Abstract
Sugilite jade is an emerging jade material in the jewelry market with various shades of purple in appearance, and there is currently a gap in research on the blue and pink hues it possesses. The mineralogical and colorimetric characteristics of South African sugilite jade were studied using polarized light microscopy, X-ray fluorescence, X-ray powder diffraction, electron microprobe, infrared spectroscopy, and UV-Vis spectroscopy to explore the causes of its color. The primary mineralogical component of sugilite jade is sugilite, with minor constituents including pectolite-serandite, aegirine, alkaline amphibole, and alkaline feldspar. The purple hue of sugilite jade is attributed to the presence of Mn3+ on the A site within the crystal structure of sugilite. It has been observed that the lightness and chroma of this color are positively correlated with the Mn content. The blue color is caused by Cu2+ in pectolite and the pink color is attributed to Mn2+ in serandite. The color of sugilite is primarily influenced by its broad absorption peak in the UV-visible spectrum, which occurs between 500 and 700 nm. The intensity of this peak is directly proportional to the concentration of Mn3+, which determines the lightness of sugilite jade. The presence of Cu2+ results in a shift of the absorption peak to a higher wavelength, imparting a bluer color to the mineral.
Similar content being viewed by others
Introduction
Sugilite jade, also known as Luvulite and Royal Azel in the jewelry market, is composed mainly of sugilite, with common accessory minerals including pectolite, aegirine, quartz, and amphibole1. It was first discovered in Japan in 1944 and is colorless to light yellow2. The characteristic purple gem-grade variety was found in 1979 in a large layered manganese ore body in the Wessels Mine in South Africa3. In addition, sugilite has also been found in Madhya Pradesh, India4 Mont Saint-Hilaire in Quebec, Canada5 Hoskins Mine and Woods Mine in Australia6,7 near Castagnola and Faggiona, Liguria, in Italy8 and in alkaline blocks in the Darai Pioz region of Tajikistan9. Sugilite jade is mostly metamorphic in origin, and purple sugilite is often found in Mn-rich skarn1,3,10. Currently, more than 90% of the purple sugilite jade on the Chinese market comes from South Africa10.
Sugilite jade exhibits remarkable color diversity, with commercial varieties predominantly ranging from light to deep purple, alongside significant occurrences of pink and blue-purple specimens. The most valuable variety in the Chinese market is the pink type, commercially termed “sakura pink” or “peach blossom”, which commands higher prices than certain varieties of jadeite and nephrite. Among purple specimens, the most prized is the intense “royal purple” variety.
Current research confirms that the purple coloration originates from sugilite, the primary mineral constituent10,11,12. However, the chromatic contributions of associated minerals to other hues (pink, blue, orange, and even green tones) remain poorly understood. Microscopic and electron microprobe analyses reveal that sugilite typically coexists with aegirine, quartz, and pectolite1,10,13. These associated minerals - appearing white (quartz, pectolite) or pale green-gray (aegirine) in fibrous, granular, or vein structures - dilute sugilite’s purple coloration, resulting in lighter overall tones10. Distinct dark impurities include blackish braunite and brown limonite, easily distinguishable from the host purple matrix1,14. Additional minerals reported in South African specimens - including andradite, wollastonite, vesuvianite, magnesio-arfvedsonite, calcite, richterite, and hydroxyapophyllite - occur as sporadic minerals rather than consistent associates, thus minimally affecting bulk coloration in gem-quality material1,9.
The premium valuation of vividly colored “royal purple” and “sakura pink/peach blossom” varieties underscores the economic importance of precise color classification. Chromatic properties fundamentally derive from sugilite, a hexagonal bicyclic silicate with ideal formula KNa₂(Al, Mn³⁺)₂(Li, Al, Fe³⁺)₃Si₁₂O₃₀, exhibiting purple to pink coloration1,12,15. Petrographic observations demonstrate positive correlation between purple intensity and sugilite abundance in thin sections10. Notably, Japanese Mn-free sugilite appears colorless to pale yellow, while Mn-bearing specimens from South Africa, India, and Australia display variable purple hues2. This coloration arises when Mn³⁺ substitutes for Fe³⁺ at the A-site, inducing a d-d transition that produces broad 500–600 nm absorption (yellow-green region) and consequent purple reflectance12,16. Pinkish-purple variants (e.g., Al-rich sugilite from Italy’s Cerchiara mine8) occur when Mn³⁺ occupies the A-site alongside increased Al³⁺/decreased Fe³⁺ ratios17. No verified occurrences of blue-purple sugilite single crystals have been documented to date.
To compare the color of sugilite jade with its mineral composition and chemistry content data and find the cause of different hues, this paper uses colorimetry to quantify the color of sugilite jade samples. In recent years, colorimetry has played an important role in gemology, involving color-change garnets18,19 spessartine20 cordierite21 ruby22 colored diamonds23 tourmaline24 silicified coral25 chrysoprase26 jadeite27,28,29 agate30 turquoise31 etc. The system widely used to explore gem colorimetry is the CIE1976 L* a* b* uniform color space, which is the latest system recommended by the International Commission on Illumination for quantitative color characterization32. The color of the sample is characterized by the L*, a*, and b* values in the CIE1976 L* a* b* color space, which were calculated in accordance with the CIE 1976 standard33. The parameter L* correlates with the total reflectance of the sample, where 0 represents pure black and 100 corresponds to pure white. The coordinates a* and b* indicate the relative intensities of red-green and yellow-blue chromatic components, respectively: positive a* values denote a red shift, while negative a* values indicate a green shift; similarly, positive b* values reflect a yellow tendency, whereas negative b* values suggest a blue tendency. For instance, a sample exhibiting high reflectance in the 600–700 nm range (red light) typically demonstrates a significantly positive a* value. Conversely, strong absorption in the 400–500 nm region (blue light) generally results in a negative b* value. Chroma C* and hue angle h° can be calculated from a* and b* as follows:
To correlate these colorimetric parameters with the underlying mineralogical and chemical factors, a multi-technique approach was employed. Specifically:
Infrared spectroscopy and XRD were used to resolve the principal mineral phases;
Gemological microscopy and SEM-BSE characterized the mineral structures and spatial distributions;
Electron microprobe and XRF quantified major and trace elements in sugilite and associated minerals;
UV-Visible spectroscopy identified electronic transitions of coloring ions.
