Abstract
The short-term conversion rate of a non-exudative macular neovascularization (ne-MNV) to an exudative stage in age-related macular degeneration (AMD) was evaluated in a single-center, retrospective case control study and associated imaging characteristics described, using multimodal imaging. A total of 241 Caucasian patients with unilateral, treatment-naive exudative neovascular AMD were screened for the presence of a ne-MNV in the fellow eye between March 2016 and May 2022. Eyes with a confirmed ne-MNV on indocyanine green angiography and/or optical coherence tomography angiography (OCTA) were monitored using structural OCT for signs of exudation. The main outcome was to evaluate multimodal imaging biomarkers to identify predictors of exudative conversion.
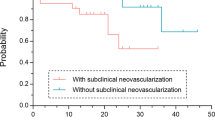
Among 241 study eyes, 40 (16.6%) showed ne-MNV at baseline, all classified as type 1 MNV. During follow-up, 13 patients (32.5%) progressed to an exudative stage, with a mean time to exudation of 12.6 months (range: 2.6–22.4 months). The presence of a shallow irregular retinal pigment epithelial elevation (SIRE) was the only statistically significant feature associated with an increased risk of conversion to exudation. (p = 0.012) Patients with ne-MNV and a baseline SIRE are at increased risk for exudation. SIRE can be easily identified and followed up with structural OCT, providing a valuable marker for monitoring ne-MNV activation.
Similar content being viewed by others
Introduction
Nonexudative macular neovascularization (ne-MNV) is defined as the presence of a treatment-naive vascular tissue ingrowth into the outer retina or sub-retinal pigment epithelial (RPE) space without evidence of exudation.1 In the 1970s, histopathological studies already documented cases of clinically dry age-related macular degeneration (AMD) with underlying neovascularization in post-mortem eyes. This was later confirmed in vivo by indocyanine green angiography (ICGA) in the 1990s. However, due to the invasive nature and limited accessibility of ICGA, this entity initially received limited attention. 2,3
The advent of Optical Coherence Tomography (OCT), and particularly OCT-Angiography (OCTA), has rekindled interest in ne-MNV. Numerous studies now aim to identify OCT and OCTA-based biomarkers that might predict the progression of a nonexudative AMD to an advanced, exudative stage. 4,5 A shallow irregular RPE elevation (SIRE), which can be regarded as a subtype of the double layer sign (DLS), was highly associated with ne-MNV. The combination of enface OCTA and B-scan with flow further increases the sensitivity and specificity for diagnosing ne-MNV. 6 Furthermore, the dimensional growth of the neovascular lesion was identified as the most critical indicator for future exudation and a greater vascular density at baseline as well as a hypointense halo around the lesion were associated with a higher risk of exudation. 6,7,8,9
As a diagnosed ne-MNV represents a strong risk factor for future exudation, the incidence of its exudative conversion is of high clinical interest. Recent studies reveal substantial variability in conversion rates, ranging from 6.6 to 80.0%. 9,10,11 The wide range in reported conversion rates likely reflects differences in inclusion criteria, imaging technologies, definitions of nonexudative MNV and exudation, as well as variability in follow-up duration and study design across cohorts.12 This raises the question of whether specific characteristics seen in OCT are associated with exudative progression in ne-MNV.
This study, therefore, aims to investigate the conversion rate from nonexudative to exudative MNV within a predefined patient cohort confirmed to have ne-MNV by both ICGA and OCTA. Additionally, we examined whether certain OCT-based biomarkers are more prevalent in patients who undergo exudative conversion, assessing their predictive potential.
Methods
In this retrospective study, all patients diagnosed with unilateral ne-MNV were screened for specific characteristics and their conversion rate to exudation at the tertiary medical retina unit, Clinic Landstraße (Vienna Healthcare Group, Vienna, Austria) between March 2016 and May 2022. Enrolled patients presented with exudative AMD in one eye, while the fellow eye had a dry AMD. The study adhered to the Declaration of Helsinki, and all patients provided informed consent. Ethics committee rules that approval was not required for this study, as stated in the Federal Hospitals Act §15a Abs. 3a.
Clinical examinations
On the study day, each patient underwent a comprehensive ophthalmic evaluation by a senior medical retina specialist. This included best corrected visual acuity measurement, dilated slit-lamp biomicroscopy, indirect fundus examination (Haag-Streit AG, Switzerland), Spectral-Domain (SD)-OCT (Carl Zeiss Meditec AG, Germany), ICGA (Spectralis HRA, Heidelberg, Germany) and Swept Source (SS)-OCTA (DRI OCT Triton Plus; Topcon Corporation, Tokyo, Japan). In addition, the device automatically takes a color fundus photograph after OCTA image acquisition is completed. Macular cubes with dimensions of 4.5 × 4.5 mm, 6 × 6 mm, or 9 × 9 mm were captured centered on the fovea and eye tracking was used to maintain fixation across all devices.
Diagnosis of ne-MNV
At the initial presentation, images of all patients with a unilateral exudative nAMD diagnosis were reviewed for the presence of a ne-MNV and the workflow is outlined in Fig. 1. Ne-MNV was identified through multimodal imaging by two medical retina experts, either showing a plaque on ICGA or a neovascular network or double layer sign (DLS) with flow on SS-OCTA. For identification of a DLS, the corresponding OCT B-scan was evaluated with a color-coded B-scan overlay to detect increased flow signals. Automated segmentation was utilized, with manual adjustments made only when the MNV network could otherwise not be visualized to its full extend. In case of disagreement between the two graders over the presence of a ne-MNV, a senior retina specialist provided the final assessment.
