Abstract
This study aims to investigate associations between PM10, PM2.5, and NO2 and maternal thyroid hormones. We investigated 443 pregnant women in Zhejiang, China with repeated examinations of serum thyroid hormones at 10, 17, and 32 weeks. Individual exposures to ambient air pollution were retrospectively calculated by inverse distance weighting interpolation. Multivariate linear mixed models were applied to estimate the association between air pollutants and thyroid hormones. PM10 exposure was positively associated with TSH (β: 0.077 [95% CI: 0.003, 0.152]), and negatively associated with FT3 (β: -0.041 [-0.057, -0.025]) and FT4 (β: -0.036 [-0.055, -0.017]) during the 0–90 lag days (per IQR). Similarly, increased exposure to PM2.5 was associated with decreased FT3 (β: -0.016 [-0.032, -0.001]), and increased Tg (β: 0.147 [0.025, 0.269]). Evaluated NO2 levels were associated with decreased FT3 (β: -0.026 [-0.039, -0.014]) and FT4 (β: -0.036 [-0.052, -0.020]), as well as increased Tg (β: 0.129 [0.011, 0.247]). The results indicate that exposure to ambient PM10, PM2.5, and NO2 throughout the entire pregnancy adversely affects thyroid hormones, implying potential health implications of air pollution on maternal thyroid function.
Similar content being viewed by others
Introduction
Thyroid hormones play a crucial role in various life stages, particularly during pregnancy and fetal development. During gestation, maternal thyroid hormone demand increases due to alterations in thyroid hormone metabolism, changes in binding proteins, and the requirements for placenta transfer and fetal utilization1. Thyroid hormones are essential regulators of both maternal metabolic processes and fetal growth and neurodevelopment2. Maintaining adequate thyroid hormone levels throughout pregnancy is therefore vital for supporting maternal health and ensuring healthy offspring development. Maternal thyroid hormone insufficiency has been associated with adverse health outcomes, including gestational hypertension, pre-eclampsia3, preterm birth4, fetal growth restriction2, and impaired neurodevelopment in children5. Given these significant risks, it is crucial to identify and understand modifiable factors, particularly environmental disturbances, that may disrupt maternal thyroid function during pregnancy. It is essential for developing strategies to reduce the burden of thyroid-related adverse pregnancy outcomes6,7,8.
Ambient air pollution, particularly particulate matter (PM) and nitrogen dioxide (NO2), has emerged as a critical global environmental concern with significant public health implications. According to the Global Burden of Disease, ambient and household air pollution contribute to approximately 6.7 million premature deaths annually9. In China, ambient PM pollution ranks as the fourth leading risk factor for both mortality and disability-adjusted life-years (DALYs)10. Growing evidence suggests that PM and NO2 exposure may disrupt thyroid function11,12 and contribute to adverse maternal and fetal outcomes13, including gestational hypertensive disorders14, gestational diabetes mellitus15, preterm birth, low birth weight, and stillbirth16,17. PM can absorb toxic substances, including endocrine-disrupting chemicals18, which may dysregulate the hypothalamic-pituitary-thyroid (HPT) axis and induce hepatic transthyretin in female rats19,20. Similarly, NO2 exposure has been linked to oxidative stress and inflammation, which can interfere with thyroid hormone regulation21.
In recent years, accumulating research has explored the association between PM10, PM2.5 and NO2 exposure and maternal thyroid hormone levels. Studies focusing on early pregnancy exposure have reported that PM2.5 and NO2 may disrupt maternal thyroid function22, whereas PM10 appears to have no significant effect23. Another cohort study found that first-trimester PM2.5 exposure was significantly associated with hypothyroxinemia, but no such link was observed for NO2 [25. While these studies suggest a potential relationship between ambient air pollution and maternal thyroid function status, most existing research has been limited to early pregnancy. To date, only a few studies have comprehensively examined maternal thyroid function across all three trimesters (including the early, mid and late pregnancy) in relation to air pollutant exposure.
In this study, we collected maternal serum samples at three gestational time points (first, second, and third trimesters) for a comprehensive thyroid function assessment. Individual exposure levels to air pollutants prior to blood collection were estimated using inverse distance weighting (IDW) interpolation. Our primary aim was to evaluate the association between prenatal exposure to ambient air pollutants (PM10, PM2.5, and NO2) and maternal thyroid function (TSH, FT3, FT4, and Tg) across all pregnancy trimesters within a prospective birth cohort from Zhejiang, China.