This systematic approach decouples the respective contributions of sugilite chemistry and paragenetic minerals to color variability.
To investigate the origin of coloration, 25 sugilite jade samples from South Africa were selected, covering a spectrum from pink-purple to blue-purple—a range that represents both commercially significant and scientifically puzzling varieties. Particular attention was given to the transition between pink and blue hues, as these variations remain poorly understood. Most samples exhibited relatively uniform coloration, minimizing interference from heterogeneous color zoning.
For consistent spectroscopic analysis, the samples were prepared as polished plates (ranging from 3 × 5 mm² to 13 × 13 mm², with a thickness of 1.7–2 mm) to ensure uniform surface reflectance (Fig. 1). This standardized preparation allowed for reliable comparisons across colorimetric and compositional datasets.
Results and discussion
Color quantification analysis
The color parameters of 25 samples were measured using an MDIS-f8 dual integrating sphere spectrometer. The results showed lightness L* ranging from 22.76 to 59.57, chromaticity coordinates a* from 0.11 to 27.34 and b* from − 31.57 to − 1.97, chroma C* from 6.74 to 41.14, and hue angle h° from 289.9° to 346.1°. The colors were plotted in the CIE 1976 L*a*b*uniform color space (Fig. 2a), where C* represents the distance from the origin, and h° is the angle between the + a* axis and the projected point. Figure 2b illustrates the sample color distribution, showing purple to blue-purple hue variations.
Bivariate correlation analysis was performed to examine the relationships between C*, h°, a*, and b*. The Pearson correlation coefficient (r) was used to quantify the strength and direction of the relationships, where r ranges from − 1 to 1. A value closer to 1 or − 1 indicates a stronger correlation, while 0 suggests no linear relationship34. The results showed a strong negative correlation between b* and C* (r = − 0.94), a strong positive correlation between a* and C* (r = 0.89), a moderate negative correlation between a* and h° (r = − 0.48), and no significant correlation between b* and h°.
To assess how well the regression models fit the data, the coefficient of determination (R²) was used, where R² ranges from 0 to 1. A higher R² value indicates that the regression line better explains the variability in the data34. The analysis revealed R² = 0.73 for a* versus C*, R² = 0.84 for b* versus C*, and R² = 0.53 for b* versus h°(Fig. 2c and d). These findings suggest that both red (+ a*) and blue (− b*) hues significantly influence chroma (C*), with blue having a slightly stronger effect. Additionally, h° first decreases and then increases as the blue hue deepens.
(a) Color distribution of 25 sugilite jade samples in the CIE 1976 L* a* b* uniform color space. (b) Schematic diagram of the color range of samples. (c) The relationship between the chroma C* and the colorimetric coordinates a* and b*. (d) The relationship between the hue h° and the colorimetric coordinates a* and b*.
Mineral composition analysis
Appearance under the microscope
Mineral structure of samples under the microscope. (a) Sample 1 has patchy light gray and black minerals on the surface. (b) Sample 14 has gray minerals and purple and light purple minerals distributed in clumps. (c) Sample 5 is almost cryptocrystalline, and a purple sample with a fibrous structure can be seen in some parts. (d) Sample 10 is a light purple sample with cryptocrystalline gangue. (e) Sample 12 has purple minerals distributed in a blue matrix with a fibrous structure, and the purple minerals have a good degree of morphism. (f) Sample 25 has pink, purple, and brown minerals, and a fibrous structure can be seen in some parts.
In the purple samples, purple, white, and gray minerals are found together in clumps and stars, displaying granular structures (Fig. 3a, b) or cryptocrystalline structures (Fig. 3c, d) with better transparency. Some samples also contain black and brown-red minerals (Fig. 3a). Additionally, Fig. 3d demonstrates that the veins contain cryptocrystalline minerals within the light-colored granular structure minerals. In the blue-purple sample, purple minerals are distributed in the blue matrix in clumps, exhibiting fibrous structures, and some exhibit a good degree of etherealization (Fig. 3e). The pink-purple sample has a fibrous structure with some brown minerals associated (Fig. 3f).
Scanning electron microscope
(a) SEM BSE of sample 2, PEC is distributed in the form of stars in sugilite (SUG). (b) SEM BSE of sample 4, SUG is distributed in the form of gravels in the matrix of RIC. (c) SEM BSE of sample 12, SUG and PEC are distributed in a fibrous and oriented manner. (d) SEM BSE of sample 14, SUG, PEC and AEG are distributed in a granular manner. (e) SEM BSE of sample 15, SUG and PEC are distributed in a granular manner. (d) SEM BSE of sample 17, SUG, AEG and QRZ are distributed in a granular manner.
To see the mineral contents and texture of sugilite jade with different colors, 6 color-representative samples were selected to make rock-thin sections for SEM BSE observations. They are sample 2 (dark blue-purple), 4 (pink-purple), 14 (containing a large amount of gray minerals), 12 (blue-purple), 15 (blue-purple), and 17 (blue-purple).
SEM BSE photos show the distribution of different minerals in the samples (Fig. 4). Sugilite in sample 2 accounts for more than 80% of the entire sample (Fig. 4a). Sample 4 presents a blastopsammitic texture. The gravels are sugilite (particle size is about 1 mm), occupying about 70% of the sample, and are euhedral to semi-euhedral, with riebeckite in the matrix (Fig. 4b). Sample 12 has a blastoporphyritic texture. Large porphyroblastic morphology (grain size of about 1 mm), which are actually metamorphic residual pseudophases, can be seen in fine-grained granoblastic crystals. Sugilite and pectolite are arranged fibrous-oriented, with sugilite accounting for about 60% (Fig. 4c). Sample 14 has a coexisting of sugilite, aegirine, and pectolite, showing an anhedral granular texture with 70% of sugilite (Fig. 4d). Sample 15 shows coexisting granular pectolite and sugilite, with 40% of sugilite (Fig. 4e). Sample 17 contains quartz, sugilite, and aegirine, showing anhedral granular texture, with sugilite occupying about 50% (Fig. 4f).
