Abstract
A semi-quantitative UV visible spectroscopy (UV-Vis) method for detecting small organic molecules (SOM) in amorphous calcium phosphate (ACP) is presented. Although SOM, such as citric acid and glutamic acid, are widely used during calcium phosphate synthesis to modulate their properties and enhance bioactivity, their quantification has not been previously reported. In this study, ACP containing SOM (citric acid, itaconic acid and ascorbic acid) were synthesized, dissolved in 1 M orthophosphoric acid, and analyzed by UV spectroscopy. Distinct absorption maxima (λmax) were observed: 200 nm for itaconic acid, 209 nm for citric acid, and 244 nm for ascorbic acid within the ACP matrix, aligning with those of the corresponding individual standards. Quantification revealed significant variations in SOM content of ACP matrix, with citric acid showing the highest amount (16%), followed by ascorbic acid (1.23%) and itaconic acid (0.3%). These differences are attributed to variations in their chemical structure, reactivity, and interaction with calcium phosphate. This simple and reliable approach not only provides insights into SOM concentration in ACP’s but also lays the foundation for extending this technique to other calcium phosphate phases, such as octacalcium phosphate (OCP) and hydroxyapatite (HAp), thereby advancing material characterization and optimization for bone tissue engineering.
Similar content being viewed by others
Introduction
Bone tissue engineering (BTE) has made significant strides in recent decades, driven by an improved understanding of bone biology and material design1,2. The field focuses on the dynamic relationship between cells and the extracellular matrix, where material properties play a crucial role in promoting bone regeneration3. The next generation of biomaterials for BTE focuses on multifunctionality by incorporating therapeutic agents, such as drugs, bioactive ions, or biomolecules to enhance healing and stimulate tissue growth4,5.
Among various biomaterials, calcium phosphates (CaP) have been extensively studied due to their similarity to the mineral phase of bone. CaPs can incorporate a wide range of bioactive ions (e.g., Mg2+, Sr2+, Zn2+), enhancing their osteoconductive, osteoinductive, angiogenic, and antimicrobial properties6. The incorporation of these ions into CaP and their quantitative analysis is relatively straightforward due to their chemical compatibility, stability, and established techniques such as Inductively Coupled Plasma Mass Spectrometry (ICP-MS), Atomic absorption spectroscopy (AAS), and X-ray Fluorescence (XRF).
Organic components, such as proteins, growth factors, or small organic molecules (SOM) like carboxylic acids, alongside the calcium phosphate structure improves its bioactivity, cell adhesion, and overall biocompatibility7. Small organic molecules (SOM), particularly carboxylic acids, serve as bio-signals that promote cell proliferation, differentiation, and tissue regeneration8,9,10. Additionally, the presence of SOM can regulate the material’s degradation rate, ensuring a more controlled release of ions - crucial for bone healing processes. This hybrid approach creates a more dynamic and responsive biomaterial, enhancing integration in bone tissue and facilitating resorption assisted repair11. Most studies involving SOM focus on metastable calcium phosphates like Amorphous Calcium Phosphate (ACP) and Octacalcium phosphates (OCP), with relatively few reports addressing materials like hydroxyapatite (HAp). Despite their advantages, a significant gap remains in the analytical techniques available for quantifying SOM contents of such CaPs. Intriguingly, since 1983 till 2025, 33 studies have reported the synthesis of calcium phosphates (including ACP, OCP and HAp) containing SOM. While qualitative evaluations of these hybrid materials are conducted, the quantification of incorporated SOM is often neglected (Supplementary information Table S1).
In order to understand the role of SOM contained in CaPs, it is essential to quantify their content and establish correlations between SOM concentration and key material properties, including biological behavior, degradation rates, ion release profiles, crystallinity, and cellular interactions. Without quantitative data, it is difficult to understand and thus control the performance of respective CaPs, thereby limiting the ability to optimize materials for specific biomedical applications. Analytical techniques such as electrophoresis for the determination of small organic molecules in the presence of inorganic ions, as well as other colorimetric analyses, typically operate at near neutral or basic pH (≥ 6.5) However, these conditions are unsuitable for the complete dissolution of CaPs, posing a challenge for accurate SOM quantification12,13. Consequently, there is a lack of standardized procedures for measuring SOM in CaP’s.
