Abstract
Heart rate recovery (HRR) and fast HRR, believed to reflect parasympathetic reactivation, have been recognized as powerful predictors of all-cause mortality. In this study we propose a modified fast HRR parameter T30HRR and investigate if otherwise healthy subjects with type 1 diabetes (T1D) have attenuated HRR. Eighteen T1D subjects (T1D = 15 ± 7 years, HbA1c = 58 ± 0.8 mmol/mol) and thirty-five healthy matched control subjects underwent maximal cardiopulmonary exercise test. After cessation of exercise T30, T30HRR and monoexponential decay as well as HRR at 10, 20, 30, 60, 120 and 300 s were assessed. The T1D subjects had diminished HRR described by T30HRR (12.6 ± 3.9 bpm vs. 16.4 ± 4.3 bpm, p = 0.013), T30 (520 ± 263 s vs. 361 ± 133 s, p = 0.022), HRR20 (9.2 ± 3.5 vs. 11.6 ± 3.1 bpm, p = 0.046), HRR30 (13.0 ± 4.5 bpm vs. 16.3 ± 4.2 bpm, p = 0.035) and HRR60 (23.0 ± 6.8 bpm vs. 28.4 ± 7.1 bpm, p = 0.041) when compared to the control subjects. Subjects with T1D exhibited attenuated fast HRR suggesting that vagal reactivation may be diminished, and exercise may unmask subclinical symptoms in otherwise healthy T1D subjects.
Similar content being viewed by others
Introduction
Heart rate recovery (HRR) is a parameter that is commonly used to describe how much heart rate (HR) decreases after cessation of exercise. The clinical relevance of HRR has been studied at different intensities and exercise settings in health and those with various health conditions, but also other triggers have been used, such as initial rise in HR after standing up in the orthostatic test1,2,3. HRR has been also used to monitor athletic performance and training status4. HRR has been commonly measured at 60–120 s and has been found to be powerful predictor of cardiovascular events, all-cause mortality and death2,5,6. In addition, fast HRR and especially 10-second HRR after cessation of exercise have been shown to be predictive for all-cause and coronary artery disease mortality7.
Both parasympathetic and sympathetic branches of the autonomic nervous system modulate HR. The fast HRR taking place immediately or shortly after cessation of exercise is mainly attributable to parasympathetic reactivation8. Whereas, the other important component, sympathetic withdrawal, becomes a potent modulator later in the recovery period9. While parasympathetic reactivation dominates usually in the first 30 s and sympathetic withdrawal later in the recovery period, both systems contribute to HRR during the entire recovery period indicating complex autonomic nervous system modulation which may vary with exercise intensity10. In traditional exercise settings, HR attenuates to steady-state condition in few minutes and resembles a mono-exponential or bi-exponential decay10,11,12.
As HRR is mainly attributable to the interplay between sympathetic and parasympathetic nervous system activations it is suitable to assess autonomic nervous system modulation for subjects at risk of cardiovascular events or with chronic diseases such as type 1 diabetes (T1D). HRR has been long considered a predictor of cardiovascular and all-cause death in diabetes group mainly consisting of type 2 diabetes patients13. In T1D, due to inadequate blood glucose control and elevated glycemic profile, long-term symptoms such as cardiovascular diseases and neuropathy may develop in time. These signs are often characterized as calcification of blood vessels, increase in resting blood pressure and sympathetic tonus, deterioration of parasympathetic activation and decrease in resting heart rate variability14. Pre or mild hypertension already increases sympathetic drive while also reduces the cardiac vagal drive and thus may have pronounced effects in T1D subjects15.
While the clinical significance of the traditional longer term HRR has been challenged by the fast HRR, shorter time span HRR parameters have suffered from poor reproducibility11,16,17. Exercise modality and intensity may reduce reproducibility but also environmental and methodological aspects may confound HRR parameters4.
In this study, we sought to investigate HRR and especially short-term HRR parameters during recovery after maximal cardiopulmonary exercise test (CPET) in T1D subjects and healthy controls. We present result of an alternative fast HRR parameter, which is a modification to T30 parameter earlier suggested by Imai et al.8. Finally, related to T1D, HRR may reveal subclinical alterations in T1D subjects when the cardiovascular system has been under acute exercise stress.