All samples show obvious metamorphic textures. As the distribution area of sugilite increases, the color of sugilite jade deepens. The color concentration of the sugilite particles themselves also affects the samples’ color. The blue-toned sugilite jade samples (12, 15, and 17) all contain pectolite, possibly an influencing factor of the hue of sugilite jade.
X-ray diffraction
X-ray diffraction analysis was performed on a series of representative sugilite jade samples spanning the full color range, encompassing dark purple (sample 1), medium purple (sample 3), light purple (samples 7 and 8), blue-purple (sample 16), and pink-purple (sample 25) varieties. The diffraction patterns revealed significant variations in mineralogical composition across the sample set. Characteristic sugilite diffraction features were identified in samples 1, 3, and 7, whereas sample 8 consisted predominantly of quartz (Fig. 5a). Samples 16 and 25 exhibited diffraction peaks corresponding to both pectolite and sugilite phases, with sample 25 displaying more intense sugilite peaks (Fig. 5b).
Prior to XRD analysis, samples 25 and 16 were manually ground using an agate mortar to achieve a particle size below 200 mesh (< 75 μm). Although this preparation method typically ensures sufficient powder averaging, minor residual coarse grains or slight preferred orientation effects may persist, potentially contributing to observed intensity discrepancies in certain reflections. Despite these intensity variations, the 2θ peak positions showed good agreement with reference patterns. The phase assignments for samples 25 and 16 were further corroborated by complementary FTIR data, which clearly distinguished between pectolite and sugilite signatures.
The observed mineralogical differences among the samples appear to correlate with their color variations. Pectolite, with a chemical formula of NaCa2Si3O8(OH), is a common paragenetic mineral in sugilite jade1,14,35,36. While typically white in appearance, pectolite can develop blue hues when containing Cu²⁺ ions37. This phenomenon is particularly relevant given the documented occurrence of copper-bearing blue pectolite coexisting with sugilite in South Africa’s Wessels mining region38. Additionally, manganese substitution in pectolite can lead to the formation of serandite1,14,39 an isostructural mineral where Mn replaces Ca in the crystal lattice40. The presence of Mn²⁺ in serandite is known to produce rose-red to pink coloration, potentially explaining the pink hues observed in certain samples. These findings suggest that the color variations in sugilite jade may be attributed to the presence and chemical composition of associated pectolite/serandite minerals.
Infrared spectroscopy
Infrared spectroscopy was used to identify the main mineral components of all sugilite jade samples (Fig. 6a, b, c). The infrared spectroscopy measurements employed a 1 mm spot size (Fig. 7a), with analyses deliberately focused on the dominant color regions to ensure consistency with bulk characterization methods (UV-vis spectroscopy and colorimetry). The data demonstrate consistent FTIR spectra from regions with almost identical coloration and texture, confirming relatively uniform mineral composition. As most samples showed homogeneous coloration, FTIR effectively characterized their dominant mineral phases.
Accessory minerals exhibit special absorption bands. The purple marked area in Fig. 6a is the absorption spectrum of sugilite. Sugilite belongs to the osumilite-milarite group with a double-ring structure, as shown in Fig. 7b, c. The silicon-oxygen tetrahedron shares an O anion with two other T(1) tetrahedrons to form a hexagonal ring. All tetrahedrons point in the same direction (such as beryl), and the top anion of the ring shares another ring with the T(1) tetrahedron to form a [Si12O30] double hexagonal ring16. The vibration of the silicon-oxygen group of cyclic silicates can be divided into terminal oxygen and bridging oxygen. Each silicon forms two Si-O-Si type bonds and two Si-O type bonds. Therefore, the vibration mode can be divided into νs Si-O-Si, νasSi-O-Si, νsO-Si-O, νasO-Si-O, and δSi-O vibration. In the range of 600–830 cm−1, the vibration frequencies of νasSi-O-Si, νsO-Si-O, and νasO-Si-O are relatively high, roughly in the range of 800–1200 cm−1. δSi-O bending vibration is below 600 cm−1, and together with νM-O vibration, it cannot be specified. Generally, the maximum frequency of hexagonal ring vibration can reach 1200 cm−116. Therefore, the peaks at 1099, 1068, and 1037 cm−1 of sugilite correspond to Si-O-Si antisymmetric stretching vibration, O-Si-O antisymmetric stretching vibration, and O-Si-O stretching vibration; the peaks at around 784, 692, 590, and 565 cm−1 are due to Si-O-Si stretching vibration; and 503, 476, 457, and 420 cm−1 correspond to Si-O bending vibration and M-O vibration. The specific vibration frequency is affected by the Si-O-Si bond angle.
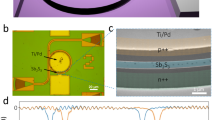
(a) The 1 mm diameter tested area on the dominant color regions. (b) Crystal structure of suglite projected along the c-axis. The blue double hexagonal ring of T(1)O4 (T(1) = Si) is formed by connecting the units of LiO4 (green) of the T2 tetrahedron and FeO6 (possibly Fe3+, Mn3+, Al3+, dark purple) of the A-site octahedron. The C site (light purple) is occupied by K. (c) Crystal structure of sugilite projected perpendicular to the c-axis. The two double-ring units formed by the T1 tetrahedron are stacked on each other, and the gap between them is occupied by Na (yellow) on the T2-coordinated C site.
Figure 6b shows the spectrum with obvious pectolite peaks. Both pectolite (NaCa2[Si3O8(OH)]) and serandite (NaMn2[Si3O8(OH)]) are chain silicates. The 1200–800 cm−1 peaks are stretching vibrations of silicon-oxygen tetrahedrons, and the bending vibrations of silicon-oxygen tetrahedrons and other structural vibrations occur at lower wave numbers. The peaks at 945, 975, and 1008 cm−1 are Si-O-Si antisymmetric stretching vibrations, the peak at 657 cm−1 is Si-O symmetric stretching vibrations, and the peaks at 507 and 407 cm−1 are Si-O-Si bending vibrations.
