Abstract
This study aims to elucidate the potential mechanisms underlying the therapeutic effects of Bletilla striata in glioma. Targets of Bletilla striata and glioma were predicted using TCMSP, SwissTargetPrediction, GeneCards, and other databases. A “drug-ingredient-target” network and protein–protein interaction (PPI) network were constructed, with core targets identified via topological analysis. Functional enrichment (GO/KEGG, DAVID), molecular docking, and experimental validations (MTT, scratch assay, RT-PCR, Western blot) were performed. A total of 11 active ingredients of Bletilla striata, 456 corresponding targets, and 2,830 glioma-related targets were identified. Nine core targets (AKT1, STAT3, mTOR, etc.) were identified. GO analysis indicated that these targets were primarily involved in phosphorylation, protein binding, and the positive regulation of RNA polymerase II transcription. KEGG analysis highlighted key pathways, including pathways in cancer, the PI3K-AKT signaling pathway, and microRNA-related regulatory mechanisms in cancer. Molecular docking analysis demonstrated high binding affinities between active ingredients and core targets, particularly AKT1 and mTOR. Functional assays showed that Blestriarene A, a key active compound in Bletilla striata, significantly suppressed glioma cell proliferation and migration. RT-PCR results indicated that treatment with varying concentrations of Blestriarene A for 48 h downregulated AKT mRNA expression while upregulating mTOR mRNA expression. Western blot analysis further confirmed a reduction in PI3K, AKT, and mTOR phosphorylation following treatment. Network pharmacology and in vitro experiments suggest that Blestriarene A anti-glioma effects by modulating the PI3K/AKT/mTOR signaling pathway.
Similar content being viewed by others
Introduction
Glioma, a tumor originating from glial cells in the brain, accounts for 30–40% of all intracranial tumors and represents the most prevalent primary intracranial malignancy in adults1. Its common subtypes include diffuse astrocytoma, oligodendroglioma, and others2. The annual incidence is approximately 6.4 per 100,000 individuals, with glioblastoma (GBM), classified as WHO grade 4, exhibiting an incidence of 4.03 per 100,000, constituting 50.1% of all primary malignant central nervous system tumors3. GBM is characterized by near-universal mortality, with a 5-year survival rate of only 5%4. Despite the current standard of care—comprising surgical resection, radiotherapy, and chemotherapy—gliomas remain highly malignant, and patient survival remains dismally short5. Recent advances in glioma research have intensified efforts to identify novel therapeutic targets and develop targeted therapies.
Traditional Chinese medicine (TCM) offers distinct advantages in glioma management, particularly in symptom relief, mitigation of radiotherapy- and chemotherapy-induced adverse effects, and overall improvement of patient quality of life. The integration of TCM with Western medicine has demonstrated significant synergistic effects6. According to TCM theory, glioma pathogenesis reflects a dynamic interplay of deficiency and excess, where deficiency constitutes the underlying condition and excess manifests as pathological changes. TCM treatment principles focus on expelling pathogenic factors while reinforcing the body’s vital energy. Given its cerebral localization, glioma is closely linked to the liver, spleen, and kidneys, with its progression influenced by various pathological factors, including wind, phlegm, blood stasis, deficiency, and cancerous toxins. Currently, mainstream Western medical interventions include surgery, radiotherapy, chemotherapy, and tumor-treating fields6. For patients ineligible for or unwilling to undergo surgery, TCM-based therapies provide alternative treatment avenues. The Expert Consensus on Integrated Traditional Chinese and Western Medicine Clinical Diagnosis and Treatment of Glioma (Shanghai) outlines recommended TCM treatment strategies, underscoring their potential role in glioma management.
Bletilla striata (Thunb. ex Murray) Rchb. F., a perennial herbaceous plant of the Orchidaceae family, exhibits astringent, hemostatic, anti-inflammatory, and tissue-regenerating properties7. Clinically, it is widely utilized for its antibacterial, anti-tumor, and wound-healing effects8. Several bioactive compounds in Bletilla striata have demonstrated anti-tumor activity. Studies indicate that Bletilla striata, in combination with transarterial chemoembolization, reduces tumor volume in hepatocellular carcinoma9. Additionally, it enhances the antitumor efficacy of docetaxel10 and exhibits cytotoxic activity against A549 non-small cell lung cancer cells11. Moreover, Bletilla striata has been reported to exert neuroprotective effects against isoflurane-induced neuronal injury by modulating the PI3K/AKT signaling pathway12, suggesting a potential therapeutic avenue for gliomas. The pharmacological complexity of TCM arises from its multi-target, multi-component interactions, making it challenging to attribute its efficacy to a single compound or pathway. The therapeutic effects of TCM often stem from the synergistic actions of multiple bioactive components and their regulatory networks. Modern network pharmacology, integrating drug-target interactions with biological networks, offers a systematic approach to predicting the active ingredients and mechanisms of TCM. Shanshan Gong’s study, employing network pharmacology and in vivo experiments, demonstrated that Bletilla striata alleviates ulcerative colitis via the EGFR/PI3K/AKT signaling pathway13. Similarly, Yue Wang’s research on chronic skin ulcers, combining Bletilla striata with Galla Chinensis, identified AKT1, TNF, and EGFR as core targets, implicating its role in suppressing inflammation and oxidative stress14. These findings underscore network pharmacology as a powerful tool for elucidating the mechanisms of action of TCM.