Methods
Study population
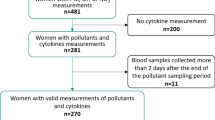
This study was conducted within a prospective population-based birth cohort in Zhejiang, China, from August 2019 to December 2021. We recruited 446 eligible pregnant women from two cities in Zhejiang, Qujiang and Jiaojiang (Fig. 1). The inclusion criteria were: (a) being between 18 and 45 years old; (b) having a gestation period of under 10 weeks; (c) having lived locally for at least one year with plans to continue residing for the next three years. The exclusion criteria were: (a) taking iodine supplementation; (b) receiving iodine-containing contrast media within the past year; (c) having a history of thyroid disease or other chronic diseases. Participants were enrolled during their first-trimester prenatal visit at local maternal and child health care hospitals. After providing written informed consent for biological sample collection and study participation, trained interviewers administered standardized questionnaires to collect sociodemographic and medical history data. Fasting blood and spot urine samples were collected during routine prenatal care visits at three time points: first trimester (approximately 10 weeks), second trimester (17 weeks), and third trimester (32 weeks). All samples were immediately stored at −80 ℃. In the final analysis, after excluding participants without blood samples (missing thyroid hormone values, n = 3), 443 participants were included in the first trimester, 411 in the second trimester, and 368 in the third trimester. The study was approved by the Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention.
Spatial distribution of the studied area. The map was created using ArcGIS Desktop 10.8 (https://www.esri.com/en-us/arcgis/products/arcgis-desktop/resources).
Assessment of air pollution
Individual exposure to ambient air pollutants (PM2.5, PM10, and NO2) was estimated using the IDW approach. Daily pollutant concentrations during 2019–2021 were obtained from national environmental monitoring stations in Zhejiang Province, provided by the Zhejiang Environmental Protection Department. Meteorological data, including daily temperature and relative humidity, were obtained from the Zhejiang Meteorological Administration. Both station locations and participants’ residential addresses were geocoded into longitude and latitude coordinates. Using the IDW approach, we calculated daily mean exposure levels of PM2.5, PM10, and NO2 for each participant. To address long-term exposure effects, we computed the 90-day moving average (0–90 days prior to thyroid function examination) as the primary exposure window. Additionally, we evaluated shorter-term exposure periods (0–9, 10–14, and 70–90 days before examination) to identify potential critical windows of susceptibility.
Assessment of thyroid function
Thyroid function was evaluated at three follow-up visits by measuring serum concentrations of thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), and thyroglobulin (Tg). Blood samples were centrifuged at 3000 rpm for 10 min, and the serum cryopreserved at −80 ℃ until analysis, which was performed within one month of collection. Serum TSH, FT3, FT4, Tg, thyroglobulin antibody (TgAb), and thyroid peroxidase antibody (TPOAb) were measured using an electrochemiluminescence immunoassay on a Cobas e411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany) with manufacturer-provided calibration materials, reagents, and quality controls. The quality control procedure was strictly followed before, during, and after testing, with two daily quality control runs per assay. The coefficient of variation was 1.23% and 0.67% for TSH at 1.50 mlU/L and 7.90 mlU/L, 1.37% and 1.16% for FT3 at 6.00 pmol/L and 23.80 pmol/L, 1.89% and 1.83% for FT4 at 15.60 pmol/L and 34.20 pmol/L, 1.39% and 1.34% for Tg at 21.00 µg/L and 78.00 µg/L, 4.78% and 4.11% for TPOAb at 26.00 IU/mL and 78.00 IU/mL, 3.08% and 2.40% for TgAb at 72.00 IU/mL and 164.00 IU/mL. All samples were analyzed only after quality control validation to ensure reliability. The limits of detection (LOD) were 0.005 mlU/L for TSH, 0.6 pmol/L for FT3, 0.5 pmol/L for FT4, and 0.04 µg/L for Tg measurements. While no FT3, FT4, and Tg measurements were below their LODs, TSH concentrations in 7 samples (0.57%) were below the LOD, and were substituted with one-half of the LOD (0.0025 mlU/L).
Assessment of covariates
The study collected demographic data including age (years), education level (high school or lower, college or higher), ethnicity (Han, others), occupation (non-manual labor, manual labor), and annual income (< 100,000 yuan, ≥ 100,000 yuan). Pre-pregnancy body mass index (BMI) was calculated as weight (kg) divided by the height squared (m2). Lifestyle factors included smoking, passive smoking, and alcohol consumption (all categorized into yes or no). Since iodine is essential for thyroid hormone synthesis, we assessed iodized salt use (iodized salt, non-iodized salt, or both), and frequency of iodine-rich food consumption (never, < 1/week, 2–7/week, or > 7/week), with the latter primarily comprising seaweed (e.g., kelp, laver). TPOAb status was classified as positive (≥ 34 IU/mL) or negative (< 34 IU/mL), and TgAb status was categorized as positive (≥ 115 IU/mL) or negative (< 115 IU/mL).
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) for normally distributed data or as median (interquartile range, IQR) for non-normally distributed data. Categorical variables were reported as frequencies (percentages). Spearman correlation analysis was used to assess the correlation between pollutants during different trimesters. In the first, second, and third trimesters, there were 61, 63, and 79 missing values on covariates, respectively. These missing values were addressed using multiple imputations by chained equations (MICE) with the R package ‘mice’ (version 3.17.0).