Figure 6c shows the spectrum with quartz peaks. The peak at 1158 cm−1 is Si-O antisymmetric stretching vibrations, and the peak at 813 cm−1 is Si-O-Si stretching vibrations. The spectrum peak of sample 11 is special, with a peak position similar to that of albite. It is speculated to be a paragenetic mineral of sugilite9. The infrared results are preliminary distinguished samples containing the pectolite-serandite series and samples containing quartz.
Mineral chemistry analysis
EPMA
Electron probe experiments were carried out to check the chemical composition of sugilite and associated minerals. The sampling positions are marked in the SEM BSE photos (Fig. 8). Table 1 shows the EPMA results.
The EPMA data were compared with the previously measured sugilite jade paragenetic mineral values. It was found that points 2 − 1, 4 − 1, 12 − 1, 12 − 3, 15 − 2, and 17 − 2 were sugilite; points 12 − 2, 14 − 1, and 15 − 1 were pectolite; points 2–2, 14 − 2, and 17 − 3 had high Fe content and were aegirine; point 4 − 2 was magnesium-rich arfvedsonite; and point 17 − 1 was SiO2.
ED-XRF
ED-XRF enables rapid detection of chemical elements in sugilite jade. Quantitative analysis of 25 samples was performed using data from the dominant color regions to align with IR integrated results. Despite minor heterogeneity, most samples show uniform surface coloration. Test locations are illustrated in Fig. 7a.
The sample test results showed that the weight% (wt%) of the main elements in 25 samples ranged from w(Si) = 42.12–94.50, and the weight% of other elements ranged from w(Fe) = 0.15–31.13, w(Ca) = 0.00-49.94, w(K) = 0.24–23.70, w(Mn) = 0.09–9.03, w(Cu) = 0.00-0.186, w(Al) = 0.00-9.638 and w(Cr) = 0.00-0.07. Among them, Cr, Mn, Fe, and Cu are transition metal elements usually related to color. The elements in all purple samples are Mn and Fe, consistent with previous studies12,17. In addition, the K and Al contents of samples 10, 11, and 24 are significantly higher. By comparing the infrared spectrum and ED XRF data, it can be further confirmed that the three contain alkaline feldspar. These samples also consistently have a high brightness L*.
Color origin analysis
Bivariate correlation analysis between ED-XRF element content and color
The color properties of sugilite jade were characterized through UV-Vis spectroscopy and colorimetry (MDIS-f8), while its phase composition was determined by XRD—all of which provide bulk (whole-sample) measurements. Similarly, IR spectroscopy and XRF (both with a 1 mm spot size) also yielded macro-scale data, though at higher spatial resolution than the integrated UV-Vis/colorimetric results. In contrast, EPMA offers micro-scale resolution, making it unsuitable for direct correlation with these bulk techniques.
To ensure consistent comparisons, XRF data (1 mm spots) were selected for quantitative correlation with sugilite’s color parameters, as both datasets represent macro-scale averages. Measurements were conducted on the sample’s dominant color region to align with the UV-Vis and colorimetry results, which reflect the sample’s overall L*a*b* values and bulk absorption characteristics.
The samples contained associated minerals (pectolite, quartz, aegirine, arfvedsonite, and feldspar) alongside sugilite (Fig. 9a). To isolate the influence of transition metals (Mn, Fe, Cu) on sugilite’s color, the dataset was partitioned into three groups for separate analysis (Table 2).
Group 1 is for analyzing the coloration mechanism of Mn in sugilite, excluding samples 14 and 17, which contain a large amount of aegirine; sample 4, containing amphibole; and samples 9, 12, 13, 15, 16, 18, 19, and 25 containing pectolite-serandite to ensure the measured Mn mainly comes from sugilite. The reason is that the amounts of Mn in aegirine, amphibole, and pectolite-serandite series are all higher than those in sugilite1,37 while feldspar minerals and quartz contain less Mn41.
Group 2 is to observe the relationship between Fe content and color, which excludes samples 14 and 17 containing aegirine and sample 4 containing amphibole to ensure that the measured Fe mainly came from sugilite. The Fe content in aegirine and amphibole can be higher than that in sugilite, while pectolite, alkaline feldspar, and quartz contain less Fe37,41 which is negligible compared with sugilite.
Group 3 is to check the relationship between the Cu content in pectolite and the color of sugilite jade. In the previous EPMA data of sugilite jade, there is no Cu in sugilite, aegirine, and alkaline feldspar1,3,4,42,43 while Cu is a common coloring element in blue pectolite37. Therefore, it is inferred that the blue hue of sugilite jade comes from pectolite containing Cu. Based on this, sample 4 with amphibole and samples 10, 11, and 24 with feldspar are excluded because the composition of amphibole varies complexly, and Na+ in feldspar may exchange with Cu+, leaving the Cu content uncertain44. In addition, because the Ca content of the pectolite-serandite series is generally much higher than that of other associated minerals1,14,16,45 the amount of Ca roughly explains the pectolite-serandite series’ content. In order to avoid the interference of pink-toned serandite on the Ca quantification, samples containing pectolite-serandite but with a pink hue (9, 18, 19, 25) were also excluded.
All test results are two-tailed, and the Pearson correlation coefficient r can determine the linear relationship between the two data groups: when |r|<0.05, the two data are correlated.
The results of the bivariate analysis in Table 3 show that w (Mn) in sugilite is negatively correlated with lightness L*, and their relationship is shown in Fig. 9b (goodness of fit RMn2 = 0.93), indicating that the more Mn content in sugilite, the lower the sample lightness L* and the darker the color. w (Mn) has no obvious correlation with C*. When 24.67 < C* < 32.91, the Mn content of the sample is the largest, and the overall content is high. More Mn does not lead to more saturated colors.
The results of the bivariate analysis in Table 4 show that w (Fe) in sugilite is negatively correlated with lightness L* (Fig. 9b, RFe2=0.97), indicating that the more Fe content in sugilite jade samples, the lower the L* and the darker the color. w(Fe) is positively correlated with chroma C* (Fig. 9c, RFe2=0.97).
