Abstract
Cadmium (Cd(II)) ions are hazardous, non-biodegradable pollutants known for their bioaccumulative nature and severe risks to human health and aquatic ecosystems. This study presents the synthesis and application of a Co0.89Mg0.79Mn1.46O3.98@C nanocomposite, fabricated employing the Pechini sol–gel approach and subsequently calcinated at 600 oC (C600) and 800 oC (C800), for enhanced elimination of Cd(II) ions from aqueous environments. The C600 and C800 samples exhibited maximum uptake capacities of 280.11 mg/g and 206.19 mg/g, respectively. X-ray diffraction (XRD) analysis confirmed a cubic structure for both samples, with crystallite sizes of 51.66 nm (C600) and 87.09 nm (C800). Field emission scanning electron microscopy (FE-SEM) analysis clarified that C600 consisted of smaller, irregular grains, while C800 displayed larger, smoother particles. Elemental composition was verified by energy-dispersive X-ray spectroscopy (EDX), revealing elevated manganese and cobalt content in C800, attributed to enhanced incorporation at elevated temperatures. The superior adsorption performance of C600 was ascribed to its reduced crystallite size in addition to greatest surface area. Regeneration studies demonstrated excellent desorption efficiencies using 3 M HCl: 99.69% for C600 and 99.35% for C800, with both maintaining high removal efficiency over five adsorption–desorption cycles. Thermodynamic assessments indicated the process is exothermic, spontaneous, and dominated by physisorption. Kinetic modeling followed the pseudo-second-order model, while isotherm analysis fit the Langmuir-type isotherm, suggesting single-layer adsorption occurring uniformly across the adsorbent surface.
Similar content being viewed by others
Introduction
The presence of heavy metals in water sources poses a significant global environmental concern resulting from industrial activities, agricultural runoff, and improper waste disposal. Industries such as mining, electroplating, and battery manufacturing release toxic heavy metals into water systems, leading to widespread pollution1,2,3.
Heavy metals are hazardous due to their persistence in the environment and their ability to bioaccumulate in living organisms, posing serious risks to human health and ecosystems. Prolonged exposure to heavy metals can cause organ damage, neurological disorders, and developmental issues in humans, while aquatic life suffers from toxicity and habitat degradation4,5,6. Among heavy metals, cadmium is particularly dangerous due to its high toxicity even at low concentrations. Cadmium can infiltrate water bodies through industrial effluents, fertilizer runoff, and leaching from landfills. It accumulates in aquatic organisms and enters the food chain, threatening human health by causing kidney damage, bone disorders, and carcinogenic effects.
The removal of Cd(II) ions from contaminated water is, therefore, a critical environmental and public health challenge7,8. Various methods are employed for eliminating toxic metal ions from aqueous systems, comprising adsorption9,10chemical precipitation11 membrane filtration12 electrochemical techniques13 as well as bioremediation14. Among these, adsorption stands out for its efficiency, simplicity, and cost-effectiveness. Unlike other methods, adsorption does not generate harmful byproducts and can be applied at a wide range of concentrations. Additionally, it is adaptable to various adsorbents, making it a preferred choice for water treatment15.
Metal oxide nanoparticles have emerged as highly effective adsorbents for heavy metals due to their high surface area, tunable surface properties, and strong affinity for metal ions. These properties enable nanoparticles to achieve superior removal efficiencies compared to conventional materials16,17,18.
There are several methods available for the synthesis of metal oxide nanoparticles, each offering distinct advantages depending on the desired particle size, morphology, crystallinity, and application. Common techniques include hydrothermal synthesis19co-precipitation20 combustion21 sol-gel processes22 and microwave-assisted synthesis23. Among these, the Pechini sol-gel method has gained significant attention due to its simplicity, cost-effectiveness, and ability to produce highly homogeneous and nanoscale metal oxides with controlled stoichiometry24. This method involves the formation of a polymeric precursor through the complexation of metal ions with a chelating agent such as tartaric acid, followed by polyesterification with a polyhydroxy alcohol like polyethylene glycol. The Pechini method offers excellent control over the chemical uniformity at the molecular level, which is crucial for achieving consistent physicochemical properties in the final nanomaterial. Therefore, it is widely used for preparing advanced metal oxide nanostructures for applications in catalysis, sensors, energy storage, and environmental remediation.
Several adsorbent materials have been developed for the removal of Cd(II) ions from aqueous media, each demonstrating varying levels of adsorption capacity and functional characteristics. For instance, the aminopropyltriethoxysilane/zeolite W composite has shown a high adsorption capacity of 253.50 mg/g, attributed to the synergistic effect of surface functionalization and the porous structure of the zeolite25. Alginate/chitosan beads also exhibit notable performance, with a capacity of 207.00 mg/g, benefiting from the biocompatibility and abundant functional groups present in both polymers26. On the other hand, polyethyleneimine, despite being rich in amine functionalities, offers a relatively low adsorption capacity of 22.73 mg/g, likely due to limited surface area and weak interaction with Cd(II) ions27. Additionally, magnetic dithiocarbamate-functionalized reduced graphene oxide demonstrates an adsorption capacity of 116.28 mg/g, combining magnetic recovery with effective chelation28. Although these materials represent important advancements, there remains a pressing need for the development of novel adsorbents with higher efficiency, stability, environmental safety, and cost-effectiveness.
The Co-Mg-Mn oxide system was selected in this work based on the individual adsorption potential of each metal oxide29,30,31,32,33,34. Cobalt oxide offers redox activity and surface reactivity, magnesium oxide contributes high surface area and stability, and manganese oxide introduces variable oxidation states for enhanced interaction with pollutants. These oxides have been successfully applied in previous adsorption studies, and their combination in a stable spinel structure is expected to provide improved performance for Cd(II) removal.