Materials and methods
In total 53 subjects, 18 subjects with T1D and 35 healthy controls, were enrolled to the study. Inclusion criteria for the T1D study group (DM, N = 18) were absence of major diabetes-related complications, history of T1D more than 3 years but less than 25 years, body mass index less than 35 kg/m2, age = 18–50 years (inclusive) and non-smoking status. Three subjects were on a mild hypertension medication (valsartan or valsartan/amlodipine) and cholesterol medication (rosuvastatin or atorvastatin) at the time of study visit. For the control group (CON, N = 35), the inclusion criteria were absence of all long-term chronic complications, overall good health, age between 18 and 50 years (inclusive), BMI between 18 and 35 kg/m2 and non-smoking status.
This study is part of the “Diabetes mellitus - Exercise and Stress” (DIAMES study, Project number 409/2019), which was approved by the Ethics Committee of Northern Savo Hospital District on 22nd of June 2021 and followed the principles of the Declaration of Helsinki. All subjects signed a dated written informed consent form before participation to any of the study assessments. This research study uses data from the passive head-up tilt test and CPET measurements. All subjects underwent the measurements in the same study visit and the measurement conditions were stable across tests and subjects.
Blood glucose and haemoglobin A1c (HbA1c, analysed with Cobas 8000 (c 702) – analyser; Hitachi High Technology Co) were taken at the beginning of the study visit. To exclude potential effects of hypoglycemia or hyperglycemia in the DM group, glucose level was kept between 5.0 and 13.9 mmol/L during the entire study visit. For CPET, appropriate means to control glucose level were taken according to guidelines18.
Subjects underwent a passive tilt test with 5-minute supine resting period and a 5-minute upright position at 70-degree angle. Resting values in supine position for systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and HR are reported. Results for the tilt test are reported elsewhere19. None of the T1D subjects experienced adverse events related to the orthostatic test or an orthostatic hypotension defined as a blood pressure drop of 20/10 mmHg in SBP/DBP respectively.
To reach maximal exertion, subject cycled an incremental exercise test protocol (3 min rest followed by 35 W/3 min for male and 25 W/3 min for female) to volitional fatigue using a cycle ergometer (Ergoline Ergoselect 200 K, ergoline GmbH, Bitz, Germany). During CPET, subjects performed inspiratory capacity maneuvers on each load. After cessation of exercise, Subject continued to cycle for 6 min at minimum load (35 W for male and 25 W for female) to record active recovery for HRR assessment. Two-lead ECG was recorded using ME6000 (Bittium, Oulu, Finland) with sampling frequency of 2000 Hz. Respiratory flow and gas analysis was measured using flow and gas analyzer (Metamax 3b-r2, Cortex-medical, Leipzig, Germany), which was calibrated before each measurement. Trained exercise physiologist supervised the test, ensured that the subject reached maximal intensity based on respiratory exchange ratio, oxygen uptake plateauing, and HR. Peak oxygen uptake was determined using 30-second window. Predicted values for peak oxygen uptake and maximum HR were derived according to Hansen et al.20 and 220 - age, respectively.
Heart rate recovery
Heartbeats were detected primarily from the Lead II ECG data using Kubios HRV software21. Beat detections were visually verified to ensure that only normal-to-normal heartbeat intervals (RR intervals) were included in HR assessment. The automatic beat correction algorithm proposed by Lipponen and Tarvainen22 was utilized for correcting abnormal beat intervals. A 5-minute recovery period was placed at the onset of recovery.
Following parameters were calculated from the recovery period: HRR after 10 (HRR10), 20 (HRR20), 30 (HRR30), 60 (HRR60), 120 (HRR120) and 300 (HRR300) seconds from the start of recovery period. In addition, decay constant of a single monoexponential fit (HRdecay), T30 by Imai et al.8 and a modified version of the T30 (T30HRR) were computed.