This study aims to investigate the therapeutic mechanisms of Bletilla striata in gliomas. Network pharmacology was employed to predict potential targets and signaling pathways associated with its anti-glioma effects. Molecular docking was further conducted to evaluate the binding affinities between key bioactive compounds of Bletilla striata and core targets. Given previous evidence of its cytotoxic effects15,16, functional validation was performed using the SNB19 glioma cell model to further elucidate its key molecular targets and pathways, providing mechanistic insights into its therapeutic potential.
Materials and methods
Network pharmacology prediction
Identification of active components and targets of Bletilla striata
Active components were initially screened from the TCMSP database17 (https://old.tcmsp-e.com/tcmsp.php) based on oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18. Additional active components were identified through a comprehensive literature review. The corresponding targets of these active compounds were retrieved from the TCMSP, SwissTargetPrediction18 (http://swisstargetprediction.ch/) and TCMSID19 (https://tcm.scbdd.com/home/index/) databases.
Identification of glioma-related targets
Glioma-related targets were identified by querying “Glioma” in the GeneCards20 (https://www.genecards.org/), DisGeNET21 (https://www.disgenet.org/), OMIM22 (https://www.omim.org/), and TTD23 (https://db.idrblab.net/ttd/) databases.
Identification of the intersection targets between Bletilla striata and glioma
The targets associated with Bletilla striata and glioma were imported into the VENNT2.1.0 online tool (https://bioinfogp.cnb.csic.es/tools/venny/) to identify overlapping targets, which were considered potential therapeutic targets of Bletilla striata for glioma treatment. A Venn diagram was generated to visualize the intersections.
Construction of the PPI network
The shared targets of Bletilla striata and glioma were further analyzed using the STRING 12.0 database24 (https://cn.string-db.org/) to construct a protein–protein interaction (PPI) network. The PPI network data were imported into Cytoscape 3.10.0 (https://cytoscape.org/) for topological analysis, with core targets identified through Cytoscape plugins, including MCODE and CytoHubba.
GO functional analysis and KEGG pathway enrichment analysis
Gene Ontology (GO) functional annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed using the DAVID database (https://david.ncifcrf.gov/)25,26,27,28. The top 20 enriched terms were visualized as bubble and bar charts using the Microbiome Analysis Platform (https://www.bioinformatics.com.cn/). (The cut-off value for P-value is 0.05.)
Molecular docking
Molecular docking was conducted to assess the binding affinity between core targets and active compounds of Bletilla striata. The 3D structures of the active compounds were retrieved from the PubChem database29 (https://pubchem.ncbi.nlm.nih.gov/), while protein structures were sourced from the PDB database30 (https://www.rcsb.org/). Docking simulations were performed using AutoDock1.5.6 (https://autodock.scripps.edu/), and the results were visualized in PyMOL3.1.0 (https://pymol.org/).
Cell experiments
Cell culture
The SNB19 human glioma cell line was obtained from Chengdu Jinboxin Biotechnology Co., Ltd. (Chengdu, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; KeyGEN, Nanjing, China) supplemented with 10% fetal bovine serum (FBS; Gibco, NY, USA), 0.1 mg/mL streptomycin, and 100 U/mL penicillin. The cultures were maintained in a humidified atmosphere at 37 °C with 5% CO₂. Following standard cell passage protocols, cells were subcultured at 80% confluence.
Drug
Blestriarene A was purchased from Sichuan Aiqi Biotechnology Co., Ltd. (Chengdu, China). For experimental use, the compound was dissolved in dimethyl sulfoxide (DMSO; Solarbio, Beijing, China) to prepare a 50 mM stock solution, which was subsequently diluted with culture medium to achieve the desired working concentrations. The final DMSO concentration in all treatments did not exceed 0.1% (v/v), which was also maintained in corresponding vehicle controls.