New materials should aim to overcome the limitations of existing systems and provide scalable solutions for the sustainable removal of cadmium and other toxic heavy metals from contaminated water. Hence, in this study, a novel ternary metal oxide nanocomposite, Co0.89Mg0.79Mn1.46O3.98@C, was successfully synthesized using the Pechini sol–gel method for the efficient removal of cadmium ions from aqueous media. This synthesis route offers precise control over the composition, crystallinity, and particle morphology of the resulting material. The combination of cobalt, magnesium, and manganese within a single oxide framework provides a unique structure with enhanced surface functionality and adsorption potential. Additionally, the incorporation of carbon plays a crucial role in improving the surface area, porosity, and dispersion of the metal oxides, which collectively enhance the accessibility of active sites and facilitate more efficient interaction with cadmium ions. Unlike conventional adsorbents, the thermally tailored Co0.89Mg0.79Mn1.46O3.98@C nanocomposite exhibits superior performance in removing Cd(II) ions from aqueous media, outperforming many previously reported materials in the literature. Furthermore, the nanocomposite demonstrates excellent regeneration ability and retains its efficiency over multiple adsorption–desorption cycles. These findings represent a significant advancement in water treatment technologies and highlight the potential of this material as a sustainable and highly effective solution for heavy metal remediation.
Experimental
Chemicals
The materials employed in this work were of analytical-grade quality and sourced from Sigma-Aldrich. All chemicals were used as received without further purification. Magnesium nitrate hexahydrate (Mg(NO₃)₂·6 H₂O, ≥ 98%), manganese acetate tetrahydrate (Mn(CH₃COO)₂·4 H₂O, ≥ 99%), and cobalt acetate tetrahydrate (Co(CH₃COO)₂·4 H₂O, ≥ 98%) were used as metal precursors. Polyethylene glycol 400 (PEG 400, ≥ 99%) was employed as a crosslinker. Sodium hydroxide (NaOH, ≥ 98%) was used for pH adjustment, while cadmium nitrate tetrahydrate (Cd(NO₃)₂·4 H₂O, ≥ 99%) served as the source of cadmium ions. Hydrochloric acid (HCl, 37%, ACS reagent grade) was used in desorption studies and for pH adjustment, and potassium chloride (KCl, ≥ 99%) was utilized to determine the point of zero charge (pHPZC).
Synthesis of Co0.89Mg0.79Mn1.46O3.98@C nanocomposite
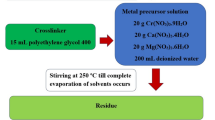
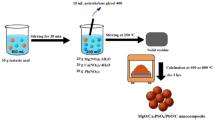
The synthesis of Co0.89Mg0.79Mn1.46O3.98@C was conducted utilizing the Pechini-type sol–gel processing technique chemical approach, as illustrated in Fig. 1. Primarily, an aqueous solution containing tartaric acid (37 g in 100 mL deionized water) was prepared, where tartaric acid functioned as the chelating agent. In addition, 20 g each of Mg(NO3)2.6H2O, Mn(CH3COO)2.4H2O, and Co(CH3COO)2.4H2O were fully solubilized in 250 mL of deionized water. Besides, the solution of tartaric acid was dropwise incorporated into the metal salt solution under persistent stirring for a duration of 1 h. Thereafter, 20 mL of polyethylene glycol 400 (as a crosslinker) was introduced into the subsequent blend, which was then heated at 250 °C under continuous stirring until the solvents evaporated completely. The resulting powder underwent calcination at 600 and 800 °C for 3 h, resulting in the formation of C600 and C800 nanoparticles, respectively.
The temperatures of 600 and 800 °C were selected to compare the effect of moderate versus higher calcination on structure and adsorption behavior. A calcination temperature of 600 °C was chosen as the lower boundary because it is sufficient for forming the spinel structure. Lower temperatures result in incomplete crystallization and the production of amorphous phases.
Instrumentation
The characterization and analysis in this study were performed using advanced instrumentation. The crystalline structure of all synthesized products was analyzed utilizing an X-ray diffraction diffractometer (PANalytical, X’Pert PRO, Netherlands). The structural features and compositional analysis of the products were analyzed with a field emission scanning electron microscope integrated with energy-dispersive X-ray spectroscopy (EDS/FE-SEM) (JSM-IT800 Schottky, JEOL Ltd., Tokyo, Japan). Besides, atomic absorption spectroscopy (Palo Alto, VARIAN®, SpectrAA 20 Plus spectrometer, California, USA) was employed for evaluating cadmium ion concentration at a wavelength of 228.8 nm.
Adsorption of Cd(II) ions from aqueous solutions
The batch adsorption method was utilized for Cd(II) ion removal from aqueous media, with experimental parameters provided in Table 1. For each experiment, the adsorbent was incorporated into the prepared Cd(II) solution and continuously stirred employing a magnetic stirrer to facilitate homogeneous blending. The contact time, pH, temperature, and adsorbent dose were varied according to the experimental conditions specified in Table 1.
Upon completion of each experimental run, the adsorbent was separated using a centrifuge. The filtrate was analyzed for Cd(II) ion concentration at a wavelength of 228.8 nm using an atomic absorption spectrophotometer. The values of %R (removal efficiency) and Q (adsorption capacity) were computed based on Eqs. 1 and 2, respectively35,36.
In these equations, Co (mg/L) represents the initial concentration of Cd(II) ions, Ce (mg/L) is the residual concentration of Cd(II) ions, V (L) denotes the volume of the solution, while W (g) represents the mass of the adsorbent applied.
Kinetic models
To evaluate the rate and mechanism of Cd(II) ion adsorption, the pseudo-first-order and pseudo-second-order kinetic models were applied. These models provide insight into the interaction dynamics between adsorbate and adsorbent. The pseudo-first-order kinetic model assumes that the rate of occupation of adsorption sites is directly proportional to the number of unoccupied sites. The model is expressed by Eq. 328,37.
where, Qt (mg/g) is the amount of Cd(II) ions adsorbed at time t (min), Qe (mg/g) is the adsorption capacity at equilibrium, and K1 (1/min) is the rate constant.
The pseudo-second-order kinetic model, on the other hand, assumes that chemisorption is the rate-limiting step involving valence forces through sharing or exchange of electrons. It is represented by Eq. 428,37.
where, K2 (g/mg.min) is the pseudo-second-order rate constant.
To further investigate the adsorption mechanism, the Weber–Morris intra-particle diffusion model was applied using the Eq. 538.
where, KI (mg/(g. min0.5)) is the intra-particle diffusion rate constant, and C (mg/g) is the intercept related to the boundary layer thickness.
Isotherm models
To describe the equilibrium behavior of Cd(II) ion adsorption onto the nanocomposites, the Langmuir and Freundlich isotherm models were utilized. The Langmuir model assumes monolayer adsorption onto a homogeneous surface with a finite number of identical sites. It is expressed by Eq. 639,40.
where, Qmax (mg/g) is the maximum adsorption capacity, and K3 (L/mg) is the Langmuir constant.