Maximum HR was calculated as the maximum HR of a 10 s moving average filtered HR data. HRR values were calculated based on mean of HR within window of ± 2.5 s for 10 s, 20 s and 30 s timepoints, and ± 5.0 s for 60 s, 120 s and 300 s timepoints. Short-term HRRs describe the fast HRR that is known to be mainly driven by parasympathetic reactivation. In contrast, longer-term HRRs are more influenced by sympathetic withdrawal, which is more dominant after 30–40 s from the start of recovery period.
Imai et al.8 suggested a parameter T30 to study fast HRR. The parameter is calculated as the negative reciprocal of the linear regression slope, when the line was fitted to natural logarithm transformed HR data within 30 s timespan. In addition, a single monoexponential fit has been used to characterize the curvature of the HR recovery by means of HRdecay. The non-linear exponential function was fitted using the damped least squares approach that is also known as the Levenberq-Marquadt algorithm. HRdecay describes how much time it takes to recover 67% of the HR. The monoexponential curve assumes that HR reaches a steady state in time. An example of the fitted curve is presented in the Fig. 1a.
Finally, we present a modified version of the T30 parameter (T30HRR) to overcome possible problems with least squares fitting and to enhance interpretability. Firstly, by fitting a linear line to a 30-second window of log-transformed HR repeatedly over the first 40-seconds, the steepest slope within the first 40 s after cessation of exercise is captured. This procedure reduces the potential bias of the linear fit, caused by delayed onset of HR recovery after cessation of exercise. Secondly, the interpretability of the T30 parameter was modified as the original T30 has units of seconds and a slower HRR is indicated by an increase in this parameter. In the computation of the T30HRR parameter after finding the steepest slope, the fitted values are reverse transformed to yield beats per minute interpretation. An example of the fitting the steepest slope is presented in the Fig. 1b. In the calculation of T30HRR, abnormal heartbeats are discarded within the analysis window.
Statistical analysis
Data are either presented as mean and standard deviation or as median and interquartile range (IQR). Demographics, blood pressure values and CPET data were assessed with the two-sample T-test assuming unequal variances. If the Anderson-Darling test yielded non-normal distribution for either of the groups, the Wilcoxon Rank-sum test was used to compare them.
Analysis of Covariance was used to compare HRR parameters between the groups, and supine rest SBP was considered as the covariate to account for potential confounding effect of blood pressure on HRR. Normality of the residuals was visually checked, and homogeneity of slopes was verified. For HRdecay parameter, the homogeneity of slopes was not met and thus separate regression slopes, to account the effect of Supine SBP, were fitted for each group. Significance level was set to 0.05 for all parameters. Statistical analyses were computed with MATLAB R2024b.
Results
The demographics are presented in Table 1 and indicate that the groups are comparable in terms of anthropometry, sex and age. The DM group possessed normal glucose level (9.0 ± 3.4 mmol/mol) at the time of study visit and overall good long-term glycemic profile as shown by HbA1c (58 ± 9 mmol/mol or 7.4 ± 0.8%). Blood pressure measurements and HR at supine rest and cardiopulmonary exercise test results are presented in Table 2. In Supine position, the DM group had higher SBP (131 ± 14 mmHg vs. 123 ± 12 mmHg, p value = 0.043) and MAP (95 ± 9 mmHg vs. 89 ± 8 mmHg, p value = 0.027) when compared to the CON group. The study groups were not statistically different in terms of oxygen uptake and HR parameters derived from the CPET.
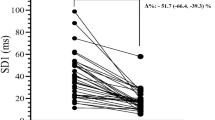
HRR results after cessation of exercise are presented in the Table 3 and the parameters are presented at the individual level in the Fig. 2. The DM group had attenuated fast HRR in terms of T30 (520 ± 263 s vs. 361 ± 133 s, p value = 0.022) and T30HRR (12.6 ± 3.9 bpm vs. 16.4 ± 4.3 bpm, p value = 0.013) when compared to the CON group. Also, HRR20 (9.2 ± 3.5 bpm vs. 11.6 ± 3.1 bpm, p value = 0.046), HRR30 (13.0 ± 4.5 bpm vs. 16.3 ± 4.2 bpm, p value = 0.035) and HRR60 (23.0 ± 6.8 bpm vs. 28.4 ± 7.1 bpm, p value = 0.041) suggest attenuated recovery in the DM group, whereas longer time span HRR parameters and HRdecay differences were not statistically significant.