Reagents
The 0.25% trypsin solution (containing EDTA, without phenol red and calcium-magnesium) and MTT reagent were sourced from Chengdu Jinboxin Biotechnology Co., Ltd. UltraStart Universal SYBR qPCR Master Mix and additional MTT reagent were supplied by Chengdu Xinnuobiotechnology Co., Ltd. (Chengdu, China). Antibodies against PI3K (1:1000), p-AKT (1:5000), AKT (1:5000), p-mTOR (1:2000), and mTOR (1:5000) were obtained from Proteintech (Wuhan, China), p-PI3K (1:1000) was purchased from Affbiotech (Jiangsu, China), and secondary antibodies (1:5000) was acquired from Boster (Wuhan, China). Primers for PI3K (Forward: 5′-AAACAGAGCCAAAGGGAAGG-3′, Reverse: 5′-ATACCAGCCACAAAGGCTTC-3′), AKT (Forward: 5′-ATCGCTTCTTTGCCGGTAT-3′, Reverse: 5′-TCTTGGTCAGGTGGTGTGAT-3′), mTOR (Forward: 5′-GCGACACCGAATCAATCAT-3′, Reverse: 5′-TTTCTTCATGGGTCCTGTTT-3′), β-Actin (Forward: 5′-CATGTACGTTGCTATCCAGGC-3′, Reverse: 5′-CTCCTTAATGTCACGCACGAT-3′) were synthesized by Zhejiang Youkang Biotechnology Co., Ltd. (Zhejiang, China).
MTT assay for cell viability
For the MTT assay, SNB19 cells in the logarithmic growth phase were digested with 0.25% trypsin and resuspended in a complete culture medium. A total of 1 × 103 cells were seeded per well in a 96-well plate and incubated for 24 h. Upon reaching 60% confluence, the culture medium was replaced with fresh medium containing varying concentrations of Blestriarene A (5, 10, 15, 20, and 40 µM). After 48 h of incubation, 10 µL of MTT reagent was added to each well and incubated for an additional 4 h. The supernatant was then carefully removed, and 100 µL of DMSO was added to dissolve the formazan precipitate by shaking for 10 min. Optical density at 490 nm was measured using a microplate reader (Thermo scientific, MA, USA), and cell viability was calculated.
Scratch assay
For the scratch assay, SNB19 cells in the logarithmic growth phase were digested with 0.25% trypsin and resuspended in a complete culture medium. A total of 5 × 105 cells were seeded into 6-well plates and cultured to full confluence. A 200 µL pipette tip was used to create two perpendicular scratches guided by a ruler, intersecting at predetermined reference points. After discarding the culture medium, cells were gently washed 2–3 times with PBS to remove detached cells. Depending on the experimental group, either a drug-containing medium or a 2% serum medium was added. Images of identical scratched regions were acquired at 0 h (baseline) and subsequent time points using an inverted phase-contrast microscope (Olympus; Tokyo, Japan) equipped with a 4 × objective lens. Cells were then incubated at 37 °C with 5% CO2. At 0 h, 12 h, 24 h, and 48 h, the same regions were observed under a microscope, and the scratch width was measured and analyzed.
RT-PCR detection
Cells in the logarithmic growth phase were digested with 0.25% trypsin and resuspended in a complete culture medium. A total of 5 × 105 cells were seeded into 6-well plates and cultured until reaching 70% confluence. Following the removal of the culture medium, a drug-containing or a complete culture medium was added according to the experimental design. After 48 h of incubation, total RNA was extracted, and RT-PCR analysis was conducted using β-actin as an internal control. Relative gene expression levels were quantified using the 2−ΔΔCT method.
Western blot detection
For Western blot analysis, cells at 70% confluence were treated under the same conditions as described above. After 48 h, total protein was extracted, subjected to SDS-PAGE, and transferred onto a membrane. The membrane was blocked and incubated overnight at 4 °C with the primary antibody. Following three washes with TBST, the membrane was incubated with the secondary antibody at room temperature for 1 h, followed by an additional three washes with TBST. The protein bands were visualized using an Odyssey infrared imaging system (LI-COR Biosciences, NE, USA). Band intensities were quantified using Image J software (National Institutes of Health, MD, USA).
Statistical analysis
Data analysis was performed using GraphPad Prism 8 (https://www.graphpad-prism.cn/) and Excel. Statistical significance between two groups was assessed using an unpaired two-tailed Student’s t-test, while comparisons among multiple groups were conducted using one-way analysis of variance (ANOVA). A p value < 0.05 was considered statistically significant.