The Freundlich model assumes heterogeneous surface energies, where the binding sites are not equivalent. It is given by Eq. 739,40. Also, Qmax was calculated from it using Eq. 839,40.
where, K4 (mg/g)(L/mg)1/n) is the Freundlich constant related to adsorption capacity, and n is a dimensionless heterogeneity factor indicating adsorption intensity.
Desorption and reusability studies
The desorption of cadmium ions from the adsorbent was investigated utilizing hydrochloric acid (HCl) as the recovery solution. Regeneration experiments were performed by treating the Cd(II)-laden adsorbent with 50 mL of HCl at concentrations of 1, 2, and 3 M. Cd(II) ion desorption (% D) was determined based on Eq. 941.
In the aforementioned equation, Cd (mg/L) represents the concentration of Cd(II) ions in the recovery solution while Vd (L) refers to the volume of the recovery solution.
Five consecutive adsorption/desorption cycles were conducted to assess the adsorbent’s regeneration and reuse potential for Cd(II) ion removal. Batch experiments were conducted using 150 mg/L Cd(II) ion solutions with a total volume of 100 mL. In addition, the adsorbent dose was 50 mg, and the disposal experiments were implemented at an initial temperature of 298 K and an initial pH of 7.5. The contact times were 50 min for C600 and 70 min for C800. These specific conditions were selected based on the results of preliminary optimization studies to ensure maximum adsorption performance. Maintaining a constant dose, temperature, and pH enabled accurate comparison of the two nanocomposites under controlled conditions, while the individual contact times reflect the equilibrium durations identified for each sample, ensuring that the adsorption capacities reported are at their optimum.
Point of zero charge (pHPZC) of Co0.89Mg0.79Mn1.46O3.98@C nanocomposites
The point of zero charge (pHPZC) of Co0.89Mg0.79Mn1.46O3.98@C products was established using a controlled batch adsorption technique in which KCl functioned as the stabilizing electrolyte41. Different 50 mL solutions containing KCl (0.1 M) were prepared with initial pH values (pHI) set to a range between 2 and 12 using 0.1 M HCl and/or NaOH. A predetermined quantity of the nanoparticles (0.05 g) was added to each solution, and the mixture were magnetically agitated at ambient temperature for 20 h for facilitating equilibrium establishment. After equilibration, the final pH (pHf) was measured. The difference in pH (△pH) was calculated for each solution. The pHPZC was assessed by graphing △pH versus pHI, where the pHPZC corresponds to the point where △pH = 0.
Results and discussion
Characterization
The X-ray diffraction (XRD) patterns of the C600 and C800 samples are displayed in Fig. 2, highlighting their crystalline structures. Characteristic peaks were detected at diffraction angles (2Ɵ) of 18.46, 30.53, 35.88, 37.55, 43.65, 54.03, 57.82, 63.38, 66.52, 71.88, 74.91, and 75.97°, which correspond to the Miller indices (111), (220), (311), (222), (400), (422), (511), (440), (531), (620), (533), and (622), respectively. The XRD analysis showed that these peaks align with the standard JCPDS No. 01-084-8309, confirming the formation of cobalt magnesium manganese oxide (Co0.89Mg0.79Mn1.46O3.98) with a cubic spinel crystal system. The mean crystal sizes of the C600 and C800 products are 51.66 nm and 87.09 nm, respectively. The rise in mean crystal size with higher calcination temperature can be attributed to enhanced thermal energy at elevated temperatures, which promotes particle growth. This growth leads to larger crystalline structures in the C800 sample compared to the C600 sample.
The EDX spectra of the C600 and C800 samples, shown in Fig. 3, provide insights into their elemental compositions. Table 2 summarizes the atomic percentages of carbon, oxygen, magnesium, manganese, and cobalt in the samples. For the C600 sample, the atomic percentages are 3.1% for carbon, 43.2% for oxygen, 13.7% for magnesium, 22.7% for manganese, and 17.3% for cobalt. In comparison, the C800 sample contains 2.5% carbon, 38.3% oxygen, 12.6% magnesium, 27.0% manganese, and 19.6% cobalt. The observed changes in the elemental composition between the two samples indicate a decrease in carbon, oxygen, and magnesium content, accompanied by an increase in manganese and cobalt percentages as the calcination temperature rises. These changes can be attributed to the enhanced thermal energy during calcination at 800 oC, which promotes the decomposition of residual organic matter (reducing carbon) and the reduction of lattice oxygen. Additionally, the higher temperature facilitates the incorporation of manganese and cobalt into the crystal lattice, improving their distribution and possibly forming more stoichiometric and thermodynamically stable phases. This shift in elemental distribution reflects the structural evolution and compositional refinement of the samples with increasing calcination temperature.
With increasing calcination temperature, slight changes in the relative elemental composition were observed. These may be due to surface migration or incorporation of metal ions into the crystalline lattice. A reduction in surface Mg or Mn content at higher temperature may lower the density of surface-active sites, such as hydroxyl or oxygenated groups, affecting the adsorption of Cd(II) ions. This could partly explain the expected lower adsorption capacity of C800 compared to C600.
Figure 4 presents the surface morphology observed via FE-SEM for the C600 (Fig. 4A) and C800 (Fig. 4B) samples, revealing their distinct morphological characteristics. The C600 sample exhibits irregular polygonal shapes with some clusters of smaller grains distributed throughout the structure, while the C800 sample shows predominantly rounded and oval-shaped grains with a smoother appearance. The analysis indicates that the mean grain size for the C600 and C800 nanostructures is 380.59 nm and 503.42 nm, respectively, demonstrating an improvement in grain size with elevated calcination temperatures. These observations suggest that the thermal treatment plays a critical role in shaping the microstructural features of the materials.
Also, the FE-SEM images revealed that the C600 sample exhibited a more porous and loosely packed structure with fine particles, which contributes to a higher surface area and greater exposure of active adsorption sites. In contrast, the C800 sample showed more compact, aggregated particles with smoother surfaces, reducing accessibility to internal pores. This morphological difference explains the expected superior adsorption performance of C600, as its microstructure facilitates better diffusion and interaction of Cd(II) ions with available sites.
