 (2017).
Fernandes, A. C. et al. Benefits and risks of fish consumption for the human health. Revista De Nutrição. 25, 283–295 (2012).
You, C. H. et al. The relationship between the fish consumption and blood total/methyl-mercury concentration of costal area in Korea. Neurotoxicology 33(4), 676–682 (2012).
Liang, P. et al. Plasma mercury levels in Hong Kong residents: in relation to fish consumption. Sci. Total Environ. 463, 1225–1229 (2013).
Kim, M. J. et al. Associations between plasma metabolites and heavy metal exposure in residents of environmentally polluted areas. Environ. Int. 187, 108709 (2024).
Sarandy, M. M. et al. l-arginine and l-citrulline supplementation accelerates second intention wound healing in iNOS knockout mice. J. Funct. Foods. 100, 105395 (2023).
Shannon, O. M. et al. Nitric oxide, aging and aerobic exercise: sedentary individuals to master’s athletes. Nitric Oxide. 125, 31–39 (2022).
Ledo, A. et al. The bioactivity of neuronal-derived nitric oxide in aging and neurodegeneration: switching signaling to degeneration. Free Radic Biol. Med. 162, 500–513 (2021).
Martínez-González, K. et al. Citrulline supplementation improves spatial memory in a murine model for Alzheimer’s disease. Nutrition 90, 111248 (2021).
Moniello, G. et al. Role of L-Arginine supplementation in long Covid-related fatigue and depression in elderly outpatients. EUR. PSYCHIAT. 67(Suppl 1), S106–S106 (2024).
Le Melledo, J. M. et al. L-Arginine and NOS activity, a complex relationship. Psychopharmacol. (Berl). 238(4), 1223–1224 (2021).
Szigetvari, P. D. et al. The effects of phenylalanine and tyrosine levels on dopamine production in rat PC12 cells. Implications for treatment of phenylketonuria, tyrosinemia type 1 and comorbid neurodevelopmental disorders. Neurochem Int. 171, 105629 (2023).
Chen, J. et al. Urinary metabolite signatures for predicting elderly stroke survivors with depression. Neuropsychiatr Dis. Treat. 17, 925–933 (2021).
Kofler, M. et al. A reduced concentration of brain interstitial amino acids is associated with depression in subarachnoid hemorrhage patients. Sci. Rep. 9(1), 2811 (2019).
Ingoglia, F. et al. Creatine metabolism in patients with urea cycle disorders. Mol. Genet. Metab. Rep. 29, 100791 (2021).
Pazini, F. L. et al. The possible beneficial effects of creatine for the management of depression. Prog Neuropsychopharmacol. Biol. Psychiatry. 89, 193–206 (2019).
Xue, W. et al. Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics. Food Sci. Hum. Well. 13(1), 472–481 (2024).
Benham, R. S. et al. α2-containing γ-aminobutyric acid type A receptors promote stress resiliency in male mice. Neuropsychopharmacology 46(12), 2197–2206 (2021).
Acknowledgements
The authors are grateful to all students of Binzhou Medical University who participated in this study.
Funding
Peng Lu was sponsored by Taishan Scholar Foundation (tsqn202211228) and National Science Foundation of China (42307549).
Author information
Authors and Affiliations
Contributions
M.F., W.X., T.Z., Y.D., P.L., planned the study and drafted the manuscript. J.Z., M.F., P.L., contributed to data analysis and interpretation. J.Z., Y.M., J.W., P.X., S.F., Y.G., P.L., participated in manuscript revision. All authors have given final approval for the manuscript to be published and have agreed to be responsible for all aspects of the manuscript.
Corresponding author
Ethics declarations
Consent to participate
Written informed consent was obtained from all participants.
Institutional review board statement
The study was approved by the Binzhou Medical University ethics committee (NO.2019075). All experiments were performed in accordance with relevant specified guidelines and regulations. Informed consent was obtained from all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feng, M., Xu, W., Zhao, T. et al. A longitudinal metabolomics study on blood mercury and depressive symptoms. Sci Rep 15, 26312 (2025). https://doi.org/10.1038/s41598-025-09017-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-09017-8