Discussion
After cessation of the maximal cardiopulmonary exercise test, subjects with T1D exhibited attenuated fast HRR during recovery period when compared with the matched control subjects. While the sympathetic activity was elevated to the extreme level due to maximal exercise, HRR at 120 s and 300 s were similar between the groups, indicating similar HR recovery and sympathetic withdrawal at time spans when the sympathetic withdrawal is most prominent. The delayed fast HRR may be related to attenuated parasympathetic reactivation that is most dominant in the first 40 s, which is captured by the fast HRR parameters. While T1D subjects in this study were in general in good health and the diabetes was well-controlled, under maximal physical stress and in the presence of mild hypertension the underlying alterations in the autonomic nervous system may be unmasked.
The presented parameter T30HRR, T30 by Imai et al.8 HRR30 and HRR60 suggest that HRR rate is decreased shortly after cessation of exercise in the DM group. The difference between the DM group and the CON group is insignificant after 10 s but the difference becomes statistically significant later after 20-seconds and up to 60-seconds from the cessation of exercise. Vagal activity has been described to be most prominent in the first 30 s after cessation of exercise10; however, in an exercise setting such as repeated sprints, T30 parameter has been fitted from 10 s to 40 s after onset of recovery3. It is plausible that T30HRR, which captures the steepest descent within the first 40 s, may characterize the vagal reactivation better over other fast HRR parameters. Furthermore, the recovery period on the analysis phase was placed at the onset of HR decrease to diminish the possible carryover effect of sympathetic action and its confounding effect on T30 and HRRs.
In longer time span of 120–300 s, the groups exhibited similar HRR. Given that in the longer periods HRR is more affected by sympathetic withdrawal, the difference in vagal activation may be masked. This is supported by HRdecay indicating the overall HRR is similar between the groups. However, concerns on the validity of monoexponential fit in short recovery period have been raised by Bartels-Ferreira et al.12 and reproducibility of the measure by Fecchio et al.11. Also, maximal exercise and active recovery period increases and preserves sympathetic activity to the recovery period making the recovery trend likely less adequate for the first order exponential fit23.
The DM group had modestly elevated blood pressure in the supine rest, a sign pre-hypertension, which can be driven by multiple mechanism and interactions, especially by microalbuminuria that is known to be a major factor for hypertension24. Elevated blood pressure has been shown to delay HRR across the entire range of recovery period25,26. Moreover, slow HRR has been sensitive predictor of metabolic syndrome characterized as elevated blood pressure and fasting plasma glucose and dyslipidemia27. Thus, SBP was added as a confounding factor in the statistical analysis to mitigate the possible effect, albeit the T1D subjects were relatively young and had no diagnosed diabetes-related major complications such as microalbuminuria.
Previously, T1D subjects with relatively high HbA1c and with lower cardiorespiratory fitness than the control group have been reported to have diminished HRR at 1 min and to be associated with cardiac autonomic neuropathy28. This result is in line with our findings; however, T1D subjects in this study experienced no orthostatic hypotension or exercise intolerance and had normal supine HRV as reported by Sorola et al.19 Thus, it may be that fast HRR can be a potent marker for assessing subtle changes in vagal control. On the other hand, rats with T1D have exhibited exaggerated mechanoreflex response29. As the study used an active recovery period, T1D subjects might have had greater mechanoreceptor upregulation and thus group III afferent fiber activation which is known to contribute to the vagal control30. However, the active pedaling lasted throughout the recovery period, whereas HRR remained similar early on at 10 s and later from 120 s to 300 s. This suggests that the vagal reactivation occurred in T1DM, but it may have been delayed due to the active pedaling. Due to complexity of the vagal control, it remains unclear if the impaired or delayed vagal reactivation in T1D subjects could be attributed to the mechanoreceptor upregulation.