Figures 5 and 6 provide a detailed HR-TEM and particle size distribution analysis of the synthesized C600 and C800 samples. In Fig. 5A, the HR-TEM image of the C600 product shows clusters of nearly spherical and slightly ellipsoidal nanoparticles with partial agglomeration and relatively uniform contrast, indicating a moderately narrow particle size distribution. In contrast, Fig. 5B illustrates the HR-TEM image of the C800 sample, where the particles appear larger and exhibit more pronounced faceted morphologies, including polygonal and irregular granular shapes with a more distinct crystallographic outline. These morphological differences suggest that the increase in calcination temperature influences particle coarsening and crystallinity. Figure 6 displays the particle size distribution histograms corresponding to the HR-TEM observations of the C600 and C800 samples. Also, Fig. 6A shows the histogram for C600, where the particle sizes range from approximately 20 to 90 nm, with the majority of particles concentrated between 40 and 60 nm, resulting in a mean particle size of 48.89 nm. Besides, Fig. 6B presents the histogram for the C800 sample with a broader distribution spanning from about 30 to 90 nm and a higher frequency of particles in the 60 to 80 nm range, leading to a mean particle size of 59.97 nm. The broader and right-shifted distribution in the C800 sample confirms the growth of larger particles at higher temperatures due to thermally induced coalescence and enhanced atomic mobility during calcination at 800 °C. The observed increase in mean particle size from 48.89 nm in the C600 sample to 59.97 nm in the C800 sample can be attributed to the thermally driven grain growth mechanism, where elevated temperatures promote the diffusion of atoms and the fusion of adjacent nanocrystallites, resulting in the formation of larger and more defined particles. These findings confirm that thermal treatment parameters critically influence both the morphology and particle size distribution of the resulting nanostructures.
The FTIR spectra presented in Fig. 7 illustrate the characteristic vibrational bands of the C600 and C800 nanostructures, confirming the formation of metal oxide frameworks and the presence of residual surface functionalities. Both spectra exhibit broad absorption bands around 3400 cm⁻¹ for C600 and 3419 cm⁻¹ for C800, which correspond to the O–H stretching vibrations of surface hydroxyl groups or adsorbed water molecules, indicating the hydrophilic nature of the nanocomposites42. The absorption bands located at 1606 cm⁻¹ for C600 and 1615 cm⁻¹ for C800 are attributed to the bending vibrations of H–O–H from molecular water retained on the material surface. Additionally, the bands observed at 1398 cm⁻¹ for C600 and 1407 cm⁻¹ for C800 may be associated with residual carbon species from precursors used during synthesis. Strong absorption bands appearing at 549 cm⁻¹ in C600 and 553 cm⁻¹ in C800 correspond to M–O stretching vibrations, where M represents Co, Mg, or Mn ions within the spinel lattice, confirming the successful formation of the mixed metal oxide phase. Furthermore, the peaks observed at 463 cm⁻¹ for C600 and 468 cm⁻¹ for C800 also arise from metal–oxygen lattice bending vibrations, supporting the presence of well-structured crystalline oxide networks in both samples43.
Figure 8 displays the nitrogen adsorption–desorption isotherms of the C600 and C800 nanocomposites, where both samples exhibit type IV isotherms with clear H3-type hysteresis loops, characteristic of mesoporous materials42. According to Table 3, the C600 sample demonstrates a significantly higher BET surface area of 65.58 m²/g and a larger total pore volume of 0.0976 cm³/g compared to the C800 sample, which shows a surface area of 19.56 m²/g and a total pore volume of 0.0278 cm³/g. The reduction in surface area and pore volume for the C800 product is likely due to the sintering or partial collapse of the porous structure caused by calcination at the higher temperature of 800 °C, which leads to grain growth and a denser material morphology. Additionally, the average pore diameters of 3.20 nm for C600 and 2.58 nm for C800 indicate that both materials fall within the mesoporous range, defined by pore diameters between 2 and 50 nm, though the slight reduction in mean pore size for C800 may also reflect structural densification during thermal treatment. These observations confirm that thermal processing conditions strongly influence the surface textural properties of the synthesized nanocomposites, impacting their adsorption capacity and potential performance in applications such as catalysis or pollutant removal.
Adsorption of Cd(II) ions from aqueous solutions
Influence of pH
The data illustrates the pH-dependent elimination efficiency of Cd(II) ions for the C600 and C800 samples and provides insights into their surface charge behavior. Figure 9 shows that the removal efficiency (% R) of Cd(II) ions increases with rising pH for both samples. At pH 2.5, the removal efficiency for C600 is 1.95%, slightly higher than the 1.77% observed for C800. Upon increasing the pH to 3.5, the remediation efficiency of C600 rises significantly to 15.23%, while C800 shows a lower efficiency of 7.79%. At pH 4.5, C600 achieves 34.48% removal, almost double the 19.57% recorded for C800. The trend continues, with C600 consistently outperforming C800 as pH increases. At pH 5.5, the removal efficiency of C600 reaches 57.64%, compared to 39.49% for C800. At pH 6.5, C600 achieves 76.38%, significantly surpassing the 48.85% observed for C800. At pH 7.5, C600 displays a near-complete elimination efficiency of 90.49%, while C800 achieves 66.51%. It is important to note that pH values above 7.5 were not investigated, as cadmium ions are known to precipitate as Cd(OH)2 under alkaline conditions. This precipitation can obscure the adsorption mechanism and lead to misleading conclusions about removal efficiency. Accordingly, the pH range was restricted to 2.5–7.5 to ensure that the observed removal was solely due to adsorption rather than chemical precipitation.
Adsorption mechanism
Figure 10 provides the point of zero charge (pHPZC) for both samples by analyzing the relationship between △pH and initial pH (pHI). The pHPZC for C600 is observed at approximately pH 5.9, while C800 shows a slightly higher pHPZC at pH 6.3. Under the corresponding pHPZC values, the respective surfaces of C600 and C800 are positively charged, resulting in diminished electrostatic interactions with electropositive Cd(II) ions and consequently lower removal efficiencies as shown in Fig. 117,45. Above their respective pHPZC, the removal efficiency for both C600 and C800 increases due to the development of a negative surface charge, which enhances the electrostatic interaction with the positively charged Cd(II) ions, as represented in Fig. 117,45. The smaller crystalline size of C600 provides a higher surface area and more active sites, enabling stronger negative surface charge above its pHPZC and promoting electrostatic interaction with Cd(II) ions. Similar findings have been reported in the literature, where materials with smaller crystallite sizes exhibited superior adsorption performance due to their higher accessible surface areas and reactivity7,44,45. In contrast, the larger crystalline size and higher pHPZC of C800 result in reduced surface area and weaker electrostatic interactions, leading to less efficient removal of Cd(II) ions under similar conditions compared with C6007,45,46.