Results
Active components and related targets of Bletilla striata
Nine active components of Bletilla striata were identified based on OB and DL criteria using the TCMSP database. Additionally, two compounds—phenanthrene, 9,10-dihydrophenanthrene, and bibenzyl enantiomers—were included from literature sources due to their reported antineuroinflammatory and cytotoxic activities, bringing the total to eleven active components (Table 1). A total of 456 Bletilla striata-associated targets were retrieved from the TCMSP, SwissTargetPrediction, and TCMSID databases (Fig. 1A).
Prediction of potential targets for Bletilla striata in the treatment of glioma
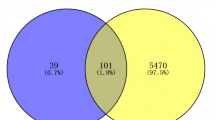
A total of 2830 glioma-related targets were obtained from the GeneCards, DisGeNET, OMIM, DrugBank, and TTD databases. Using Venny 2.1.0 software, an overlap analysis between Bletilla striata-associated and glioma-related targets identified 206 common targets, suggesting that Bletilla striata may exert therapeutic effects against glioma through multi-target synergy (Fig. 1B).
Construction of the PPI network
The 206 shared targets were imported into the STRING database to construct a PPI network, which was subsequently visualized in Cytoscape 3.10.0 (Fig. 1C). The network comprises 206 nodes and 4,313 edges, where nodes represent target genes and edges denote protein interactions, with edge thickness indicating the strength of data support. Topological analysis using Cytoscape 2.2 plugins, including MCODE and Cytohubba, identified nine core targets (Fig. 1D). These core targets—AKT1, STAT3, TP53, ESR1, CASP3, CCND1, HIF1A, BCL2, and mTOR (Fig. 1E)—are likely to play a pivotal role in Bletilla striata’s potential therapeutic effects against glioma.
Screening of core targets of Bletilla striata in Glioma. A Bletilla striata—active components—targets map (the inner circle represents the 11 active components of Bletilla striata, while the outer oval represents the 456 associated targets). B Venn diagram illustrating the intersection between Bletilla striata active component-associated targets and glioma-related targets. C PPI network diagram. D Intersection analysis performed using Cytoscape 3.10.0, incorporating the Cytoscape2.2, MCODE, and Cytohubba plugins. E The nine core targets identified from the intersection.
GO enrichment analysis of targets
The 206 intersecting targets of Bletilla striata were analyzed using the DAVID database, yielding 1114 GO enrichment results, including 823 biological processes (BP), 103 cellular components (CC), and 188 molecular functions (MF). The top 10 GO enrichment terms were selected and visualized in a bubble chart (Fig. 2A). The biological enrichment analysis highlights key processes involved in glioma treatment, including phosphorylation, positive regulation of transcription by RNA polymerase II, protein phosphorylation, signal transduction, and negative regulation of apoptosis.
KEGG pathway enrichment analysis of targets
KEGG pathway enrichment analysis identified 168 signaling pathways, with the top 20 pathways selected for visualization in a bar chart (Fig. 2B). Notably, key pathways include cancer-related signaling pathways, the PI3K-AKT signaling pathway, microRNA regulation in cancer, proteoglycans in cancer, human papillomavirus infection, and chemical carcinogenesis-receptor activation. These findings suggest that Bletilla striata exerts its therapeutic effects against glioma through a multi-pathway regulatory mechanism.
“Component-target-pathway” network construction
A “Component-Target-Pathway” network was constructed using Cytoscape 3.10.0 (Fig. 2C). The network comprises 11 active components (represented by dark green hexagons), 206 target genes (light green rectangles), and 20 pathways (pink octagons), interconnected by 1526 edges, illustrating the complex interplay between Bletilla striata’s bioactive compounds, their molecular targets, and key signaling pathways. KEGG pathway analysis was performed using KEGG database26,27,28.
Enrichment Analysis of KEGG Pathways and GO Terms, and “Component-Target-Pathway” Network Construction. A GO analysis bubble chart. B KEGG analysis bar chart. C “Component-Target-Pathway” network diagram, where dark green hexagons represent Bletilla striata active components, light green rectangles denote potential targets, and pink octagons correspond to key signaling pathways.