To estimate the associations between air pollutant exposures (PM10, PM2.5, and NO2) and maternal thyroid hormones (TSH, FT3, FT4, and Tg), we employed multivariate linear mixed models with a subject-specific random intercept to account for repeated measurements within each participant24. The normality of thyroid hormone levels was visually tested through histograms, which showed that TSH, FT3, FT4, and Tg were skew-distributed. To ensure normality or approximate normality of the outcome measures, we applied statistical transformations according to their distribution. Specifically, TSH and Tg were square root transformed, while FT3 and FT4 were log-transformed for analysis due to skewed distribution. Models were adjusted for potential confounders including maternal age, education levels, ethnicity, occupation, annual income, pre-pregnancy BMI, smoking status, passive smoking status, alcohol consumption, iodized salt use, iodine-rich food consumption, and meteorological factors (mean temperature and relative humidity during the corresponding exposure period).
We categorized PM10, PM2.5, and NO2 exposures into quintiles (Q1 to Q5) and computed regression coefficients with 95% confidence intervals (CIs), using the lowest quintile (Q1) as the reference. Additionally, we treated air pollutants as continuous variables in linear mixed models to calculate the effects of each interquartile range (IQR) increase in exposure on thyroid hormone levels. To assess linear trends (P trend), we incorporated the median values of each quintile as continuous variables in the models.
To evaluate the robustness of our results, we performed several sensitivity analyses: (1) excluding TPOAb-positive or TgAb-positive participants to account for potential autoimmune interference with thyroid function; (2) removing participants diagnosed with thyroid disease (including hypothyroidism or subclinical hypothyroidism) during pregnancy to minimize confounding by medical interventions; (3) constructing two-pollutant models incorporating pollutants with Spearman correlation coefficients ≤ 0.75 to control for potential co-pollutant confounding while maintaining model stability; and (4) examining exposure windows at 0–9, 10–14, and 70–90 lag days before thyroid hormone measurements to assess temporal variations in pollutant effects.
All statistical analyses were performed using R software (version 3.6.3). A two-sided p-value < 0.05 was considered statistically significant.
Results
Table 1 presents the demographic characteristics of the 443 participants during the first trimester. On average, each participant underwent 2.76 thyroid hormone examinations, with a range of 1 to 3. The mean age at examination was 29.17 years (SD = 4.81). Regarding pre-pregnancy BMI, 13.54% (n = 60) were underweight, while 19.41% (n = 86) were overweight or obese. Approximately half of the participants (n = 204, 46.05%) had at least a college education. The majority were of Han ethnicity (n = 432, 97.52%). About two-thirds were manual laborers (n = 296, 66.82%) or had an annual household income exceeding 100,000 yuan (n = 285, 64.33%). Over a quarter (n = 119, 26.86%) reported passive smoking exposure, whereas only 1.81% (n = 8) were current smokers. Alcohol consumption was reported by 6.55% (n = 29). The proportions of TPOAb and TgAb positivity were identical (n = 39, 8.80% each).
Table 2 presents the distribution of ambient levels and thyroid hormone concentrations across pregnancy trimesters. The median (25th−75th percentile) concentrations of TSH, FT3, FT4, and Tg were 1.44 (0.90, 2.09) mU/L, 4.32 (3.84, 4.83) pmol/L, 13.93 (12.24, 16.08) pmol/L, and 12.11 (6.77, 19.83) µg/L, respectively. Serum TSH levels exhibited an increasing trend across trimesters, whereas FT3 and FT4 levels declined. Tg levels were highest during the third trimester. For the 0–90 days average exposure preceding examination, median (25th−75th percentile) ambient levels were 43.47 (37.89, 51.74) µg/m3 for PM10, 25.64 (20.72, 28.97) µg/m3 for PM2.5, and 24.07 (20.84, 29.80) µg/m3 for NO2. The corresponding median (25th−75th percentile) temperature and relative humidity were 20.00 ℃ (13.40, 25.70) and 76.00% (66.25%, 86.00%), respectively. Spearman correlations among air pollutants by trimester are shown in Figure S1. PM2.5 and PM10 showed strong correlations across all trimesters. Spearman correlation coefficients were 0.83, 0.92, and 0.95 for the first, second, and third trimesters, respectively.