Table 5 shows that w (Ca) and w (Cu) negatively correlate with the hue h° of group 3 samples. The more Ca and Cu the sample contains, the smaller h° is, and the bluer the color is (RCa2=0.98, RCu2=0.94), as shown in Fig. 9d.
In addition, since both sugilite and serandite contain Mn, it is difficult to obtain the relationship between the Mn content in serandite and the pink tone of the sample based on ED-XRF data alone. However, infrared and XRD analysis show that pink pectolite-serandite-containing samples (9, 18, 19, 25) all show higher Ca and Mn contents.
(a) The distinguishing samples’ mineral composition logic. (b) The L* and w(Mn) of group 1 are negatively correlated; the L* and w(Fe) of group 2 are negatively correlated. (c) There is no obvious correlation between the chroma C* and w(Mn) of group 1, showing a positive correlation first and then a negative correlation; the chroma C* and w(Fe) of group 2 samples are positively correlated. (d) The hue angle h° and w(Ca) and w(Cu) of group 3 samples are all negatively correlated.
UV-Visible spectroscopy analysis
To investigate the influence of transition metal elements on the coloration of sugilite jade, UV-VIS spectroscopy was performed on all samples. Figure 10 presents the absorption spectra obtained in diffuse reflection mode, revealing a broad absorption band between 500 and 700 nm with significant variation in bandwidth and intensity among samples.
The purple sugilite jade samples exhibit an absorption band centered at 540–580 nm, consistent with characteristic Mn³⁺ transitions observed in other purple minerals46,47,48,49. This feature corresponds to the ⁵Eg→⁵T2g transition resulting from Jahn-Teller splitting of Mn³⁺ at the ⁵T2g energy level50. In blue-purple samples, this absorption band broadens and undergoes a bathochromic shift, with the peak maximum reaching approximately 650 nm (Fig. 10a). The shifted peak position aligns with known Cu²⁺ absorptions in pectolite (655–699 nm) and tourmaline (700–900 nm), attributable to the ²Ag→²Bg transition of Cu²⁺ in the associated pectolite (Fig. 10b)51,52.
The 500–700 nm spectral region was selected for focused analysis due to its consistently high absorption intensity across all samples and its critical role in governing coloration through dominant electronic transitions. Within this range, variations in the absorption maximum wavelength (λmax) arise from crystal field effects in both sugilite and pectolite. Notably, a robust inverse relationship exists between hue angle (h°) and λmax (R2 = 0.99, Fig. 11a), where hypsochromic shifts (decreasing λmax) correlate with elevated h° values and diminished blue hues.
Further examination of Group 3 samples reveals a key structural influence: XRF-derived Ca and Cu content both exhibit positive correlations with the λmax (RCa2=0.95, RCu2=0.96, Fig. 11b). The trends—manifested as decreasing λmax values with increasing Ca and Cu concentrations—provide direct evidence for Cu²⁺ substitution in pectolite components, thereby validating prior mechanistic conclusions.
A correlation is observed between the samples’ lightness (L*) and the intensity of the 500–700 nm absorption band. Given the strong background absorption present in most samples, the integrated band intensity, Sum (Σ(Maximum, Onset, Endpoint intensities)), across the 500–700 nm range serves as the analytical parameter. Results demonstrate a significant negative correlation between Sum and L* (RL2=0.99, Fig. 11c), where samples with lower overall absorption intensity exhibit lighter coloration.
(a) Significant negative correlation between hue angle (h°) and λmax (absorption maximum wavelength): Red-shifted bands correspond to blue-purple coloration. (b) Positive correlations between λmax and both w(Cu) & w(Ca) in Group 3. (c) Inverse relationship between summed band intensities (peak + onset + end, 500–700 nm) and lightness (L*). (d) Correlations between spectral indices (SumG1, SumG2) and chromophore contents. SumG1 = Σ(Maximum, Onset, Endpoint intensities) of 500–700 nm bands (Group 1) vs. w(Mn). SumG2 = Σ(Maximum, Onset, Endpoint intensities) of 500–700 nm bands (Group 2) vs. w(Fe). (e) Relationship between the 500–700 nm band area S and chroma C*. The chroma increases with the 500–700 nm peak area increasing.
Analysis of Group 1 and Group 2 samples reveals distinct relationships between SumG1/Mn content and SumG2/Fe content. Mn content displays a strong positive correlation with SumG1 (RMn²=0.99), while Fe content shows no significant correlation with SumG2 (RFe²=0.48). These findings indicate that higher absorption values correspond to darker sample colors and increased Mn concentrations, as illustrated in Fig. 11d.
The UV-Vis spectra of samples 1, 18–22 exhibit a smooth shoulder peak at about 500 nm, which merges with the main 500–700 nm absorption band, altering its shape and consequently affecting color (Fig. 10b). Infrared spectroscopy confirms the presence of pectolite-serandite components in these samples. The observed absorption feature at 519.5 nm is attributed to Mn²⁺ in serandite50.
Previous studies establish a relationship between chroma (C*) and the area of the main absorption peak26. For the blue-purple samples, variability in the endpoint positions of the broad absorption band necessitates a modified approach: The peak area (S, 500–700 nm) is calculated between the starting point and a normalized position on the descending slope of the peak. While this method yields somewhat dispersed data, a clear positive correlation emerges between C* and the derived peak area (RS2=0.88, Fig. 11e).
Additionally, weak absorption features are observed in the 300–500 nm range, with synchronous intensity variations at 360 nm and 410 nm but stable intensity at 432 nm. Notably, Samples 4, 12, 13, 15, and 16 completely lack the 410 nm feature with negligible 360 nm absorption, while Samples 8, 9, 18, 20, and 21 show no detectable 432 nm absorption. All iron in sugilite exists exclusively as Fe³⁺ in octahedral A-sites16,53 with the characteristic 348, 362, 412, and 418 nm peaks arising from Fe³⁺ or Mn²⁺ d-d transitions17,54; however, EPR spectroscopy confirms Mn²⁺ occurs only in pectolite-serandite components, not in sugilite itself17,35. The synchronous 362 nm and 410 nm peaks—covarying with the broad 472–487 nm band—are attributed to Mn²⁺ transitions (⁶A₁g→⁴T₂g(⁴D) and ⁶A₁g→⁴A₁g,⁴E_g(⁴G))55,56 whereas the persistent 432 nm feature, prevalent in blue-purple samples and independent of the 362/410 nm peaks, aligns with Fe³⁺ transitions (⁶A₁g→⁴A₁g,⁴E_g(⁴G)) rather than Mn²⁺ pathways56,57.