Molecular docking
Molecular docking analysis was performed to assess the binding affinity between the bioactive components of Bletilla striata and the predicted core target proteins. Nine core targets—AKT1 (PDB ID: 6hhh), STAT3 (PDB ID: 6nuq), TP53 (PDB ID: 3exj), ESR1 (PDB ID: 1GWQ), CASP3 (PDB ID: 2xyg), CCND1 (PDB ID: 2W96), HIF1A (PDB ID: 1h2m), BCL2 (PDB ID: 2w3l), and, mTOR (PDB ID: 3ML9)—were selected as receptor proteins. Based on the “Component-Target-Pathway” network analysis, the five key components with the highest degree values were identified: 2,3,4,7-tetramethoxyphenanthrene, 3-(p-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene, 4,7-dihydroxy-1-p-hydroxybenzyl-2-methoxy-9,10-dihydrophenanthrene, Blestriarene A, and Blestriarene B, which were used as molecular ligands for docking simulations. As shown in Fig. 3A, all active components exhibited binding energies below − 4 kcal/mol with their respective targets, indicating strong binding affinities. Among these, Blestriarene A demonstrated the most favorable binding energy with core targets. As presented in Fig. 3B and C, Blestriarene A forms multiple interactions with AKT1 and mTOR, including hydrogen bonding, H–π interactions, and π–π stacking, suggesting its strong potential for modulating these key oncogenic pathways.
Molecular docking and visualization of Bletilla striata active components with target proteins. A Heatmap of binding energies between Bletilla striata active components and target proteins. B Molecular docking visualization of Blestriarene A with AKT1. C Molecular docking visualization of Blestriarene A with mTOR.
Inhibition of glioma cell proliferation by the active compound blestriarene A from Bletilla striata
To evaluate the biological effects of Blestriarene A on glioma cells, SNB19 cells were treated with increasing concentrations (0, 5, 10, 15, 20, and 40 µM), followed by MTT assays to assess cell viability. As shown in Fig. 4A, Blestriarene A significantly inhibited glioma cell proliferation at 20 µM and 40 µM compared to the control group. Moreover, the inhibitory effect was concentration-dependent, with an IC50 value of 40 µM. Based on these results, subsequent mechanistic investigations were conducted using low (20 µM), medium (40 µM), and high (60 µM) concentrations to elucidate the molecular mechanisms underlying Blestriarene A’s anti-glioma effects.
Inhibition of glioma cell migration by the active compound blestriarene A from Bletilla striata
To evaluate the impact of Blestriarene A on glioma cell migration, SNB19 cells were treated with increasing concentrations (0, 20, 40, and 60 µM), and migration was assessed at 0, 12, 24, and 48 h. As shown in Fig. 4B, all tested concentrations significantly inhibited cell migration, with stronger suppression observed at higher concentrations (Fig. 4C–E). Notably, exposure to 60 µM Blestriarene A for 24 h resulted in substantial cellular damage, suggesting a potent inhibitory effect on glioma cell motility and potential anti-metastatic activity.
The active compound blestriarene A from Bletilla striata downregulates AKT mRNA expression and upregulates mTOR mRNA expression
To investigate the regulatory effects of Blestriarene A on the mRNA expression of PI3K, AKT, and, mTOR, SNB19 cells were treated with varying concentrations (0, 20, 40, and 60 µM) for 48 h, and their mRNA expression levels were quantified by RT-PCR, using β-actin as the internal control. Compared to the control group, Blestriarene A downregulated AKT mRNA expression while upregulating mTOR mRNA levels (Fig. 4F–H).
The active compound blestriarene A from Bletilla striata reduces the expression of phosphorylated PI3K, AKT1, and mTOR proteins
To further assess its impact at the protein level, Western blot analysis was conducted to measure the expression of PI3K, AKT1, and mTOR after 48 h of Blestriarene A treatment. Compared to the control group, Blestriarene A treatment led to a dose-dependent reduction in the phosphorylation levels of PI3K, AKT, and mTOR, as evidenced by the decreased p-PI3K/PI3K, p-AKT/AKT, and p-mTOR/mTOR ratios (Fig. 4I–L).
Mechanistic Study of the Inhibitory Effects of Blestriarene A on Glioma. A Cytotoxic effects of Blestriarene A on SNB19 cells at different concentrations. B Microscopic images of glioma cell migration, scale bar = 100 μm. C–E Quantification of glioma cell migration rates. Relative mRNA expression levels of F PI3K, G AKT, and H mTOR measured by RT-PCR. I Representative Western blot images. Quantification of J p-PI3K/PI3K, K p-AKT/AKT, and L p-mTOR/mTOR ratios, expressed as relative intensity. All data are presented as mean ± standard deviation (*p < 0.05 vs. control, **p < 0.01 vs. control).
Discussion
Glioma is the most prevalent tumor of the central nervous system31. Despite the availability of clinical treatments—including surgical resection, radiotherapy, chemotherapy, and targeted therapy—patient prognosis remains poor32. Additionally, glioblastoma treatment imposes a substantial economic burden on patients, healthcare institutions, and society due to its high per capita medical costs. In recent years, TCM has demonstrated certain advantages in glioma management by improving clinical symptoms, mitigating the adverse effects of radiotherapy and chemotherapy, and improving patient quality of life. Various bioactive compounds in Bletilla striata have exhibited anti-tumor properties, with studies indicating therapeutic effects against liver, breast, and colorectal cancers33. This study applied a network pharmacology approach to identify the core targets, biological functions, and signaling pathways involved in Bletilla striata’s anti-glioma effects, followed by in vitro validation to elucidate its molecular mechanisms.