The associations between maternal exposure to PM10, PM2.5, and NO2 and serum thyroid hormone concentrations are presented in Fig. 2 and Table S1. The models were adjusted for age, education, ethnicity, occupation, annual income, pre-pregnancy BMI, current and passive smoking status, and alcohol consumption. Linear mixed models revealed that PM10 exposure was significantly associated with elevated TSH, reduced FT3, and decreased FT4. PM2.5 exposure showed a negative association with FT3 and a positive association with Tg. Higher NO2 levels were associated with lower FT3 and FT4, along with increased Tg (all P trend < 0.05). In the adjusted model, an IQR increase in PM10 exposure (28.58 µg/m3) was associated with a 0.077 mIU/L (95% CI: 0.003, 0.152) increase in TSH levels on the square root scale, and a 0.041 pmol/L (95% CI: −0.057, −0.025) decrease in FT3 levels and a 0.036 pmol/L (95% CI: −0.055, −0.017) decrease in FT4 levels, both on the log10 scale. An IQR increase in PM2.5 exposure (16.00 µg/m3) was associated with a 0.016 pmol/L (95% CI: −0.032, −0.001) decrease in FT3 levels on the log10 scale, and a 0.147 µg/L (95% CI: 0.025, 0.269) increase in Tg levels on the square root scale. Similarly, higher NO2 levels were associated with a decrease of 0.026 (95% CI: −0.039, −0.014) in FT3, a decrease of 0.036 pmol/L in (95% CI: −0.052, −0.020) FT4 both on the square root scale, alongside an increase of 0.129 µg/L (95% CI: 0.011, 0.247) in Tg levels on the square root scale, per IQR increase in NO2 levels (14.67 µg/m3).
Association of ambient air pollutant exposure during the 0–90 days prior to thyroid examination and thyroid function. Note: Separate regression models were conducted for square-root transformed TSH and Tg, and for log10-transformed FT3 and FT4. *p < 0.05, **p < 0.01. ***p < 0.001. Models were adjusted for age, education, ethnicity, occupation, income, pre-pregnancy BMI, smoking status, passive smoking status, drinking status, intake of iodized salt, intake of iodine-rich food, average temperature and relative humidity.
The sensitivity analysis demonstrated the robustness of our results. Excluding TPOAb-positive or TgAb-positive women did not significantly change the observed relationships between exposure to PM2.5, PM10, or NO2 and maternal thyroid hormone concentrations (Table S2). Similarly, removing women who developed thyroid disease during pregnancy had no substantial effect on the associations between air pollutant exposure and thyroid hormone levels (Table S3). In the two-pollutant models, the associations for PM2.5, PM10, and NO2 remained statistically significant after mutual adjustment for other pollutants (Table S4). When examining different exposure periods (Table S5), significant associations with maternal thyroid hormones were only observed for PM10, PM2.5, and NO2 exposures during the 70–90 day lag period, while weaker associations were found for exposures during the 0–9 and 10–14 day periods before examination.
Discussion
Our study investigated the effects of prenatal exposure to ambient air pollution (PM10, PM2.5, and NO2) on maternal thyroid function throughout the entire gestation period. After adjusting for potential confounders, we found that higher exposure to air pollutants was associated with reduced FT3 and FT4 levels, as well as elevated TSH and Tg levels. These results provide further evidence supporting the association between prenatal ambient air pollutants and maternal thyroid function.
The association between ambient air pollutant exposure and maternal thyroid hormone levels remains a subject of ongoing debate. Regarding PM, a prospective study of 8077 pregnant women in Shanghai, China, demonstrated that a 10 µg/m3 increase in PM2.5 during the second trimester was related to a decrease of 0.73% (95% CI: −1.25%, −0.20%) in FT4 levels4. Similarly, a study of 921 pregnant women in Wuhan, China, revealed that higher PM2.5 exposure during the first trimester was linked to lower maternal FT4 levels, though no significant associations were observed between PM2.5 and TSH or FT3, nor between PM10 and any thyroid hormones23. Additionally, a multi-cohort study of 9931 participants from four European and one US cohort suggested that a 5 µg/m3 increase in PM2.5 exposure was related to increased odds of hypothyroxinemia (OR = 1.21, 95% CI: 1.00, 1.47)25. Consistent with these prior findings, our study also identified adverse effects of PM10 and PM2.5 on maternal thyroid hormones. Nevertheless, unlike previous studies, we observed a relatively stronger effect of PM10 compared to PM2.5. Given the high correlation between PM10 and PM2.5, the specific PM fraction responsible for these effects remains unclear. Several factors may explain these inconsistencies. First, our study assessed exposure across all three trimesters, while most previous studies focused mainly on the first trimester. Additionally, variations in exposure assessment methods, exposure windows, thyroid hormone biomarkers (e.g., serum vs. plasma), and statistical methods may contribute to divergent findings. Moreover, the health effects of PM may vary depending on its sources and toxicological composition, including airborne metals and sulfates, which have been linked to neurotoxic effects26,27, and could partially account for the observed discrepancies.