The FTIR spectrum of C600, as illustrative example, before Cd(II) adsorption (Fig. 7A) shows bands at 549 cm⁻¹ and 463 cm⁻¹, which are assigned to M–O stretching and bending vibrations, respectively, where M = Co, Mg, or Mn. The band at 1398 cm⁻¹ corresponds to the C = O stretching of surface carboxylic groups. The band at 1606 cm⁻¹ is due to H–O–H bending of adsorbed water. The broad band near 3400 cm⁻¹ is attributed to O–H stretching vibrations of surface hydroxyl groups. After Cd(II) adsorption (Fig. 12), the band at 1398 cm⁻¹ shifted slightly to around 1385 cm⁻¹, and the band at 3400 cm⁻¹ shifted to approximately 3380 cm⁻¹ with reduced intensity. These changes suggest that carboxyl and hydroxyl groups were involved in the interaction with Cd(II) ions, likely through surface complexation. The band at 1606 cm⁻¹ remained almost unchanged, indicating that molecular water was not involved in the adsorption. No significant change was observed for the bands at 549 and 463 cm⁻¹, confirming that the structure remained stable42,43.
Influence of contact time
Figure 13 illustrates the time-dependent removal efficacy of Cd(II) ions for C600 and C800 products. At a contact time of 10 min, the remediation efficiency for C600 is 69.92%, considerably greater than the 33.25% observed for C800, indicating a faster adsorption rate for C600. At 50 min, C600 reaches its equilibrium removal efficiency of 89.75%, showing a plateau region where no additional increase in adsorption happens. In contrast, C800 demonstrates slower adsorption kinetics, achieving a removal efficiency of 54.54% at 50 min and reaching its equilibrium efficiency of 65.49% at 70 min. The difference in equilibrium times and removal efficiencies highlights the superior performance of C600, attributed to its smaller crystalline size and higher surface area, which provide more active sites for Cd(II) ion adsorption compared to C800. After reaching equilibrium durations of both samples, no substantial changes in disposal efficacy are observed due to the full occupation of the adsorbent surfaces.
In the case of C800, a slight irregular increase in adsorption percentage was observed between 60 and 70 min. This behavior may be attributed to localized restructuring of surface-active sites or delayed accessibility of internal pores due to the more compact microstructure of C800. Additionally, small measurement fluctuations near the equilibrium point, when the system becomes less dynamic, can occasionally cause minor deviations in the adsorption curve. Such behavior has been observed in similar oxide-based systems and does not affect the overall trend or equilibrium value46.
Figure 14 shows the kinetic modeling results, where Fig. 14A represents the pseudo-first-order rate model while Fig. 14B illuminates the pseudo-second-order rate model. Besides, the data from Table 4 demonstrate that the experimental uptake capacity (QExp) for C600 (269.24 mg/g) and C800 (196.48 mg/g) closely aligns with the computed Qe values generated from the pseudo-second-order model (273.22 mg/g and 194.55 mg/g, respectively). Additionally, the pseudo-second-order rate model displays higher correlation coefficients (R2) for both C600 (0.9997) and C800 (0.9999), compared to the pseudo-first-order model. These findings confirm that the removal process obeys the pseudo-second-order kinetic model, suggesting that the removal is primarily governed by the availability of uptake sites and their interaction with Cd(II) ions.
As shown in Fig. 15, the plots for both C600 and C800 exhibit good linearity, which suggests that intra-particle diffusion contributes to the overall adsorption process. However, the lines do not pass through the origin, as confirmed by the non-zero intercept values listed in Table 4. This indicates that intra-particle diffusion is not the sole rate-limiting mechanism; rather, other processes such as boundary layer film diffusion are also involved47. Additionally, the higher intercept observed for C600 implies a stronger influence of external mass transfer resistance compared to C800.
Influence of solution temperature
Figure 16 clarifies the impact of solution temperature on the remediation efficacy of Cd(II) ions utilizing C600 and C800 products. At a temperature of 298 K, C600 achieves a remediation efficiency of 89.75%, considerably greater than the 65.49% recorded for C800, reflecting the remarkable remediation accomplishment of C600 at diminished temperatures. Upon increasing the initial temperature to 328 K, the disposal efficacy declines for both products, with C600 showing a reduced efficiency of 76.13% and C800 decreasing to 52.21%. This decline in performance with increasing temperature suggests an exothermic adsorption process, where lower temperatures favor the disposal of Cd(II) ions. The consistently higher disposal efficacy of C600 compared to C800 at both temperatures highlights its greater adsorption capacity, stemming from its larger available surface area and smaller crystalline size, providing more active centers for ion remediation.
The thermodynamic constants for the remediation of cadmium ions utilizing C600 and C800 products were investigated using Eqs. 10, 11, and 1249.
In these equations, Kd refers to the distribution coefficient, Qe (mg/g) represents the equilibrium adsorption capacity, Ce (mg/L) is the equilibrium concentration, ΔS° (kJ/mol·K) refers to the entropy change, ΔH° (kJ/mol) is defined as the enthalpy change, ΔG° (kJ/mol) refers to the Gibbs free energy change, R refers to the universal gas constant (8.314 J/mol·K), and T refers to the temperature in Kelvin.
Figure 17 presents the plot of Van’t Hoff, which depicts the interdependence between 1/T and lnKd, while thermodynamic parameters are outlined in Table 5 for both products. The negative values of ΔH° (− 28.15 kJ/mol in the case of C600 and − 14.97 kJ/mol in the case of C800) indicate that the remediation process is exothermic. Since ΔH°<40 kJ/mol, the adsorption is primarily physical in nature. The negative ΔG° values across all temperatures demonstrate that the remediation process is spontaneous, while the positive ΔSo values (0.0705 kJ/mol.K in the case of C600 and 0.0388 kJ/mol.K in the case of C800) indicate that the remediation method is thermodynamically achievable.