A total of 11 active components of Bletilla striata were identified, with 206 overlapping targets shared between Bletilla striata and glioma. Nine core targets—AKT1, STAT3, TP53, ESR1, CASP3, CCND1, HIF1A, BCL2, and mTOR—were identified through topological analysis. GO enrichment analysis revealed that Bletilla striata’s therapeutic effects are associated with key biological processes such as phosphorylation, positive regulation of transcription by RNA polymerase II, signal transduction, protein binding, ATP binding, and protein kinase activity, all of which play pivotal roles in glioma pathogenesis. KEGG pathway analysis further highlighted its involvement in cancer-related pathways, the PI3K/AKT signaling cascade, chemical carcinogenesis-receptor activation, neurodegeneration-associated pathways, and glioma-specific pathways. Molecular docking analysis demonstrated that Blestriarene A, a major bioactive compound of Bletilla striata, exhibits strong binding affinity with core targets such as AKT1 and mTOR, both of which are key regulators of glioma cell proliferation, migration, and metabolism. AKT, a serine/threonine kinase, is a central player in glioma progression and invasiveness, with three highly conserved isoforms: AKT1, AKT2, and AKT334. Among these, AKT1 is implicated in multiple oncogenic processes that drive malignant transformation35. mTOR, an atypical serine/threonine kinase, forms two distinct complexes—mTORC1 and mTORC2—both of which contribute to glioma pathophysiology36. Additionally, inhibiting mTOR signaling enhances glioma cell resistance to hypoxia-induced cell death37. The PI3K/AKT/mTOR pathway is among the most frequently dysregulated signaling cascades in glioma, playing a crucial role in tumorigenesis, disease progression, and therapeutic response38. Accumulating evidence suggests that suppression of glioma cell proliferation and tumorigenicity can be achieved by inhibiting autophagy through PI3K/AKT/mTOR pathway activation39. Given its central role in glioma biology, targeting the PI3K/AKT signaling axis represents a promising therapeutic strategy.
Blestriarene A, an active component derived from Bletilla striata, was selected for in vitro antitumor assays based on prior findings. Glioma cells were treated with varying concentrations of Blestriarene A to evaluate its impact on proliferation. MTT assays confirmed a dose-dependent inhibitory effect on glioma cell proliferation, while wound healing assays demonstrated suppressed cell migration. Given the identified core targets and signaling pathways, further validation was conducted to elucidate whether Blestriarene A exerts its antiglioma effects via the PI3K/AKT/mTOR pathway. RT-PCR analysis revealed a downregulation of AKT mRNA expression and an upregulation of mTOR mRNA expression, suggesting that Blestriarene A may suppress PI3K activity, thereby attenuating AKT activation and reducing AKT mRNA levels. Concurrently, it may activate the mTOR pathway through alternative mechanisms, necessitating further investigation. Western blot analysis demonstrated a reduction in phosphorylated PI3K, AKT1, and mTOR protein levels, indicating that Blestriarene A disrupts the phosphorylation cascade within the PI3K/AKT/mTOR axis. We found a contradiction between mRNA and protein expression of mTOR. We speculate that this mRNA upregulation may be due to the strong inhibition of the PI3K/AKT pathway activating a negative feedback loop, prompting glioma cells to upregulate mTOR gene transcription to compensate for the loss of pathway activity (similar to the feedback mechanism in previous studies where rapamycin inhibition of mTOR induced upregulation of IRS-1 protein and activated AKT phosphorylation, protein kinase activity, and downstream signaling. Additionally, studies have found that when rapamycin fails to effectively block the mTORC1 target 4EBP1, it leads to feedback activation of the PI3K-AKT signaling pathway40,41). Meanwhile, the decrease in mTOR protein levels suggests that Blestriarene A may have interfered with mTOR protein activity, thereby inhibiting the initiation of protein translation. This mechanism may be similar to how rapamycin affects mTOR proteins, but the specific mechanism requires further validation42. This phenomenon has provided a new direction for follow-up research. The current study, through the integration of network pharmacology, molecular docking, and in vitro experiments, has suggested that Blestriarenne A might exert anti-glioma effects by inhibiting the PI3K/AKT/mTOR signaling pathway. However, the precise mechanisms of action and clinical translational potential require further validation. The primary limitation of this research lies in its exclusive reliance on in vitro cell models and computational simulations, with insufficient pharmacokinetic data and a lack of in vivo pharmacodynamic validation using animal models. Specifically, the drug’s ability to penetrate the blood-brain barrier and its long-term toxicity profile have not been fully characterized. To address these limitations, future research will prioritize the establishment of physiologically relevant animal models for in vivo studies, with the dual objectives of systematically evaluating the compound’s therapeutic efficacy, pharmacokinetic profile, and toxicity thresholds within a holistic physiological context. This integrated in vitro-in vivo cross-validation strategy is expected to not only deepen the mechanistic understanding of the compound’s action but also generate clinically translatable data for preclinical evaluation, thus accelerating the translation process from basic research to clinical applications.