HRR may be an important clinical marker for T1D as studies on type 2 diabetes patients suggest that reduced HRR is a predictor of cardiovascular diseases and mortality13,31. In addition, HRR at post 1-minute exercise has predicted incident of type 2 diabetes in men and has been linked to reduced exercise capacity27,32. These findings suggest that HRR can be similarly impaired in T2D subjects as in T1D subjects reported by us and by Turker et al.28 and may be a consequence of autonomic dysfunction.
Fortunately, HRR can also be improved as long-term exercise interventions have shown positive effects in type 2 diabetes subjects and cardiac heart failure patients33,34. In contrast, also a short-term non-invasive technique called ischemic precondition has improved post-exercise vagal reactivation in endurance runners according to Sabino-Carvalho et al.35. Therefore, HRR may be of clinical relevance in T1D clinical practice and exercising can improve HRR, but the exercise modality, intensity, and reported parameters should be standardized to allow adequate use in the clinical practice.
Although the recent clinical relevance of fast HRR in predicting cardiovascular incidents and all-cause mortality and the support to include HRR to routine exercise testing, the reliability of the fast HRR parameters has not been optimal5,11,36. It can be argued that the shorter analysis timeframe is more susceptible to noise and bias. Fast HRR parameters can be more influenced by the analysis trigger point, i.e. the onset of recovery, as exercise modality and intensity may have varied sympathetic carryover effect to the recovery period. Also, traditional HRR measured as difference of HR between two timepoints, may be less sensitive if similar averaging has been used as for longer HRR time intervals. On the other hand, if the comparison would be done only on 1–5 heart beats, traditional HRR could be more prone to impulsive errors. With the T30HRR parameter, the calculation minimizes some of the possible sources of variability with the fast HRR parameters. T30HRR controls sympathetic carryover effect by finding the steepest 30 s descent in the first 40 s, assuming that this descent in HR is mainly attributable to the vagal reactivation. The parameter also uses all datapoints within the timespan instead of simply comparing maximal HR to the relative time point. Thus, noise at the relative timepoint induces less bias to the results. In contrast, T30HRR may be more susceptible to artefact beats in general as the regression line is fitted to a 30-second window. However, the effect of noise is minimized by omitting noisy heart beats during linear regression and due to linear regression fitting over different time windows within the first 40 s. In terms of Coefficient of Variation (CV) which describes variability of the parameter, T30HRR appears to be more robust parameter compared to the T30 in both study groups (T1D: T30 CV = 51% vs. T30HRR CV = 31%, CON: T30 CV = 37% vs. T30HRR CV = 26%). Finally, when compared to the T30, The T30HRR is reverse transformed to yield more attractive and understandable beats per minute units. However, reproducibility parameters of the results cannot be calculated with the study setup. Thus, further research is needed to elaborate if T30HRR could be more sensitive and reproducible than other fast HRR parameters.
The authors consider that there are some limitations in the study. The design of the study setting does not support comparison of parameters’ performance, and hence T30HRR cannot be adequately compared to the other fast HRR parameters. In addition, a different exercise protocol could have been more befitting for the study. In more detail, inspiratory capacity maneuvers during exercise may impact subject’s performance on CPET. In addition, the use of active recovery instead of passive recovery period impacts sympathetic withdrawal during recovery, but also influence vagal reactivation. This may affect both fast HRR and overall HRR, and thus reduce the interpretability of the findings related to parasympathetic modulation.
In this article, we have presented fast HRR parameters which suggest attenuated parasympathetic reactivation in T1D subjects after maximal volitional fatigue and proposed a new fast HRR parameter T30HRR. The proposed parameter is easy to interpret and captures the steepest 30-second descent in HR within the first 40 s of recovery mitigating the possible sympathetic carryover effect to the recovery period. While this study focused on T1D, improvements in fast HRR analysis can be utilized in other patient populations where recent findings support fast HRR analysis as predictor of major complications. Thus, T30HRR parameter can be used in other target populations and exercise modalities where investigation of HRR is recommended.