ACP is more soluble than OCP and HAp. It is important to note that the research presented in this manuscript is an extension of our previously published work on the synthesis and characterization of small organic molecule (SOM)-containing amorphous calcium phosphate (ACP)14. Given the specific scope of the present study, octacalcium phosphate (OCP) and hydroxyapatite (HAp) were not included in the evaluation. Nonetheless, the authors would like to emphasize that, in principle, the semi-quantitative analysis using UV spectroscopy described here can also be extrapolated to OCP and HAp systems.
To address these limitations, in current study we present a semi-quantitative analysis of SOM in CaP’s using UV–vis spectroscopy as represented in Fig. 1. A simple synthesis approach was utilized for preparing Small Organic Molecule (SOM) - containing amorphous calcium phosphate (ACP). The synthesized SOM - containing ACP (ACP_SOM) was dissolved in an acidic medium, followed by the detection of SOM in the UV range. The SOM concentration was determined by comparing the absorbance values with a standard curve of the respective SOM generated under similar conditions.
Materials and methods
Itaconic anhydride (95%, CAS 2170-03-8), citric acid (99%, CAS 77-92-9), calcium chloride (> 97%, CAS 10043-52-4), trisodium phosphate (96%, CAS 7601-54-9), hydrochloric acid (37%, CAS 7647-01-0), ascorbic acid (99%, CAS 50-81-7), orthophosphoric acid (85%, CAS 7664-38-2), and sodium hydroxide (≥ 97.0%, CAS 1301-73-2) were sourced from Sigma Aldrich, Germany, and ascorbic acid (CAS 50-81-7) was procured from Enola, Latvia. Milli-Q® water was used throughout the experiments to avoid potential interference from impurities.
Preparation of ACP and SOM-containing ACP
SOM-containing ACP was synthesized according to the previously reported procedure. The reaction was carried out in total volume of 300 mL, starting with preparation of 150 mL of 50 mM of calcium citrate solution in Milli-Q® water followed by pH adjustment to 11.5 using 3 M of NaOH. Subsequently, 150 mL of 100 mM of trisodium phosphate solution was rapidly added under constant stirring leading to precipitation. The precipitate was further isolated by centrifugation at 3000 rpm for 5 min and washed thrice with Milli-Q® water. The resulting precipitate was frozen in liquid nitrogen followed by freeze-drying for 72 h.
For synthesis of ACP_ITN and ACP_ASC, a similar procedure was followed, except that 150 mM of calcium chloride was used as calcium precursor with addition of 150 mM of itaconic acid or ascorbic acid. The pH of the calcium precursor solutions was adjustment to 11.5 using 3 M of NaOH followed by rapid addition of equal of 100 mM trisodium phosphate solution. The precipitate was further isolated by centrifugation at 3000 rpm for 5 min and washed thrice with Milli-Q® water. The resulting precipitate was frozen in liquid nitrogen followed by freeze-drying for 72 h. Plain ACP was synthesized in similar fashion without the without addition of SOM. The obtained powders were used for characterization. The phase composition of the synthesized ACP variants and plain ACP was demined using X-ray diffraction (XRD). Diffraction data was collected using PANalytical Aeries Diffractometer (Netherlands) operated at 40 kV and 15 mA in step mode with step size 0.04°, in 2θ range from 3 to 60°. Fourier Transformed Infrared Spectroscopy (FTIR) was performed using Nicolet iS50 FTIR (Thermo Scientific, United States). Experiments were performed in transmission mode from wavenumber ranging from 4000 to 400 cm− 1 with resolution of 400 cm− 1 and average of 64 scans. Further, FTIR analysis of the native SOM like citric, ascorbic and itaconic acid were recorded.