However, some studies have reported no significant association between PM exposure and maternal thyroid function. For instance, a Belgian study of 499 mother-child pairs reported that ambient PM2.5 exposure during the third trimester was associated with decreased cord plasma TSH levels but showed no significant relationship with maternal thyroid hormone levels after delivery17. This discrepancy may be explained by differences in the timing of blood sample collection, as our study specifically examined maternal thyroid function during pregnancy rather than postpartum. Despite this difference, our findings align with the majority of previous studies, supporting the adverse effects of PM on maternal thyroid hormones during gestation. Our study contributes to the evidence on the association between air pollution and thyroid function throughout the entire pregnancy. Several studies further explored this association in relation to offspring outcomes28,29,30. A recent study found that PM2.5 exposure was associated with reduced maternal FT4 levels during both the preconception period and the first trimester, suggesting that maternal thyroid hormones may mediate the association between PM2.5 exposure and offspring neurodevelopmental outcomes31. Similarly, another study found that maternal FT4 levels mediated the association between PM2.5 exposure and fetal growth restriction during early gestation26. These findings highlight the importance of considering fetal development and growth in future research.
Regarding NO2 exposure, several studies have investigated its association with maternal thyroid function. A prospective study in Shanghai, China, demonstrated an inverse association between first-trimester NO2 exposure and maternal FT4 concentrations (β = 0.61%, 95% CI: −0.88%, −0.35%)22. Similarly, a cross-sectional study in Jinhua, China, identified a significant association between an IQR increase in NO2 and a −2.56% decrease (95% CI: −4.76%, −0.31%) in FT4 levels27. Additional research has revealed potential adverse effects of NO2 exposure in thyroid homeostasis, including thyroid nodules32 and thyroid cancer33. In our study, prenatal exposure to NO2 was observed to be correlated with decreased FT3 and FT4 levels as well as increased Tg levels, thereby further confirming its adverse effect on FT4 in previous studies. However, a study of European and US cohorts found no association between NO2 and thyroid function in pregnant women25, potentially attributable to lower ambient air pollutant concentrations in these regions compared to China. Given that PM and NO2 share common emission sources, primarily from traffic and industrial activities, the similar associations we observed between these pollutants and maternal thyroid hormone levels are biologically plausible. Further epidemiological research is warranted to validate these findings and better elucidate the impact of NO2 exposure on thyroid hormones.
Although some epidemiological studies have explored the relationship between air pollution and thyroid function, the underlying biological mechanisms remain incompletely understood. Previous studies have proposed several potential pathways through which air pollution may affect thyroid function. An in vivo study on female rats indicated that PM2.5 exposure interfered with thyroid hormone biosynthesis, biotransformation, and transport, while also altering thyroid hormone receptor levels. Additionally, it induced oxidative stress and inflammatory responses, ultimately activating the hypothalamic-pituitary-thyroid (HPT) axis, which plays a significant role in PM2.5-induced thyroid dysfunction19. Other mechanistic studies have similarly found that ambient PM exposure triggers oxidative stress and inflammatory responses, contributing to abnormal thyroid hormone levels21,34,35, which may further lead to neurological disorders36. A recent study combining 16 S rRNA gene sequencing and metabolomics in male rats revealed that PM2.5 alters the gut microbiome, affecting key metabolites associated with thyroid function and disturbing vital metabolic pathways37, emphasizing the potential role of gut-thyroid axis in PM2.5-induced thyrotoxicity38. Moreover, the hepatic endocrine axis plays a vital role in thyroid hormone homeostasis, as the liver is the primary site of thyroid hormone metabolism39. Previous research indicated that PM2.5-induced reduction of hepatic transthyretin impairs thyroid hormone transport and utilization19. In contrast, few studies have explored the mechanism linking NO2 exposure to thyroid dysfunction. NO2 has been shown to elevate systemic inflammatory biomarkers such as CRP and TNF-α, indicating that it may trigger immune sensitization and inflammatory responses40,41. Since inflammation can disrupt the HPT axis regulation42, this may represent a pathway through which NO₂ influences the thyroid function.
The current study has the following strengths. First, thyroid hormone assessment was based on multiple serum measurements throughout pregnancy. The repeated measurements of maternal thyroid hormones during early, mid, and late pregnancy provided a comprehensive measure of thyroid status, enabling control of intra-individual variability, increasing statistical power, and allowing the exploration of associations between ambient air pollutants and thyroid functions throughout pregnancy. Second, our study focused on a population in a developing country with relatively high levels of air pollutant exposure. Given the established link between maternal hypothyroxinemia and offspring neurodevelopmental deficits43,44,45, our findings have significant implications for developing effective intervention strategies in regions facing similar air pollution challenges. Additionally, the extensive measurement of covariates during examinations allowed for rigorous control of confounding biases, thereby strengthening the validity of our results.