Conclusion
The main component of sugilite jade is sugilite, and the accessory mineral components are mainly pectolite-serandite series, aegirine, alkaline amphibole, alkaline feldspar, and quartz. These minerals are mostly granular and short columnar (0.2–2 mm). The pectolite-serandite series is needle-shaped with white, grey, and blue color; the aegirine is black and grayish white. Quartz, feldspar, and amphibole are mostly colorless.
The colors of sugilite jade samples were measured under a D65 light source. The lightness L* was moderately negatively correlated with the Mn and Fe content, and the chroma C* was weakly positively correlated with the Mn content. Mn3+ causes the purple color in sugilite. The5Eg→5T2g transition of Mn3+ leads to an absorption peak at 560 nm. The higher the Mn3 + content, the greater the absorption peak intensity and area, the lower the L* of sugilite jade, and the higher the C*. The hue h° is significantly positively correlated with the Cu content in pectolite. The2Ag→2Bg transition of Cu2+ causes the main absorption peak at 560 nm to shift to a higher wavelength. As the Cu content increases, the h° decreases, and the hue becomes blue-purple. In addition, the Mn2+ with a higher content in serandite may have a broad peak at 500 nm, which is speculated to widen the peak at 500–700 nm and increase the peak area. It absorbs more green light, thereby strengthening the overall purple color of sugilite jade and increasing the C* and the h° at the same time, making sugilite jade more pink.
Methods
Colorimetric analysis
The color measurement was performed by an MDIS-f8 dual integrating sphere spectrometer of the China University of Geosciences (Beijing), which collects the reflection signal of the samples’ surface. Reflectance spectra of the samples were measured using an MDIS-f8 spectrophotometer (in this study) and subsequently converted to XYZ tristimulus values under D65 illuminant conditions with the CIE 1931 standard observer functions. These values were then nonlinearly transformed to obtain the CIELAB coordinates. Figures 12 and 13 indicate the principle and structure of the MDIS-f8 dual integrating sphere spectrometer.
The test conditions are: wavelength range: 380–760 nm; spectral resolution: 1 nm; CIE standard illumination: D65; observer’s view, 2°; measurement time, less 2.5 s; thermal stability temperature: 30 °C; voltage: 220 volts; and frequency: 50–60 Hz. The tested area of a single sample was a circle with a diameter of 6 mm.
Polarized light microscope observation
A polarized light microscope observed six sugilite jade rock thin sections (30 μm thick) to analyze the their mineral composition and structural characteristics. Microscopic observation and photography were completed in the laboratory of China University of Geosciences (Beijing) Jewelry College. The instrument is Canon EOS1000D polarized photography gem microscope, and the model is GI-MLPPV.
XRD diffraction analysis
The phase composition of the samples was analyzed by powder X-ray diffraction (XRD), which was completed in the Powder XRD Laboratory, China University of Geosciences, Beijing. The powder XRD data was acquired with a Rigaku Smart lab diffractometer equipped with a conventional copper target X-ray tube set to 40 kV and 200 mA, and with a graphite monochromator, where the scanning speed was 10° min−1 in the range of 3 < 2q < 70.
Sample preparation:
Samples 1, 3, 7, and 8 were analyzed directly on polished rock slabs.
Samples 16 and 25 were manually ground with an agate mortar to a particle size < 75 μm (200 mesh) before XRD analysis.
Data processing was performed using MDI Jade 9.
Infrared spectra
The test site of Infrared Spectroscopy is the Gemological Experimental Teaching Center of China University of Geosciences (Beijing, China), and the instrument we used in the experiment is BRUKER Tensor 27 (Bremen, German), a German Fourier transform infrared spectrometer. The 25 data analyzed all tested rock samples of the sugilite jade.
Test conditions: scanning range 400–4000 cm−1, scanning time of samples and Beijing is 8 SCANS, scanning speed is 7.5 kHz. Power supply 85–265 V, power frequency 47–65 Hz, temperature 18–35℃, humidity < 70%, resolution 4 cm−1, grating setting 6 mm, sample scanning times 50–100, test mode is absorption mode.
ED-XRF
The chemical composition of the samples was determined using the EDX-7000 energy dispersive X-ray fluorescence spectrometer of the School of Jewelry, China University of Geosciences (Beijing). The test conditions were: atmosphere, oxide; voltage 50 kV; 108µA; 30% DT; collimator, 1 mm. The 25 data analyzed all tested rock samples of the sugilite jade.
EPMA
The chemical composition of sugilite and associated accessory minerals was determined using a JEOL JXA-8230R electron probe microanalyzer (EPMA) at the Testing Center of the Institute of Science, China University of Geosciences (Beijing). Analyses were performed under optimized conditions with an acceleration voltage of 20 kV, beam current of 20 nA, and a focused beam diameter of 5 μm. Characteristic X-rays were measured with a peak counting time of 10 s and background counting times of 5 s (split before and after the peak). Raw data were processed using the ZAF3 program for matrix correction, calibrated against SPI certified reference materials. The laboratory environment was maintained at 23.0 °C with 38% relative humidity. The following certified standards were employed for quantification: diopside (Si, Ca), rutile (Ti), plagioclase (Al), chromite (Cr, Fe), rhodonite (Mn), olivine (Mg), albite (Na), sanidine (K), pentlandite (Ni), vanadium metal (V), celestite (Sr), chalcopyrite (Cu), cobaltite (Co), and sphalerite (Zn). The wavelength-dispersive spectrometry system was equipped with TAP, PET, and LiF diffracting crystals, with a method detection limit of 100 ppm.