Solvent identification for UV–Vis spectroscopy
ACP is completely soluble in 1 M H2PO4. Upon addition of 1 M orthophosphoric acid to the ACP samples (50–5000 µg/mL), complete dissolution was observed without any visible small aggregates or undissolved particles. In order to ensure complete solubilization, a sonication step was purposely included, with the ACP samples dissolved in 1 M orthophosphoric acid or 1 M HCl, followed by sonication for 5 min. UV spectra were recorded in the range of 190 to 400 nm using UV–Vis’s spectrophotometer (Thermo Scientific, Evolution 300, Madison, WI USA) and compared with standard curves of the respective SOMs to select appropriate solvent.
UV–vis spectroscopic analysis
Based on the results orthophosphoric acid was chosen as an optimal solvent for analysis (refer to the result and discussion section). Calibration curves for itaconic, citric, and ascorbic acids were generated by preparing standard solutions, using 1 M Orthophosphoric acid as the solvent. The linearity in absorbance versus concentration was confirmed through linear regression analysis for each SOM in the UV region (200–400 nm). The corresponding regression equations were then utilized to estimate SOM content in ACP samples based on their absorbance values.
Results and discussion
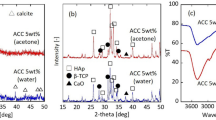
The lack of crystalline order was observed in Fig. 2A confirming X-Ray amorphous nature of synthesized ACP variants. The FTIR spectra in Fig. 2B confirms the presence of characteristic functional groups in synthesized ACP variants. Distinct vibrational bands corresponding to the phosphate groups were identified at v1 (~ 950 cm− 1), v2 (1000–1150 cm− 1), and v4 (500–620 cm− 1). Notably, the broad nature of the v4 band (500–620 cm− 1) is indicative to amorphous phase of ACP. The absence of peak splitting within the v4 region further supports the lack of crystalline phases in the synthesized samples15. The broad peak around 3000–3700 cm− 1 and 1680 − 1640 cm− 1 corresponds to stretching and bending vibration of water. In ACP_CIT the characteristic peak at 1600 cm− 1 and 1420 cm− 1 represents COO− bending and COH stretching of citrate’s carboxylic groups. Moreover, the peaks observed in ACP_ASC at 1498 cm− 1 (CH bending), 1412 cm− 1 (CH2 scissoring), 1322 cm− 1 (C–H bending), 871 cm− 1 (C–C ring stretching) and 785 cm− 1 (OH out-off plane scissoring). Lastly, the peaks in ACP_ITN 1516 and 1418 cm− 1 corresponds to symmetric and asymmetric vibration of C = O. The band observed around 870 cm− 1 and 840 cm− 1 represents the CH streaking vibration of itaconate14. Additionally, FTIR spectra of SOMs that is itaconic, citric and ascorbic acid (Figs. S1–S3) and XRD spectra of plain ACP is included in the supplementary information (Fig. S4).