Influence of initial concentration
Figure 18 clarifies the impact of the primary concentration of Cd(II) ions on the remediation efficiency of the C600 and C800 products. At an initial concentration of 50 mg/L, the disposal efficiency for C600 is 95.16%, which is higher than the 87.68% observed for C800. Upon increasing the initial concentration to 100 mg/L, the remediation efficacy is marginally reduced to 91.35% for C600 and 84.66% for C800, showing a consistent trend. At 150 mg/L, C600 maintains a higher disposal efficiency of 89.75% compared to 65.49% for C800. With further increases in concentration to 200 mg/L, the disposal efficacy decreases significantly to 67.88% for C600 and 49.32% for C800. At 250 mg/L, C600 achieves 54.64%, while C800 achieves only 39.74%. At the uppermost concentration level of 300 mg/L, the disposal efficacy drops to 45.68% for C600 and 33.46% for C800. These results indicate that both samples exhibit higher removal efficiencies at lower initial concentrations due to the abundance of reactive sites, but the efficiency decreases with increasing concentration as the adsorbent surfaces become saturated. Throughout the tested concentrations, C600 consistently outperforms C800, demonstrating its superior adsorption capacity.
Figure 19 illustrates the Langmuir isotherm plot (Fig. 19A) and the Freundlich isotherm plot (Fig. 19B) for the remediation of Cd(II) ions utilizing C600 and C800 nanostructures. Table 6 summarizes the adsorption parameters based on both models. The Langmuir model demonstrates a better fit for both samples, as indicated by the higher R2 values (0.9993 for C600 and 0.9991 for C800) compared to the Freundlich model. This suggests that the adsorption process follows the Langmuir isotherm, implying monolayer adsorption on homogeneous surface sites. The higher Qmax value for C600 compared to C800 reflects its superior adsorption capacity.
Table 7 describes a comparison of the greatest uptake capacities (Qmax) of many adsorbents for the remediation of Cd(II) ions25,26,27,28,37,49,50,51. Among the listed adsorbents, C600 exhibits the highest Qmax of 280.11 mg/g, exceeding all other materials, including the aminopropyltriethoxysilane/zeolite W composite (253.50 mg/g) and Fe3O4/orange peel-chitosan composite (251.88 mg/g). C800 also demonstrates a notable performance, with a Qmax of 206.19 mg/g, exceeding that of alginate/chitosan beads (207.00 mg/g) and the NiO-CuO/activated carbon nanocomposite (179.92 mg/g). The remarkable effectiveness of C600 and C800 may be ascribed to their optimized surface properties, because of its large specific surface area in addition to abundance of active binding positions, which enhance their interaction with Cd(II) ions. C600, in particular, benefits from its smaller crystalline size and higher adsorption capacity, making it more effective than other materials. These results highlight the potential of C600 and C800 as highly efficient adsorbents for removal of Cd(II) ions compared to conventional materials listed in Table 6.
Regeneration and reusability of nanostructures
Figure 20 illuminates the desorption efficacy of Cd(II) ions from C600 and C800 samples across varying concentrations of HCl. At 1 mol/L HCl, the desorption efficiency for C600 is 85.47%, slightly higher than the 79.06% observed for C800, indicating effective ion release even at minimal acid concentrations. As the concentration of HCl rises to 2 mol/L, the desorption efficacy improves considerably, reaching 93.05% for C600 and 91.03% for C800, highlighting the enhanced ability of the acidic medium to break bonds between Cd(II) ions and the adsorbent surfaces. At 3 mol/L HCl, nearly complete desorption is achieved, with efficiencies of 99.69% for C600 and 99.35% for C800. The remarkable desorption efficiency of HCl stems from its strong acidic nature, which provides an abundance of protons to effectively replace Cd(II) ions on the adsorbent surface through competitive adsorption, thus facilitating their release into the solution. This efficient desorption underscores the suitability of HCl as a regenerating agent for adsorbent reuse.
Although HCl was used for regeneration, the desorbed Cd(II) ions were concentrated into a small volume (50 mL), which can be safely collected and treated using standard hazardous waste protocols. This strategy avoids direct release into the environment and allows for potential recovery or secure disposal. Such regeneration methods are commonly employed to balance material reuse, cost-efficiency, and environmental safety.
Figure 21 illustrates the reusability performance of C600 and C800 samples for the remediation of cadmium ions over five adsorption-desorption cycles. In the primary cycle, the remediation efficiency for C600 and C800 is 89.75% and 65.49%, respectively, demonstrating the superior adsorption capacity of C600. After the first cycle, the removal efficacy marginally decreases to 89.35% for C600 and 65.12% for C800, indicating minimal loss in performance. By the third cycle, C600 maintains a high removal efficiency of 88.01%, while C800 shows a moderate decrease to 63.03%. At the fifth cycle, the removal efficiency for C600 and C800 drops to 85.05% and 59.65%, respectively. The results highlight the better reusability and stability of C600 compared to C800, as C600 exhibits a slower decline in removal efficiency over multiple cycles, making it more effective for repeated applications in the disposal of Cd(II) ions.
Conclusions
This study demonstrated the successful synthesis of Co0.89Mg0.79Mn1.46O3.98@C nanocomposites via the Pechini sol-gel approach, followed by calcination at 600 °C (C600) and 800 °C (C800). The XRD analysis confirmed a cubic spinel crystal structure with an increase in crystallite size from 51.66 nm for C600 to 87.09 nm for C800, highlighting the impact of calcination temperature on structural properties. SEM and EDX analyses revealed smaller and irregular grains with a higher surface area for C600 compared to larger and smoother grains for C800, alongside compositional variations due to thermal incorporation of manganese and cobalt. Batch adsorption experiments revealed maximum uptake capacities of 280.11 mg/g for C600 and 206.19 mg/g for C800, with C600 consistently exhibiting superior performance as a result of its larger surface area and reduced crystallite size. Kinetic studies indicated that the adsorption process follows the pseudo-second-order model, while isotherm analysis showed alignment with the Langmuir model, confirming monolayer adsorption on homogeneous surfaces. Thermodynamic evaluations revealed that the adsorption is exothermic, spontaneous, and principally physical. Regeneration experiments using HCl as the eluting agent achieved desorption efficiencies of up to 99.69% for C600 and 99.35% for C800 using 3 M HCl, emphasizing the efficiency of HCl in regenerating the adsorbent. Both samples retained significant adsorption capacity over five cycles.