UV-Vis spectra
The absorption spectra in the UV-to-visible (UV-Vis) range were recorded using the UV-3600 UV-visible spectrophotometer of the School of Jewelry, China University of Geosciences (Beijing). The test parameters are shown below: the test method is diffuse reflection; the range of measurement is 300–900 nm; the sampling interval is 1.0 s; the scanning speed is high; and the scanning mode is single. The 25 data analyzed all tested rock samples of the sugilite jade.
Data availability
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the author Li Pengyu (300923009@email.cugb.edu.cn) upon reasonable request.
References
Sugilite and associated metamorphic silicate minerals from Wessels mine, Kalahari manganese field (Universy of Cape Town, 1988).
Murakami, N., Kato, T., Miúra, Y. & Hirowatari, F. Sugilite, a new silicate mineral from Iwagi islet, Southwest Japan. Mineral. J. 8, 110–121 (1976).
Dunn, P. J., Brummer, J. J., Belsky, H. & Sugilite A second occurrence: Wessels mine, Kalahari manganese field. Can. Mineral. 18, 37–39 (1980).
Clark, A. M., Bearne, G. S., Fejer, E. E., Din, V. K. & Couper, A. G. Additional data on Sugilite. Mineral. Mag. 43, 947–949 (1980).
Grice, J. D., Ercit, T. S., Velthuizen, J. V., Dunn, P. J. & Poudretteite KNa2B3Si12O30, A new member of the osumilite group from Mont Saint-Hilaire, quebec, and its Crystal-Structure. Can. Mineral. 25, 763–766 (1987).
Ashley, P. M. An unusual manganese silicate occurrence at the Hoskins mine, Grenfell district, new South Wales. Aust J. Earth Sci. 33, 443–456 (1986).
Kawachi, Y., Ashley, P. M., Vince, D. & Goodwin, M. Sugilite in manganese silicate rocks from the Hoskins mine and woods mine, new South wales, Australia. Mineral. Mag. 58, 671–677 (1994).
Nagashima, M. et al. KNa2Al2Li3Si12O30, an al analogue of sugilite, from the cerchiara mine, liguria, Italy. Eur. J. Mineral. 32, 57–66 (2020). Aluminosugilite.
Cairncross, B. Connoisseur’s choice: sugilite, Wessels mine, Kalahari manganese field, Northern cape province, South Africa. Rocks Min. 92, 550–555 (2017).
Dai, H. The Study on Mineral Composition and Color Causes of Sugilite (China University of Geosciences, Beijing, 2014).
Bai, F., Luo, F., Yang, L. & Zheng, S. Study on gemological and mineralogical characteristics of Sugilite: a new Gem-quality material. China Non-Met Min. Ind. 61(66), 71–73 (2007).
Shigley, J. E., Koivula, J. I. & Fryer, C. W. The occurrence and gemological properties of Wessels mine Sugilite. Gems Gemol. 23, 78–89 (1987).
Xie, Y. Gemological and mineralogical study of purple Sugilite from South Africa. J. Shenzhen Polytech. 8, 71–73 (2009).
Moore, J. M., Kuhn, B. K., Mark, D. F. & Tsikos, H. A sugilite-bearing assemblage from the Wolhaarkop breccia, Bruce iron-ore mine, South africa: evidence for alkali metasomatism and 40Ar-39Ar dating. Eur. J. Mineral. 23, 661–673 (2011).
Gagné, O. C. & Hawthorne, F. C. Chemographic exploration of the Milarite-Type structure. Can. Mineral. 54, 1229–1247 (2016).
Armbruster, T. & Oberhansli, R. Crystal chemistry of double-ring silicates: structures of Sugilite and brannockite. Am. Mineral. 73, 595–600 (1988).
Fritsch, E. & Shigley, J. E. Causes of the purple and Pink colours of Manganoan sugilites from the Wessels mine, South Africa. Mineral. Mag. 58, 681–685 (1994).
Liu, W., Qiu, Y. & Guo, Y. Mineralogical characteristics of color-Changing Garnet and the effect of light path length on color. Sci. Adv. Mater. 16, 807–816 (2024).
Qiu, Y. & Guo, Y. Explaining colour change in Pyrope-Spessartine garnets. Minerals 11, 865 (2021).
Zhu, M. & Guo, Y. New insights into the coloration mechanism in spessartines and the impact of Munsell neutral grey backgrounds. Crystals 13, 1529 (2023).
Liu, X. & Guo, Y. Study on the Color-Influencing factors of blue iolite. Minerals 12, 1356 (2022).
Yuan, B., Guo, Y. & Liu, Z. The influence of light path length on the color of synthetic Ruby. Sci. Rep. 12, 5943 (2022).
Liu, F. K., Guo, Y., Zhao, B. & Li, X. The color origin and evaluation of natural colored diamonds. Sci. Adv. Mater. 14, 243–256 (2022).
Cui, L., Guo, Y., Tang, J. & Yang, Y. Spectroscopy characteristics and Color-Influencing factors of green Iron-Bearing Elbaite. Crystals 13, 1461 (2023).
Color Genesis and Chromatography of Yellow Silicified Corals. Ingenta Connect. https://www.ingentaconnect.com/contentone/asp/sam/2024/00000016/00000007/art00002
Jiang, Y. & Guo, Y. Genesis and influencing factors of the colour of Chrysoprase. Sci. Rep. 11, 9939 (2021).
Zhang, S. & Guo, Y. Measurement of gem colour using a computer vision system: A case study with Jadeite-Jade. Minerals 11, 791 (2021).
Liu, Z., Guo, Y., Shang, Y. & Yuan, B. Research on parameters optimization of digital imaging system in red–yellow jadeite color measurement. Sci. Rep. 12, 3619 (2022).
Liu, Z. & Guo, Y. The Effect of Munsell Neutral Value Scale on the Color of Yellow Jadeite and Comparison between AP and K-Means Clustering Color Grading Schemes. Crystals 12, 241 (2022).
Zhou, Y., Liu, Z., Zhao, Z. & Guo, Y. Quantitative study on colour and spectral characteristics of Beihong agate. Minerals 12, 677 (2022).