UV Spectral Analysis of ACP and SOM-Containing ACP: The UV spectra of plain ACP dissolved in 1 M hydrochloric acid displayed a concentration dependent increase in absorbance in UV region from 200 to 215 nm with incidence of distinct and sharp peak. Such distinct peak was not observed in case of plain ACP samples dissolved in orthophosphoric acid (Fig. 3a and b). Moreover, when SOM containing ACP when dissolved and analyzed in orthophosphoric acid distinct peaks were recorded for each of the SOM containing ACP, ACP itaconate exhibited distinct concentration dependent absorption maxima at 200 nm, ACP citrate at 209 nm, and ACP ascorbic acid at 244 nm. This indicates that orthophosphoric acid did not interfere in the quantification of SOM contained in ACP. Additionally, SOM´s investigated are stable under laboratory conditions and 1 M orthophosphoric acid used for the evaluations. Ascorbic acid specially exhibits higher stability in acidic solvents under ambient/room temperature (where heat exposure is absent). Phosphoric acid and its combinations with EDTA/methanol are commonly used for analysis of samples containing ascorbic acid, itaconic acid, and citric acid16,17,18. Furthermore, UV analysis of prepared solutions was performed the same day reducing chances of degradation. Therefore, orthophosphoric acid was utilized for further analysis. Absorption maxima (λmax) for the respective SOM (i.e., ascorbic acid, citric acid and itaconic acid) was in agreement with the experimentally observed and existing reports19,20. Additionally, during the synthesis of ACP containing different SOMs, calcium salts such as calcium chloride and calcium citrate were used; however, as described in the section “Preparation of ACP and SOM-containing ACP,” the precipitate was isolated by centrifugation at 3000 rpm for 5 min and washed three times with Milli-Q® water, effectively removing residual calcium salts from the ACP-SOM product. This prevents any interference with the analysis of the incorporated SOMs. The synthesis of ACP, was performed at pH 11.5, facilitating its precipitation making this value appropriate. SOMs such as citric acid are deprotonated at this high pH of 11.5. This facilitates interaction of their carboxylic groups with calcium ions in the medium, aiding their incorporation into the ACP phase. Following centrifugation, washing the precipitate three times with deionized water removes residual alkalinity and any unreacted calcium salts. The obtained ACP or ACP-SOM is solid at near-neutral pH without traces of unreacted calcium salts. When dissolved in 1 M H2PO4 for UV analysis, ACP fully dissolves, and at acidic pH, citric acid becomes protonated, reducing its interaction with calcium ions and thereby supporting its accurate quantification.
For SOM -containing ACP, distinct absorption maxima were recorded for each small organic molecule. Itaconic acid displayed a peak at 200 nm, citric acid at 209 nm, and ascorbic acid at 244 nm. These characteristic peaks allowed for the quantification of SOM by comparing the absorbance of ACP samples with standard calibration curves for each molecule. The linearity of the regression was confirmed with R2 values ≥ 0.99 for all calibration curves, ensuring reliable quantification as depicted in Figs. 4, 5 and 6 and S5–S7 respectively (supplementary information).
Chemical and structural properties of SOM govern their association with the amorphous calcium phosphate (ACP) matrix. The results showed that citric acid content (16%) was highest in ACP, followed by ascorbic acid (1.23%) and itaconic acid (0.3%). These differences suggest that the way each molecule interacts with the ACP matrix affects how well it integrates during synthesis.
Citric acid’s high incorporation can be attributed to its molecular structure (represented in Fig. 7), which consists of three carboxyl groups and one hydroxyl group. At basic pH citric acid is present in tribasic form which supports its chelation activity21. These functional groups provide multiple binding sites for calcium ions, facilitating a stronger chelation effect and allowing citric acid to readily integrate into the ACP matrix. The presence of three carboxyl groups enhances its ability to interact with calcium, stabilizing the structure by acting as a chelating agent that binds to calcium, thereby promoting higher incorporation into the amorphous mineral phase22.
In contrast, ascorbic acid content was much lower in the ACP matrix, which can be explained by its different molecular structure. While ascorbic acid does possess hydroxyl groups, it features only one enediol group and lacks the number of carboxyl groups as compared to citric acid. The reduced number of potential binding sites limits its chelation capability with calcium ions, resulting in less effective integration into the amorphous structure23. The enediol configuration, although important for its antioxidant properties, does not enhance binding with calcium in the same manner as multiple carboxyl groups. Itaconic acid, which demonstrated the lowest content among the three, further illustrates how molecular structure affects the integration of SOM into ACP. Itaconic acid contains two carboxyl groups but lacks hydroxyl groups, which possibly limits its potential interaction with divalent metal ions24. This structural simplicity, with fewer functional groups limits its interplay with calcium ions in the matrix. Consequently, its affiliation was lower with ACP than as compared to citric and ascorbic acids.