Conclusion
This study employed a network pharmacology platform to identify the primary active components of Bletilla striata and elucidate its mechanism of action in glioma treatment through multi-component, multi-target, and multi-pathway analyses. In vitro experiments further demonstrated that Blestrialene A its antiglioma effects via the PI3K/AKT/mTOR pathway. These findings lay the foundation for further in-depth investigations into the underlying mechanisms of Bletilla striata in glioma therapy.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- GBM:
-
Glioblastoma
- TCM:
-
Traditional Chinese medicine
- OB:
-
Oral bioavailability
- DL:
-
Drug-likeness
- PPI:
-
Protein–protein interaction
- BP:
-
Biological processes
- CC:
-
Cellular components
- MF:
-
Molecular functions
- KEGG:
-
Kyoto Encyclopedia of genes and genomes
- GO:
-
Gene ontology
References
Zeng, T., Cui, D. & Gao, L. Glioma: an overview of current classifications, characteristics, molecular biology and target therapies. Front. Biosci. (Landmark edition) 20 (7), 1104–1115 (2015).
Gestrich, C. K., Couce, M. E. & Cohen, M. L. Adult diffuse astrocytic and oligodendroglial tumors. Neurosurgery 89 (5), 737–749 (2021).
Ostrom, Q. T. et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-oncology 24 (Suppl 5), v1–v95 (2022).
Delgado-López, P. D. & Corrales-García, E. M. Survival in glioblastoma: a review on the impact of treatment modalities. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mexico 18 (11), 1062–1071 (2016).
Zhou, Y. S., Wang, W., Chen, N., Wang, L. C. & Huang, J. B. Research progress of anti-glioma chemotherapeutic drugs (Review). Oncol. Rep. 47(5). (2022).
Wang, J. et al. A review of traditional Chinese medicine for treatment of glioblastoma. Biosci. Trends. 13 (6), 476–487 (2020).
Chen, Z., Cheng, L., He, Y. & Wei, X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: a review. Int. J. Biol. Macromol. 120 (Pt B), 2076–2085 (2018).
Qiu, J. et al. Comparison of extraction processes, characterization and intestinal protection activity of Bletilla striata polysaccharides. Int. J. Biol. Macromol. 263 (Pt 1), 130267 (2024).
Qian, J. et al. Combined transarterial chemoembolization and arterial administration of Bletilla striata in treatment of liver tumor in rats. World J. Gastroenterol. 9 (12), 2676–2680 (2003).
Guan, Q. et al. Docetaxel-Loaded Self-Assembly stearic Acid-Modified Bletilla striata polysaccharide micelles and their anticancer effect: preparation, characterization, cellular uptake and in vitro evaluation. Molecules (Basel Switzerland) 21(12). (2016).
Zhou, F. et al. Antiproliferative and proapoptotic effects of phenanthrene derivatives isolated from Bletilla striata on A549 lung Cancer cells. Molecules (Basel Switzerland) 27(11). (2022).
Zhao, G., Li, K., Chen, J. & Li, L. Protective effect of extract of Bletilla striata on isoflurane induced neuronal injury by altering PI3K/Akt pathway. Transl. Neurosci. 9, 183–189 (2018).
Gong, S., Lv, R., Fan, Y., Shi, Y. & Zhang, M. The potential mechanism of Bletilla striata in the treatment of ulcerative colitis determined through network pharmacology, molecular docking, and in vivo experimental verification. Naunyn. Schm. Arch. Pharmacol. 396 (5), 983–1000 (2023).
Wang, Y., Ding, T. & Jiang, X. Network Pharmacology study on herb pair Bletilla striata-Galla chinensis in the treatment of chronic skin ulcers. Curr. Pharm. Des. 30 (17), 1354–1376 (2024).