Still, several limitations warrant further elaboration. First, the cross-sectional design precludes the establishment of causal relationships between ambient air pollution exposure and maternal thyroid hormone levels. While significant associations were observed, these results should be interpreted with caution. Second, the relatively modest sample size and recruitment from only two local areas, combined with the use of convenience sampling and lack of data on non-participants, may limit the ability to assess selection bias and reduce the generalizability of our findings. Additionally, the observed air pollutant levels were relatively low with limited variation, potentially restricting external validity. Our results primarily serve to confirm previously reported associations, and further large-scale, multi-center studies across diverse regions with a broader range of air pollution exposure are needed to validate these relationships. Third, individual exposure to air pollutants was estimated using the IDW method based on maternal residences during the 0–90 days prior to examination. This approach did not account for other potentially relevant locations (e.g., workplace) or physical activity patterns, which may have introduced exposure misclassification. However, since the participants were pregnant women, we assumed that they tended to maintain geographic stability to some extent. Given that we used the 0–90 day window before examination as the exposure period, the potential for bias was likely reduced. Moreover, other factors that may influence exposure levels and thyroid function7,46, such as indoor air pollution (e.g., compounds from cooking and heating), were not considered in our analysis. Future studies should consider changes in location and indoor air pollutants to better characterize individual exposures. Furthermore, although we adjusted for a series of important covariates, including the intake of iodized salt and iodine-rich foods, personal information on potential dietary and lifestyle confounders was not available. Iodine intake, in particular, was assessed through self-reported dietary questionnaires, which may have introduced recall bias and inaccuracies. Future studies should consider directly measuring iodine status, such as through urinary or serum iodine levels. Additionally, a more comprehensive assessment of these potential dietary and lifestyle confounders should be conducted to further minimize bias and improve the robustness of findings.
Conclusions
In conclusion, our study found that higher exposure to PM10, PM2.5, and NO2 was associated with lower levels of FT3 and FT4, as well as higher levels of TSH and Tg throughout pregnancy. The study underscores the potential impact of ambient air pollution on maternal thyroid function during pregnancy. Future studies should explore the causal relationships and underlying mechanisms. These findings highlight the need for enhanced air quality management and public health interventions.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Korevaar, T. I. M., Medici, M., Visser, T. J. & Peeters, R. P. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat. Rev. Endocrinol. 13, 610–622. https://doi.org/10.1038/nrendo.2017.93 (2017).
Derakhshan, A. et al. Association of maternal thyroid function with birthweight: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 8, 501–510. https://doi.org/10.1016/s2213-8587(20)30061-9 (2020).
Toloza, F. J. K. et al. Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 10, 243–252. https://doi.org/10.1016/s2213-8587(22)00007-9 (2022).
Korevaar, T. I. M. et al. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: A systematic review and Meta-analysis. JAMA 322, 632–641. https://doi.org/10.1001/jama.2019.10931 (2019).
Haddow, J. E. et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl. J. Med. 341, 549–555. https://doi.org/10.1056/nejm199908193410801 (1999).
Fernie, K. J. et al. Changes in thyroid function of nestling tree swallows (Tachycineta bicolor) in relation to polycyclic aromatic compounds and other environmental stressors in the Athabasca oil sands region. Environ. Res. 169, 464–475. https://doi.org/10.1016/j.envres.2018.11.031 (2019).
Wang, S., Romanak, K. A., Hendryx, M., Salamova, A. & Venier, M. Association between thyroid function and exposures to brominated and organophosphate flame retardants in rural central Appalachia. Environ. Sci. Technol. 54, 325–334. https://doi.org/10.1021/acs.est.9b04892 (2020).
Rogan, W. J. et al. Iodine deficiency, pollutant chemicals, and the thyroid: new information on an old problem. Pediatrics 133, 1163–1166. https://doi.org/10.1542/peds.2014-0900 (2014).
Collaborators, G. R. F. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 1223–1249. https://doi.org/10.1016/s0140-6736(20)30752-2 (2020).
Zhou, M. et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 394, 1145–1158. https://doi.org/10.1016/s0140-6736(19)30427-1 (2019).
Valdés, S. et al. Ambient air pollution and thyroid function in Spanish adults. A nationwide population-based study (Di@bet.es study). Environ. Health. 21, 76. https://doi.org/10.1186/s12940-022-00889-1 (2022).
Liu, J. et al. Association between ambient air pollution and thyroid hormones levels: A systematic review and meta-analysis. Sci. Total Environ. 904, 166780. https://doi.org/10.1016/j.scitotenv.2023.166780 (2023).
Howe, C. G. et al. Association of prenatal exposure to ambient and Traffic-Related air pollution with newborn thyroid function: findings from the children’s health study. JAMA Netw. Open. 1, e182172. https://doi.org/10.1001/jamanetworkopen.2018.2172 (2018).
Sears, C. G. et al. The association of traffic-related air and noise pollution with maternal blood pressure and hypertensive disorders of pregnancy in the HOME study cohort. Environ. Int. 121, 574–581. https://doi.org/10.1016/j.envint.2018.09.049 (2018).
Hehua, Z., Yang, X., Qing, C., Shanyan, G. & Yuhong, Z. Dietary patterns and associations between air pollution and gestational diabetes mellitus. Environ. Int. 147, 106347. https://doi.org/10.1016/j.envint.2020.106347 (2021).