Data availability
The datasets analyzed during the current study are not publicly available due privacy statements but are available from the corresponding author on reasonable request.
References
McCrory, C. et al. Speed of heart rate recovery in response to orthostatic challenge. Circ Res 119, 666–675 (2016).
Cole, C. R., Blackstone, E. H., Pashkow, F. J., Snader, C. E. & Lauer, M. S. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl. J. Med 341, 1351–1357 (1999).
Buchheit, M., Laursen, P. B. & Ahmaidi, S. Parasympathetic reactivation after repeated sprint exercise. Am. J. Physiol. - Hear. Circ. Physiol. 293, 133–141 (2007).
Daanen, H. A. M., Lamberts, R. P., Kallen, V. L., Jin, A. & Van Meeteren, N. L. U. A systematic review on heart-rate recovery to monitor changes in training status in athletes. Int. J. Sports Physiol. Performance 7, 251–260 (2012).
Qiu, S. et al. Heart rate recovery and risk of cardiovascular events and all-cause mortality: A meta-analysis of prospective cohort studies. J Am. Heart Assoc 6(5):e005505 (2017).
Cole, C. R., Blackstone, E. H., Pashkow, F. J., Snader, C. E. & Lauer, M. S. Heart rate recovery immediately after exercise as a predictor of mortality. J. Cardiopulm. Rehabil. 20, 131–132 (2000).
van de Vegte, Y. J., van der Harst, P. & Verweij, N. Heart rate recovery 10 seconds after cessation of exercise predicts death. J Am. Heart Assoc 7(8):e008341 (2018).
Imai, K. et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J. Am. Coll. Cardiol. 24, 1529–1535 (1994).
Perini, R. et al. Plasma norepinephrine and heart rate dynamics during recovery from submaximal exercise in man. Eur J. Appl. Physiol. Occup. Physiol 58, 879–883 (1989).
Pierpont, G. L., Adabag, S. & Yannopoulos, D. Pathophysiology of exercise heart rate recovery: a comprehensive analysis. Ann Noninvasive Electrocardiol 18, 107–117 (2013).
Fecchio, R. Y., Brito, L., Leicht, A. S., Forjaz, C. L. M. & Peçanha, T. Reproducibility of post-exercise heart rate recovery indices: A systematic review. Auton Neurosci. Basic. Clin 221, 102582 (2019).
Bartels-Ferreira, R. et al. Can a first-order exponential decay model fit heart rate recovery after resistance exercise? Clin. Physiol. Funct. Imaging. 35, 98–103 (2015).
Cheng, Y. J. et al. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 26, 2052–2057 (2003).
Pop-Busui, R. Cardiac autonomic neuropathy in diabetes: A clinical perspective. Diabetes Care. 33, 434–441 (2010).
Mancia, G. & Grassi, G. The autonomic nervous system and hypertension. Circ. Res. 114, 1804–1814 (2014).
Tonello, L. et al. Correlates of heart rate measures with incidental physical activity and cardiorespiratory fitness in overweight female workers. Front. Physiol. 6, 1–11 (2016).
Dupuy, O. et al. Reliability of heart rate measures used to assess post-exercise parasympathetic reactivation. Clin Physiol. Funct. Imaging 32, 296–304 (2012).
Colberg, S. R. et al. Physical activity/exercise and diabetes: A position statement of the American diabetes association. Diabetes Care. 39, 2065–2079 (2016).
Sorola, S. et al. Orthostatic test shows higher systolic blood pressure and sympathetic response in uncomplicated type 1 diabetes patients with normal V ̇ O 2max vs. healthy controls. Clin. Auton. Res. 35, 381–391 (2025).
Hansen, J. E., Sue, D. Y. & Wasserman, K. Predicted values for clinical exercise testing. Am Rev. Respir Dis 129, S49–55. (1984).
Tarvainen, M. P., Niskanen, J. P., Lipponen, J. A., Ranta-aho, P. O. & Karjalainen, P. A. Kubios HRV - Heart rate variability analysis software. Comput. Methods Programs Biomed. 113, 210–220 (2014).