Wang, X. & Guo, Y. The impact of trace metal cations and absorbed water on colour transition of turquoise. R. Soc. Open Sci. 8, 201110 (2021).
Kirillova, N. P., Vodyanitskii, Y. N. & Sileva, T. M. Conversion l*a*b*of l*a*b*soil l*a*b*color l*a*b*parameters l*a*b*from l*a*b*the l*a*b*Munsell l*a*b*system l*a*b*to l*a*b*the l*a*b*CIE l*a*b**a*b* l*a*b*system. Eurasian Soil. Sci. 48, 468–475 (2015).
Luo, M. R. CIELAB. In Encyclopedia of Color Science and Technology (ed. Luo, R.) 1–7 (Springer Berlin Heidelberg, 2015). https://doi.org/10.1007/978-3-642-27851-8_11-1.
Kutner, M., Nachtsheim, C., Neter, J. & Li, W. Applied Linear Statistical Models (McGraw-Hill/Irwin, 2004).
Giester, G., Rieck, B. & Wesselsite SrCu[Si4O10], a further new gillespite-group mineral from the Kalahari manganese field, South Africa. Mineral. Mag. 60, 795–798 (1996).
Armbruster, T., Oberhänsli, R., Bermanec, V. & Dixon, R. Hennomartinite and kornite, two new Mn3 + rich silicates from the Wessels mine, kalahari, South Africa. Schweiz. Min. Petrog Mitt. 73, 349–355 (1993).
Woodruff, R. E. & Fritsch, E. Blue pectolite from the Dominican Republic. Gems Gemol. 25, 216–225 (1989).
Rieck, B., Pristacz, H., Giester, G. & Colinowensite BaCuSi 2 O 6, a new mineral from the Kalahari manganese field, South Africa and new data on wesselsite, srcusi 4 O 10. Mineral. Mag. 79, 1769–1778 (2015).
Gu, X. et al. Lipuite, a new manganese phyllosilicate mineral from the n’chwaning III mine, Kalahari manganese fields, South Africa. Mineral. Mag. 83, 645–654 (2019).
Takeuchi, Y., Kudou, Y. & Yamanaka, T. Crystal chemistry of the serandite-pectolite series and related minerals. Am. Mineral. 61, 229–237 (1976).
Wibberley, C. A. J. & McCaig, A. M. Quantifying orthoclase and albite muscovitisation sequences in fault zones. Chem. Geol. 165, 181–196 (2000).
Gnos, E., Armbruster, T., Villa, I. M. & Norrishite K(Mn 2 3+ Li)Si 4 O 10 (O) 2, an oxymica associated with sugilite from the Wessels Mine, South Africa: Crystal chemistry and 40 Ar- 39 Ar dating. Am. Mineral. 88, 189–194 (2003).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Crystallogr. Sect. A. 32, 751–767 (1976).
Kucha, H. Feldspar, clay, organic and carbonate receptors of heavymetals in Zechstein deposits(Kupferschiefer-type), Poland. Trans Inst. Min. Metall. Sect. B-Appl EARTH Sci 94, (1985).
Imaoka, T. et al. Chemical and lithium isotope characteristics of murakamiite and Li–rich pectolite from Iwagi islet, Southwest Japan. J. Mineral. Petrol. Sci. 116, 9–25 (2021).
Reddy, B. J., Frost, R. L., Martens, W. N., Wain, D. L. & Kloprogge, J. T. Spectroscopic characterization of Mn-rich tourmalines. Vib. Spectrosc. 44, 42–49 (2007).
Gong, N., Wang, C. & Xu, S. Color origin of Greyish-Purple tremolite Jade from Sanchahe in Qinghai province, NW China. Minerals 13, 1049 (2023).
Lu, R. Color origin of lavender jadeite: an alternative approach. Gems Gemol. 48, 273–283 (2012).
Vigier, M. & Fritsch, E. Pink axinite from merelani, tanzania: origin of colour and luminescence. J. Gemmol. 37, 192–205 (2020).
Manning, P. G. Absorption spectra of the manganese-bearing chain silicates pyroxmangite, rhodonite, bustamite and serandite. Can. Mineral. 9, 348–357 (1968).
Laurs, B. M. et al. Copper-bearing (Paraíba-type) tourmaline from Mozambique. Gems Gemol. 44, 4–30 (2008).
Sun, Y. The Relationship between the Component and Color of Blue Tone Larimar (China University of Geosciences, Beijing, 2018).
Geiger, C. A. A 57Fe Mossbauer spectroscopic study of Sugilite, KNa2(Fe3+,Mn3+,Al)2Li3Si12O30. Can. Mineral. 47, 927–931 (2009).
Rossman, G. R. Colored varieties of the silica minerals. SILICA Phys. Behav. Geochem. Mater. Appl. 29, 433–467 (1994).
Manning, P. G. An optical absorption study of the origin of colour and pleochroism in Pink and brown tourmalines. Can. Mineral. 9, 678–690 (1969).
Rossman, G. R. Spectroscopic and magnetic studies of ferric iron hydroxy sulfates: intensification of color in ferric iron clusters bridged by a single hydroxide ion. Am. Mineral. 60, 698–704 (1975).
Gibbons, R. V., Ahrens, T. J. & Rossman, G. R. A spectrographic interpretation of the Shock-Produced color change in Rhodonite(MnSiO3): the Shock-lnduced reduction of Mn(lll) to Mn(ll). Am. Mineral. 59, 177–182 (1974).
Acknowledgements
The experiments in this research were conducted in the Gemological Institute Laboratory, China University of Geosciences, Beijing. We would like to thank Ye Yuan, Yu Zhang, Mudan Xu, and Xingqi Zhao for their kind help in our experiments.
Author information
Authors and Affiliations
Contributions
P, L. conducted the experiments and wrote the original manuscript, P, L. and Y, G. performed statistical analysisand figure generation. The results were validated by Y, G. and P, L. The manuscript was reviewed by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, P., Guo, Y. The impact of mineral composition and trace metal cations on the body color of South African Sugilite Jade. Sci Rep 15, 23887 (2025). https://doi.org/10.1038/s41598-025-09281-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09281-8