Bekkar, B., Pacheco, S., Basu, R. & DeNicola, N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: A systematic review. JAMA Netw. Open. 3, e208243. https://doi.org/10.1001/jamanetworkopen.2020.8243 (2020).
Janssen, B. G. et al. Fetal thyroid function, birth weight, and in utero exposure to fine particle air pollution: A birth cohort study. Environ. Health Perspect. 125, 699–705. https://doi.org/10.1289/ehp508 (2017).
Novák, J., Vaculovič, A., Klánová, J., Giesy, J. P. & Hilscherová, K. Seasonal variation of endocrine disrupting potentials of pollutant mixtures associated with various size-fractions of inhalable air particulate matter. Environ. Pollut. 264, 114654. https://doi.org/10.1016/j.envpol.2020.114654 (2020).
Dong, X. et al. PM(2.5) disrupts thyroid hormone homeostasis through activation of the hypothalamic-pituitary-thyroid (HPT) axis and induction of hepatic transthyretin in female rats 2.5. Ecotoxicol. Environ. Saf. 208, 111720. https://doi.org/10.1016/j.ecoenv.2020.111720 (2021).
Niu, Y. et al. Fine particulate matter constituents and stress hormones in the hypothalamus-pituitary-adrenal axis. Environ. Int. 119, 186–192. https://doi.org/10.1016/j.envint.2018.06.027 (2018).
Mancini, A. et al. Thyroid hormones, oxidative stress, and inflammation. Mediators Inflamm. 2016 (6757154). https://doi.org/10.1155/2016/6757154 (2016).
Zhao, Y. et al. Air pollution exposure in association with maternal thyroid function during early pregnancy. J. Hazard. Mater. 367, 188–193. https://doi.org/10.1016/j.jhazmat.2018.12.078 (2019).
Zhang, X. et al. Association of exposure to ambient particulate matter with maternal thyroid function in early pregnancy. Environ. Res. 214, 113942. https://doi.org/10.1016/j.envres.2022.113942 (2022).
Molenberghs, G. & Verbeke, G. Linear Mixed Models for Longitudinal Data 1 edn (Springer New York, 2000).
Ghassabian, A. et al. Association of exposure to ambient air pollution with thyroid function during pregnancy. JAMA Netw. Open. 2, e1912902. https://doi.org/10.1001/jamanetworkopen.2019.12902 (2019).
Zhou, Y. et al. Early pregnancy PM2.5 exposure and its inorganic constituents affect fetal growth by interrupting maternal thyroid function. Environ. Pollut. 307, 119481. https://doi.org/10.1016/j.envpol.2022.119481 (2022).
Qiu, L. et al. Association of exposure to PM(2.5)-bound metals with maternal thyroid function in early pregnancy. Sci. Total Environ. 810, 151167. https://doi.org/10.1016/j.scitotenv.2021.151167 (2022).
Wang, X. et al. Evaluation of maternal exposure to PM(2.5) and its components on maternal and neonatal thyroid function and birth weight: A cohort study. Thyroid 29, 1147–1157. https://doi.org/10.1089/thy.2018.0780 (2019).
Chamot, S. et al. Does prenatal exposure to multiple airborne and tap-water pollutants increase neonatal thyroid-stimulating hormone concentrations? Data from the Picardy region, France. Sci. Total Environ. 905, 167089. https://doi.org/10.1016/j.scitotenv.2023.167089 (2023).
Nourouzi, Z. & Chamani, A. Characterization of ambient carbon monoxide and PM 2.5 effects on fetus development, liver enzymes and TSH in Isfahan city, central Iran. Environ. Pollut. 291, 118238. https://doi.org/10.1016/j.envpol.2021.118238 (2021).
Li, J. et al. Preconceptional and the first trimester exposure to PM(2.5) and offspring neurodevelopment at 24 months of age: examining mediation by maternal thyroid hormones in a birth cohort study. Environ. Pollut. 284, 117133. https://doi.org/10.1016/j.envpol.2021.117133 (2021).
Zhang, Y. et al. Six air pollutants associated with increased risk of thyroid nodules: A study of 4.9 million Chinese adults. Front. Endocrinol. (Lausanne). 12, 753607. https://doi.org/10.3389/fendo.2021.753607 (2021).
Park, S. J., Min, C., Yoo, D. M. & Choi, H. G. National cohort and meteorological data based nested case-control study on the association between air pollution exposure and thyroid cancer. Sci. Rep. 11, 21562. https://doi.org/10.1038/s41598-021-00882-7 (2021).
Gangwar, R. S., Bevan, G. H., Palanivel, R., Das, L. & Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: recent insights. Redox Biol. 34, 101545. https://doi.org/10.1016/j.redox.2020.101545 (2020).