Lipponen, J. A. & Tarvainen, M. P. A robust algorithm for heart rate variability time series artefact correction using novel beat classification. J. Med. Eng. Technol. 43, 173–181 (2019).
Pierpont, G. L., Stolpman, D. R. & Gornick, C. C. Heart rate recovery post-exercise as an index of parasympathetic activity. J Auton. Nerv. Syst 80, 169–174 (2000).
Jia, G. & Sowers, J. R. Hypertension in diabetes: an update of basic mechanisms and clinical disease. Hypertension 78, 1197–1205 (2021).
Aneni, E. et al. Delayed heart rate recovery is strongly associated with early and late-stage prehypertension during exercise stress testing. Am J. Hypertens 27, 514–521 (2014).
Lou, J., Shi, W. R., Dong, Y., Jin, Y. P. & Guo, X. G. The impact of delayed heart rate recovery on prevalent hypertension. Postgrad Med 133, 362–368 (2021).
Yu, T. Y. et al. Delayed heart rate recovery after exercise predicts development of metabolic syndrome: A retrospective cohort study. J. Diabetes Investig. 13, 167–176 (2022).
Turker, Y. et al. Heart rate variability and heart rate recovery in patients with type 1 diabetes mellitus. Acta Cardiol 68, 145–150 (2013).
Grotle, A. K. et al. Exaggerated mechanoreflex in early-stage type 1 diabetic rats: role of piezo channels. Am J. Physiol. - Regul. Integr. Comp. Physiol 316, R417–R426 (2019).
Nóbrega, A. C. L. & Araújo, C. G. S. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci. Sports Exerc 25, 37–41 (1993).
Sydó, N. et al. Impaired Heart Rate Response to Exercise in Diabetes and Its Long-term Significance. Mayo Clin. Proc. 91, 157–165 (2016).
Fang, Z. Y., Sharman, J., Prins, J. B. & Marwick, T. H. Determinants of exercise capacity in patients with type 2 diabetes. 28, 1643–1648 (2005).
Myers, J. et al. Effects of exercise training on heart rate recovery in patients with chronic heart failure. Am Heart J 153, 1056–1063 (2007).
Ribisl, P. M. et al. Lifestyle intervention improves heart rate recovery from exercise in adults with type 2 diabetes: results from the Look AHEAD Study. 2012:309196 (2012).
Sabino-Carvalho, J. L. et al. Ischemic preconditioning boosts post-exercise but not resting cardiac vagal control in endurance runners. Eur J. Appl. Physiol 119, 621–632 (2019).
Dewar, A., Kass, L., Stephens, R. C. M., Tetlow, N. & Desai, T. Heart rate recovery assessed by cardiopulmonary exercise testing in patients with cardiovascular disease: relationship with prognosis. Int J. Environ. Res. Public. Health 20(6):4678 (2023).
Acknowledgements
The authors express their gratitude to biomedical laboratory scientist Kirsi Saastamoinen, clinical nurse Tuula‐Riitta Mutanen, and HUMEA laboratory (www.uef.fi/humea).
Funding
This study was (funded through an EFSD award) supported by EFSD/JDRF/Lilly and by the Finnish Diabetes Foundation.
Author information
Authors and Affiliations
Contributions
MT, HT, VH, TE and MV participated to conception and design of the study. TE, SK and VH collected the data. VH and TE prepared the data for analysis. VH performed analysis and prepared tables and figures for the manuscript. VH drafted the first manuscript. All authors participated to the manuscript review process and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study is part of the “Diabetes mellitus - Exercise and Stress” (DIAMES study, Project number 409/2019), which was approved by the Ethics Committee of Northern Savo Hospital District on 22nd of June 2021.
Patient consent
All subjects signed a dated written informed consent form before participation to any of the study assessments.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hyrylä, V.V., Eronen, T., Kupari, S. et al. Attenuated fast heart rate recovery suggests delayed parasympathetic reactivation after cessation of exercise in uncomplicated type 1 diabetes patients. Sci Rep 15, 24136 (2025). https://doi.org/10.1038/s41598-025-09287-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09287-2