The observed differences in SOM incorporation underscore the role of functional groups in determining the affinity of each molecule for the ACP matrix. Molecules with a higher number of functional groups, such as carboxyl and hydroxyl groups, exhibit a greater capacity to interact with the calcium ions present in ACP. The specific arrangement and combination of these functional groups further influence the molecule’s chelation potential and integration efficiency. Citric acid, with its optimal configuration of multiple carboxyl groups, demonstrates the highest affinity for binding with calcium, whereas ascorbic and itaconic acids, with fewer or less effective binding sites, show progressively lower levels of incorporation. Additionally, the reactivity of the different functional groups influences the interaction with the ACP matrix. Citrate by virtue of its three carboxylic groups possess strong binding efficiency to divalent metal ions like Ca+ 2. Therefore, citrate molecules are able to cover the surface area of ACP in ACP citrate, blocking all the nucleation sites and hindering its conversion to hydroxyapatite (HAp)25. Conversely, the lesser reactivity and fewer binding sites in ascorbic and itaconic acids contribute to their lower content onto the amorphous mineral.
In conclusion, the chemical structure and composition of SOM significantly impact their incorporation into amorphous calcium phosphate. The findings indicate that molecules with a higher number of functional groups and a greater ability to chelate calcium ions, such as citric acid, exhibit higher incorporation, while those with fewer binding sites, like ascorbic and itaconic acids, show limited integration. These results provide valuable insights into the factors that govern the incorporation of small organic molecules in biominerals, paving the way for designing more effective biomaterials for bone tissue engineering and other biomedical applications.
Conclusion
A UV spectroscopy-based approach was developed to facilitate the semi-quantitative analysis of small organic molecules in amorphous calcium phosphate. The method enabled the detection of itaconic, citric, and ascorbic acids in ACP, providing distinct UV absorption maxima. The differences in SOM incorporation in ACP were attributed to variations in SOM chemical structure, reactivity, and the number of functional groups. This study contributes to improving the detection and quantification of small organic molecules in calcium phosphate-based materials, addressing a significant gap in the standardization of SOM analysis. UV spectroscopy offers a viable approach for semi-quantitative analysis of SOM in ACP, and further studies with alternate analytical methods involving chromatography would pave way for the better understanding of the same.
Data availability
Data Availability Statement: All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Francis, A. P. et al. A review on biomaterials-based scaffold: an emerging tool for bone tissue engineering. Mater. Today Commun. 34, 105124 (2023).
Qu, H., Fu, H., Han, Z. & Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 9, 26252–26262 (2019).
Indurkar, A., Choudhary, R., Rubenis, K. & Locs, J. Role of carboxylic organic molecules in interfibrillar collagen mineralization. Front Bioeng. Biotechnol. 11, 1150037 (2023).
Pandit, A., Indurkar, A., Locs, J., Haugen, H. J. & Loca, D. Calcium phosphates: A key to Next-Generation in vitro bone modeling. Adv. Healthc. Mater. 13 2401307 (2024).
Ferraz, M. P. An overview on the big players in bone tissue engineering: Biomaterials, scaffolds and cells. Int. J. Mol. Sci. 25, 3836 (2024).
Fosca, M., Streza, A., Antoniac, I. V., Vadalà, G. & Rau, J. V. Ion-Doped calcium Phosphate-Based coatings with antibacterial properties. J. Funct. Biomater. 14, 250 (2023).
Sokolova, V. Frontispiece biological and medical applications of calcium phosphate nanoparticles. Chem. A Eur. J. (2021). https://doi.org/10.1002/chem.202182761
Kieler, M. et al. Itaconate is a metabolic regulator of bone formation in homeostasis and arthritis. Ann. Rheum. Dis. 83, 1465–1479 (2024).
Aghajanian, P., Hall, S., Wongworawat, M. D. & Mohan, S. The roles and mechanisms of actions of vitamin C in bone: new developments. J. Bone Min. Res. Off J. Am. Soc. Bone Min. Res. 30, 1945–1955 (2015).
Xu, H. et al. Citric acid: A Nexus between cellular mechanisms and biomaterial innovations. Adv. Mater. 36, 2402871 (2024).