Liu, C., Yang, J., Du, X. & Geng, X. Filtered air intervention modulates hypothalamic-pituitary-thyroid/gonadal axes by attenuating inflammatory responses in adult rats after fine particulate matter (PM2.5) exposure. Environ. Sci. Pollut Res. Int. 29, 74851–74860. https://doi.org/10.1007/s11356-022-21102-3 (2022).
Kang, Y. J., Tan, H. Y., Lee, C. Y. & Cho, H. An air particulate pollutant induces neuroinflammation and neurodegeneration in human brain models. Adv. Sci. (Weinh). 8, e2101251. https://doi.org/10.1002/advs.202101251 (2021).
Dong, X. et al. Alterations in the gut microbiota and its metabolic profile of PM2.5 exposure-induced thyroid dysfunction rats. Sci. Total Environ. 838, 156402. https://doi.org/10.1016/j.scitotenv.2022.156402 (2022).
Fenneman, A. C., Bruinstroop, E., Nieuwdorp, M., van der Spek, A. H. & Boelen, A. A comprehensive review of thyroid hormone metabolism in the gut and its clinical implications. Thyroid 33, 32–44. https://doi.org/10.1089/thy.2022.0491 (2023).
Qatanani, M., Zhang, J. & Moore, D. D. Role of the constitutive Androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology 146, 995–1002. https://doi.org/10.1210/en.2004-1350 (2005).
Xu, Z. et al. Association between gaseous air pollutants and biomarkers of systemic inflammation: A systematic review and meta-analysis. Environ. Pollut. 292, 118336. https://doi.org/10.1016/j.envpol.2021.118336 (2022).
Zhang, X. et al. Associations of oxidative stress and inflammatory biomarkers with chemically-characterized air pollutant exposures in an elderly cohort. Environ. Res. 150, 306–319. https://doi.org/10.1016/j.envres.2016.06.019 (2016).
Roelfsema, F., Boelen, A., Kalsbeek, A. & Fliers, E. Regulatory aspects of the human hypothalamus-pituitary-thyroid axis. Best Pract. Res. Clin. Endocrinol. Metab. 31, 487–503. https://doi.org/10.1016/j.beem.2017.09.004 (2017).
Andersen, S. L., Andersen, S., Vestergaard, P. & Olsen, J. Maternal thyroid function in early pregnancy and child neurodevelopmental disorders: A Danish nationwide Case-Cohort study. Thyroid 28, 537–546. https://doi.org/10.1089/thy.2017.0425 (2018).
Korevaar, T. I. et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 4, 35–43. https://doi.org/10.1016/s2213-8587(15)00327-7 (2016).
Jansen, T. A. et al. Maternal thyroid function during pregnancy and child brain morphology: a time window-specific analysis of a prospective cohort. Lancet Diabetes Endocrinol. 7, 629–637. https://doi.org/10.1016/s2213-8587(19)30153-6 (2019).
Zhang, J. et al. In Silico approach to identify potential thyroid hormone disruptors among currently known dust contaminants and their metabolites. Environ. Sci. Technol. 49, 10099–10107. https://doi.org/10.1021/acs.est.5b01742 (2015).
Acknowledgements
The authors would like to thank the participants, clinicians and administrators of the birth cohort. All authors have read and approved the final manuscript and take responsibility for the integrity and security of the data.
Funding
This study was supported by Zhejiang Provincial Natural Science Foundation of China (LTGY23H240001), Zhejiang Provincial Project for Medical Research and Health Sciences (2024KY905), the Opening Foundation of NHC Key Laboratory of Etiology and Epidemiology (Harbin Medical University) (NHCKLEE20230908), and Science and Technology Project of Zhejiang Provincial Department of Water Resources (RC2242).
Author information
Authors and Affiliations
Contributions
F.G.: Conceptualization, Formal analysis, Validation, Visualization, Writing - Original Draft. X.P.: Writing - Review & Editing. S.G.: Data Curation, Methodology, Writing - Review & Editing. Q.X.: Investigation, Writing - Review & Editing. J.D.: Investigation, Writing - Review & Editing. X.W.: Resources, Supervision, Funding acquisition. Z.C.: Conceptualization, Supervision, Funding acquisition. Q.F.: Resources, Writing - Review & Editing. G.M.: Resources, Investigation. P.X.: Resources, Data Curation. D.X.: Resources, Data Curation. X.H.: Resources, Investigation. Y.L.: Investigation, Data Curation. Y.J.: Investigation, Data Curation. C.L.: Investigation, Methodology. Z.M.: Conceptualization, Methodology, Writing - Review & Editing, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The studies involving humans were approved by the Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, F., Pan, X., Gu, S. et al. Association of ambient air pollutants with maternal thyroid hormones during the entire pregnancy. Sci Rep 15, 23767 (2025). https://doi.org/10.1038/s41598-025-09126-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-09126-4