Data availability
The datasets generated and/or analysed during the current study are available in the [Data-of-C600-and-C800-nanocomposites] repository, [https://github.com/Ehab1986539/Data-of-C600-and-C800-nanocomposites.git].
References
Aziz, K. H. H., Mustafa, F. S., Hamarawf, R. F. & Omer, K. M. Adsorptive removal of toxic heavy metals from aquatic environment by metal organic framework (MOF): A review. J. Water Process. Eng. 70, 106867 (2025).
Baldev, Kumar, G., Sharma, V. & Nemiwal, M. Biomass-derived zirconium composite: an adsorbent for Preferential removal of heavy metals and contaminants in wastewater. J. Water Process. Eng. 69, 106778 (2025).
Ávila, F. G. et al. Removal of heavy metals in industrial wastewater using adsorption technology: Efficiency and influencing factors. Clean. Eng. Technol. 24, 100879 (2025).
Rana, P. et al. Recent advancements in polythiophene-based adsorbents for heavy metal ion removal: Modification, kinetics and mechanistic insights. Inorg. Chem. Commun. 172, 113657 (2025).
Yang, T. et al. Insights and perspectives of chitosan-based hydrogels for the removal of heavy metals and dyes from wastewater. Int. J. Biol. Macromol. 292, 139280 (2025).
Oladimeji, T. E. et al. Review on the impact of heavy metals from industrial wastewater effluent and removal technologies. Heliyon 10, e40370 (2024).
Singh, D. et al. Citric acid coated magnetic nanoparticles: Synthesis, characterization and application in removal of Cd(II) ions from aqueous solution. J. Water Process. Eng. 4, 233–241 (2014).
Hu, L. et al. Synthesis of ZIF-8 in high yield and simultaneous removal of Mn(II), Cu(II), and Cd(II): Performance and mechanism. Chem. Eng. Res. Des. 208, 217–226 (2024).
Jing, W. et al. MnFe2O4-loaded bamboo pulp carbon-based aerogel composite: Synthesis, characterization and adsorption behavior study for heavy metals removal. RSC Adv. 14, 39995–40005 (2024).
Jiménez, A., Trujillano, R., Rives, V., Vicente, M. A. & Gil, A. Removal of heavy metals from aqueous solutions by adsorption on zeolites synthesized from aluminum saline slags. Inorg. Chem. Commun. 170, 113440 (2024).
Chen, Q. et al. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process. Eng. 26, 289–300 (2018).
Xiang, H., Min, X., Tang, C. J., Sillanpää, M. & Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process. Eng. 49, 103023 (2022).
Wu, L., Garg, S. & Waite, T. D. Progress and challenges in the use of electrochemical oxidation and reduction processes for heavy metals removal and recovery from wastewaters. J. Hazard. Mater. 479, 135581 (2024).
Zhang, L. et al. Calcium self-release bioremediation system combined with microbially induced calcium precipitation for the removal of ammonium nitrogen, phosphorus and heavy metals. J. Environ. Chem. Eng. 12, 114190 (2024).
Rostami, M. S. & Khodaei, M. M. Recent advances in chitosan-based nanocomposites for adsorption and removal of heavy metal ions. Int. J. Biol. Macromol. 270, 132386 (2024).
Precious, N., Patience, T., Kweinor, E. & Rathilal, S. Review on advancing heavy metals removal: The use of iron oxide nanoparticles and microalgae-based adsorbents. Clean. Chem. Eng. 11, 100137 (2025).
Lellala, K. et al. Fe3O4 nanoparticles decorated on N-doped graphene oxide nanosheets for elimination of heavy metals from industrial wastewater and desulfurization. Diam. Relat. Mater. 150, 111746 (2024).
Singh, A. K., Hussain, A., Priyadarshi, M. & Haider, A. Heavy metals removal from synthetic and industrial wastewater using synthesized zinc oxide nanoparticles. J. Indian Chem. Soc. 101, 101145 (2024).
Tadic, M., Lazovic, J., Panjan, M., Tadic, B. V. & Lalatonne, Y. Low-coercivity behavior and biomedical potential of cube-like and rounded hematite (α-Fe2O3) nanoparticles: Insights from hydrothermal synthesis. Mater. Sci. Eng. B. 317, 118204 (2025).
Kotresh, M. G., Patil, M. K. & Inamdar, S. R. Reaction temperature based synthesis of ZnO nanoparticles using co-precipitation method: Detailed structural and optical characterization. Optik (Stuttg). 243, 167506 (2021).
Ranjithkumar, B., Sudha, D., Ranjith Kumar, E. & Alharthi, S. S. Natural honey (Mellifera) assisted combustion synthesis of zno, Ag-ZnO and Fe-ZnO nanoparticles for ethanol gas sensor applications. Ceram. Int. 50, 27679–27688 (2024).
Modrić-Šahbazović, A. et al. Role of synthesis temperature in the formation of ZnO nanoparticles via the Sol-Gel process. J. Cryst. Growth. 650, 128003 (2025).
Verma, N., Pathak, D. & Thakur, N. Eco-friendly green synthesis of (Cu, Ce) dual-doped ZnO nanoparticles with Colocasia esculenta plant extract using microwave assisted technique for antioxidant and antibacterial activity. Next Mater. 5, 100271 (2024).
Al-Kadhi, N. S. et al. Facile synthesis of mgo/zno nanocomposite for efficient removal of Alizarin red S dye from aqueous media. Inorg. Chem. Commun. 162, 112233 (2024).
Abdellaoui, Y. et al. New hybrid adsorbent based on APTES functionalized zeolite W for lead and cadmium ions removal: Experimental and theoretical studies. Chem Eng. J 499, (2024).
Kuczajowska-Zadrożna, M., Filipkowska, U. & Jóźwiak, T. Adsorption of Cu(II) and Cd(II) from aqueous solutions by Chitosan immobilized in alginate beads. J. Environ. Chem. Eng. 8, 103878 (2020).
Bagdat, S., Tokay, F., Demirci, S., Yilmaz, S. & Sahiner, N. Removal of Cd(II), Co(II), Cr(III), Ni(II), Pb(II) and Zn(II) ions from wastewater using polyethyleneimine (PEI) cryogels. J. Environ. Manag. 329, 117002 (2023).