Nomoto, T. et al. Calcium phosphate-based organic–inorganic hybrid nanocarriers with pH-responsive on/off switch for photodynamic therapy. Biomater. Sci. 4, 826–838 (2016).
Kamel, M. Y. & El-Awamry, Z. A. A colorimetric method for the determination of carboxylic acids. Microchem. J. 23, 445–452 (1978).
Xu, J., Chen, Z., Yu, J. C. & Tang, C. Simultaneous determination of inorganic anions, carboxylic and aromatic carboxylic acids by capillary zone electrophoresis with direct UV detection. J. Chromatogr. A. 942, 289–294 (2002).
Indurkar, A. et al. Small organic molecules containing amorphous calcium phosphate: Synthesis, characterization and transformation. Front Bioeng. Biotechnol. 11, 1329752 (2024).
Querido, W. et al. Fourier transform infrared spectroscopy of developing bone mineral: from amorphous precursor to mature crystal. Analyst 145, 764–776 (2020).
Nováková, L., Solich, P. & Solichová, D. HPLC methods for simultaneous determination of ascorbic and dehydroascorbic acids. TrAC Trends Anal. Chem. 27, 942–958 (2008).
Quantitative, H. P. L. C. Determination of itaconic acid residuals i | 219. https://www.tsijournals.com/abstract/quantitative-hplc-determination-of-itaconic-acid-residuals-in-polymer-hydrogels-219.html
Hotha, K. K., Patel, T., Roychowdhury, S. & Subramanian, V. Development of better-quality assay method for the citric acid and sodium citrate in ophthalmic/oral solutions and their application to deformulation studies. Am. J. Anal. Chem. 5, 1249–1260 (2014).
Krukowski, S., Karasiewicz, M. & Kolodziejski, W. Convenient UV-spectrophotometric determination of citrates in aqueous solutions with applications in the pharmaceutical analysis of oral electrolyte formulations. J. Food Drug Anal. 25, 717–722 (2017).
Gómez Ruiz, B., Roux, S., Courtois, F. & Bonazzi, C. Spectrophotometric method for fast quantification of ascorbic acid and dehydroascorbic acid in simple matrix for kinetics measurements. Food Chem. 211, 583–589 (2016).
Burel, C., Kala, A. & Purevdorj-Gage, L. Impact of pH on citric acid antimicrobial activity against gram-negative bacteria. Lett. Appl. Microbiol. 72, 332–340 (2021).
Ruiz-Agudo, E. et al. Citrate stabilizes hydroxylapatite precursors: Implications for bone mineralization. ACS Biomater. Sci. Eng. 7, 2346–2357 (2021).
Kontoghiorghes, G. J. et al. Trying to solve the puzzle of the interaction of ascorbic acid and iron: Redox, chelation and therapeutic implications. Medicines 7, 45 (2020).
Karaffa, L., Fekete, E. & Kubicek, C. P. The role of metal ions in fungal organic acid accumulation. Microorganisms 9, 1267 (2021).
Chen, Y., Gu, W., Pan, H., Jiang, S. & Tang, R. Stabilizing amorphous calcium phosphate phase by citrate adsorption. CrystEngComm 16, 1864–1867 (2014).
Acknowledgements
The authors acknowledge financial support from the European Union’s Horizon 2020 research and innovation program (H2020 Spreading Excellence and Widening Participation) under grant agreement No. 857287 (BBCE – Baltic Biomaterials Centre of Excellence).
Author information
Authors and Affiliations
Contributions
Authors Ashish Vishwas Pandit and Abhishek Rajesh Indurkar have contributed equally in conceptualizing and drafting the manuscript. Authors Dagnija Loca and Janis Locs have provided inputs for the improvement of manuscript and superivised the experimental work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pandit, A., Indurkar, A., Locs, J. et al. Semi-quantitative evaluation of small organic molecules content in amorphous calcium phosphate. Sci Rep 15, 24132 (2025). https://doi.org/10.1038/s41598-025-09232-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09232-3