Sun, M. H. et al. Phenanthrene, 9,10-dihydrophenanthrene and bibenzyl enantiomers from Bletilla striata with their antineuroinflammatory and cytotoxic activities. Phytochemistry 182, 112609 (2021).
Li, J. Y. et al. Stilbenes with anti-inflammatory and cytotoxic activity from the rhizomes of Bletilla ochracea schltr. Fitoterapia 127, 74–80 (2018).
Ru, J. et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6, 13 (2014).
Daina, A., Michielin, O. & Zoete, V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47 (W1), W357–w364 (2019).
Zhang, L. X. et al. TCMSID: a simplified integrated database for drug discovery from traditional Chinese medicine. J. Cheminform. 14 (1), 89 (2022).
Stelzer, G. et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 54, 13031–13033 (2016).
Piñero, J. et al. The disgenet knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 48 (D1), D845–d855 (2020).
Amberger, J. S., Bocchini, C. A., Scott, A. F. & Hamosh, A. OMIM.org: leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 47 (D1), D1038–d1043 (2019).
Zhou, Y. et al. TTD: therapeutic target database describing target druggability information. Nucleic Acids Res. 52 (D1), D1465–d1477 (2024).
Szklarczyk, D. et al. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51 (D1), D638–d646 (2023).
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50 (W1), W216–w221 (2022).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53 (D1), D672–d677 (2025).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. Publ. Protein Soc. 28 (11), 1947–1951 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30 (2000).
Kim, S. et al. PubChem 2023 update. Nucleic Acids Res. 51 (D1), D1373–d1380 (2023).
Burley, S. K. et al. RCSB protein data bank (RCSB.org): delivery of experimentally-determined PDB structures alongside one million computed structure models of proteins from artificial intelligence/machine learning. Nucleic Acids Res. 51 (D1), D488–d508 (2023).
Gusyatiner, O. & Hegi, M. E. Glioma epigenetics: from subclassification to novel treatment options. Sem. Cancer Biol. 51, 50–58 (2018).
Śledzińska, P., Bebyn, M., Furtak, J., Koper, A. & Koper, K. Current and promising treatment strategies in glioma. Rev. Neurosci. 34 (5), 483–516 (2023).
Xu, D., Pan, Y. & Chen, J. Chemical constituents, Pharmacologic properties, and clinical applications of Bletilla striata. Front. Pharmacol. 10, 1168 (2019).
Ahmad, Z. & Somanath, P. R. AKT isoforms in the immune response in cancer. Curr. Top. Microbiol. Immunol. 436, 349–366 (2022).
Chautard, E., Ouédraogo, Z. G., Biau, J. & Verrelle, P. Role of Akt in human malignant glioma: from oncogenesis to tumor aggressiveness. J. Neurooncol. 117 (2), 205–215 (2014).
Szwed, A., Kim, E. & Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 101 (3), 1371–1426 (2021).
Divé, I. et al. Inhibition of mTOR signaling protects human glioma cells from hypoxia-induced cell death in an autophagy-independent manner. Cell. Death Discov. 8 (1), 409 (2022).
Yu, L., Wei, J. & Liu, P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Sem. Cancer Biol. 85, 69–94 (2022).
Chen, H. et al. Xanthatin suppresses proliferation and tumorigenicity of glioma cells through autophagy Inhibition via activation of the PI3K-Akt-mTOR pathway. Pharmacol. Res. Perspect. 11 (1), e01041 (2023).
O’Reilly, K. E. et al. mTOR Inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66 (3), 1500–1508 (2006).
Sun, S. Y. et al. Activation of Akt and eIF4E survival pathways by Rapamycin-mediated mammalian target of Rapamycin Inhibition. Cancer Res. 65 (16), 7052–7058 (2005).
Choo, A. Y., Yoon, S. O., Kim, S. G., Roux, P. P. & Blenis, J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. USA 105 (45), 17414–17419 (2008).
Funding
This study was supported by the “New Quality Pharmacy Sailing Plan” Hospital Pharmacy High-Quality Development Research Fund Project from the Sichuan Provincial Pharmaceutical Industry Association (grant number: scyxh20240703).
Author information
Authors and Affiliations
Contributions
XMZ, CYZ: Conceptualization, Methodlogy. FLR, YHW: Investigation, Resources, Validation. XMZ: Writing-Original Draft. XZ: Writing-Review and Editing. CYH: Project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, F., Wang, Y., He, C. et al. The mechanism of Bletilla striata inhibiting glioma proliferation through the PI3K/AKT/mTOR signaling pathway based on network Pharmacology analysis. Sci Rep 15, 20415 (2025). https://doi.org/10.1038/s41598-025-09081-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09081-0