Fu, W. & Huang, Z. Magnetic dithiocarbamate functionalized reduced graphene oxide for the removal of Cu(II), Cd(II), Pb(II), and Hg(II) ions from aqueous solution: Synthesis, adsorption, and regeneration. Chemosphere 209, 449–456 (2018).
Zhao, N. et al. Intensive adsorption of Tetracycline by Cobalt oxide quantum dots-loaded mineral carbon. Bioresour Technol. 385, 129373 (2023).
Seid, S. M. & Gonfa, G. Adsorption of Cr(V) from aqueous solution using eggshell-based Cobalt oxide- zinc oxide nano-composite. Environ. Challenges. 8, 100574 (2022).
Praipipat, P. et al. The adsorptions of cadmium (II) ions and methylene blue dye by mangosteen (Garcinia Mangostana Linn) peels with metal oxide modifications of magnesium oxide (MgO) and titanium dioxide (TiO2). Sustain. Mater. Technol. 43, e01318 (2025).
Li, J. et al. Resource utilization of microcrystalline magnesite: Synthesis of magnesium oxide by malic acid steam-assisted decomposition method for the synergetic adsorption of congo red dye and Cu(II) ion. Environ. Res. 271, 121136 (2025).
Jiang, X. et al. Reducing arsenic mobilization in sediments: A synergistic effect of oxidation and adsorption with zirconium-manganese binary metal oxides. Water Res. 283, 123798 (2025).
Popat, P. R. et al. Enriched adsorption of Methyl orange by zinc doped lithium manganese oxides nanosorbent. Inorg. Chem. Commun. 161, 112016 (2024).
Singh, S. et al. Graphene oxide-based novel MOF nanohybrid for synergic removal of Pb (II) ions from aqueous solutions: simulation and adsorption studies. Environ. Res. 216, 114750 (2023).
Kothavale, V. P. et al. Carboxyl and thiol-functionalized magnetic nanoadsorbents for efficient and simultaneous removal of Pb(II), Cd(II), and Ni(II) heavy metal ions from aqueous solutions: Studies of adsorption, kinetics, and isotherms. J. Phys. Chem. Solids. 172, 111089 (2023).
Joseph, I. V., Tosheva, L. & Doyle, A. M. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly Ash. J. Environ. Chem. Eng. 8, 103895 (2020).
Wei, S. et al. One-step microwave-assisted synthesis of MgO-modified magnetic Biochar for enhanced removal of lead and phosphate from wastewater: Performance and mechanisms. Sep. Purif. Technol. 354, 128936 (2025).
Pan, J. et al. Synthesis and characterization of Chitosan schiff base grafted with formaldehyde and aminoethanol: As an effective adsorbent for removal of Pb(II), Hg(II), and Cu(II) ions from aqueous media. Int. J. Biol. Macromol. 281, 135601 (2024).
Manzoor, Q. et al. Polymer-assisted synthesis of ternary magnetic graphene oxide nanocomposite for the adsorptive removal of Cr(VI) and Pb(II) ions. Heliyon 10, e35204 (2024).
Al-Kadhi, N. S. et al. Facile synthesis of mnco₃/zro₂/mgco₃ nanocomposite for high-efficiency malachite green dye removal. J. Inorg. Organomet. Polym. https://doi.org/10.1007/s10904-024-03577-4 (2025). (In press.
Alghanmi, R. M. & Abdelrahman, E. A. Simple production and characterization of zno/mgo nanocomposite as a highly effective adsorbent for eliminating congo red dye from water-based solutions. Inorg. Chem. Commun. 161, 112137 (2024).
Manohar, A. et al. Characterization of a synthesized mg₀.₇ni₀.₃fe₂o₄/ceo₂/mgfe₂o₄ nanocomposite for magnetic and electrochemical applications. Colloids Surf. Physicochem. Eng. Asp. 722, 137269 (2025).
Abdelrahman, E. A. & Basha, M. T. Facile synthesis and characterization of SrCO3/MgO/CaO/CaCO3 novel nanocomposite for efficient removal of crystal Violet dye from aqueous media. Inoganics 13, 112 (2025).
Pal, S., Wareppam, B. & Singh, N. S. Stabilized surface charge by optimizing the carbonization temperature of bamboo for sustainable dye degradation at varying pH. J. Mater. Sci. Mater. Electron. 35, 1721 (2024).
Suresh Kumar, P., Korving, L., Keesman, K. J., van Loosdrecht, M. C. M. & Witkamp, G. J. Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics. Chem. Eng. J. 358, 160–169 (2019).
Abdelrahman, E. A., El-Reash, A., Youssef, Y. G., Kotp, H. M., Hegazey, R. M. & Y. H. & Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 401, 123813 (2021).
Al-Wasidi, A. S., Abdelrahman, E. A., Shah, R. K., Abdelhakim, N. A. & Saad, F. A. Facile synthesis of novel nanocomposite composed of Co3O4, mgo, and Mg3B2O6 for malachite green dye decontamination from aqueous media. Sci. Rep. 14, 1–23 (2024).
Asuquo, E., Martin, A., Nzerem, P., Siperstein, F. & Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 5, 679–698 (2017).
Jadhao, J. S. et al. Efficient removal of toxic Cd(II) ions from waste streams by a novel modified biodegradable magnetic sorbent. Chem. Inorg. Mater. 1, 100016 (2023).
Hashem, B. Y., Alswat, A. A., Ali, S. L., Al-Odaini, N. A. & Alshorifi, F. T. Facile synthesis of NiO-CuO/Activated carbon nanocomposites for use in the removal of lead and cadmium ions from water. ACS Omega. 7, 47183–47191 (2022).
Acknowledgements
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Author information
Authors and Affiliations
Contributions
Ehab A. Abdelrahman (Funding acquisition, Methodology, Conceptualization, Writing – Review & Editing), Mohamed N. Goda (Methodology, Writing – Review & Editing), Fawaz A. Saad (Writing – Review & Editing, Visualization), Abdalla M. Khedr (Writing – Review & Editing), Reem K. Shah (Visualization, Writing – Review & Editing).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abdelrahman, E.A., Goda, M.N., Khedr, A.M. et al. Thermal tuning of nanocomposites for superior cadmium ion adsorption. Sci Rep 15, 24003 (2025). https://doi.org/10.1038/s41598-025-09274-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09274-7