For patients with identified ne-MNV, each follow-up OCT B-scan was reviewed to identify intraretinal or subretinal fluid indicative of exudation. The time to exudation was calculated as the interval between initial examination and the date of follow-up where exudation was detected for the first time. ICGA and OCTA were not routinely repeated during follow-up, as treatment decisions were based on the presence of fluid on structural OCT.
Imaging characteristics
For each patient, OCT B-scans and color fundus photography were evaluated to identify characteristics such as the presence of large drusen (> 125 μm), retinal pigment epithelium (RPE) pigmentary abnormalities, subretinal drusenoid deposits (SDD), retinal pigment epithelial and outer retinal atrophy (RORA), a DLS, a SIRE, a pigment epithelial detachment (PED), subretinal hyperreflective material (SHRM) and intraretinal hyperreflective dots (HRD) as well as the subfoveal choroidal thickness measured. (Fig. 1) Baseline neovascular lesion size on ICGA and OCTA was compared between the eyes that developed exudation and those that did not. Unlike drusen, which are an accumulation of deposit below the RPE, SDDs are located between the RPE and neurosensory retina. 13 RORA was further subdivided into incomplete and complete RPE and outer retinal atrophy (iRORA and cRORA), following the Classification of Atrophy Meetings (CAM) group.14 For patients with multiple RORA regions, the atrophy with the largest diameter was measured. To distinguish SIRE from drusenoid PED, the following criteria had to be fulfilled: The length and height of the RPE elevation had to be more than 1000 μm and less than 100 μm, respectively, and was defined as SIRE by Narita et al.15 RPE elevations not meeting these criteria and lacking drusenoid characteristics were considered as classical DLS. Additional morphologic OCT-based characteristics identified included SHRM, defined as hyperreflective material in the subretinal space, and HRD, being well-defined, hyperreflective punctate lesions scattered across all retinal layers.16,17 Subfoveal choroidal thickness was measured vertically from the outer border of the hyperreflective line of the RPE/Bruch membrane band to the sclera-choroidal junction. All data were collected via charts for further analysis.
Patients were excluded if the MNV outline exceeded the scan area, images displayed severe motion artifacts, or intravitreal injection had been administered previously. The interval for follow-up visits was individualized by the specialist and was not preset by a protocol. Patients were routinely seen every 8 to 12 weeks, due to exudation in the partner eye.
Statistical analysis
Statistical analysis was performed using SPSS Statistics Version 27 (IBM, Chicago, Ill., USA). To determine statistically significant differences between categorical variables, the χ2 test was used. The effect size was assessed using Phi or Cramer-V. For significant differences and for tables larger than 2 × 2, post-hoc tests were performed using Microsoft Office Excel. For subfoveal choroidal thickness, as the only metric variable, normal distribution was determined by Shapiro–Wilk test followed by pairwise comparison using Mann–Whitney U test. Baseline MNV lesion sizes between exudation and non-exudation groups were compared using the Mann–Whitney U test, given the non-normal distribution. A p-value < 0.05 was considered statistically significant. To correct for multiple comparisons, p-values were adjusted using the Bonferroni method.
Results
A total of 241 patients with unilateral exudative nAMD were screened for ne-MNV in the fellow eye. Among the exudative eyes (n = 241), 183 (75.9%) were classified as type 1 MNV, 18 (7.5%) as type 2, 14 (5.8%) as type 3, and 26 (10.8%) as mixed-type lesions. Ne-MNV in the fellow eye was identified in 40 patients (16.6%), who formed the study cohort. The mean age of these patients was 78.6 ± 7.1 years, with 52.5% being female and equally balanced laterality of right and left eyes (45.0% vs 55.0%). A ne-MNV was detected by both ICGA and OCTA, only on ICG or only on OCTA in 25 (62.5%), 7 (17.5%) and 8 (20.0%) patients, respectively. In cases where ne-MNV was identified by only one modality (ICGA or OCTA), the diagnosis was made based on characteristic imaging features—such as sub-RPE localization and a plaque-like appearance on ICGA or flow signal under the RPE on OCTA. All patients had type 1 MNV, and all fellow eyes were under ongoing anti-VEGF treatment throughout the observation period. Patients with identified ne-MNV were followed for a mean duration of 34.6 months (range: 2.8–71 months), with most patients (90.0%) followed for over one year. During this period, 13 patients (32.5%) developed subsequent exudation with a mean time to exudation of 12.6 months (range: 2.6–22.4 months). Further categorization of the time to exudation revealed that one patient (7.7%) converted in less than 3 months, five patients (38.5%) converted after more than one year, and 7 patients (53.8%) converted between 3 months and one year. Throughout the observation period, exudation presented as subretinal fluid in 7 (53.8%), intraretinal fluid in 4 (30.8%), and both subretinal and intraretinal fluid in 2 (15.4%) eyes.
Table 1 lists the OCT and color fundus photography-based characteristics and their p-values for patients with ne-MNV, comparing those who developed exudation with those who remained inactive throughout the study. The presence of a SIRE was the only characteristic significantly associated with later exudation (p = 0.012). Patients with a SIRE who developed exudation did not show a significantly earlier time to exudation compared to those without a SIRE. (12.7 months versus 15.2 months; p = > 0.05) All patients with RORA had at least one area of atrophy greater than 250 μm, classifying them as cRORA.
Discussion
The detection of ne-MNV and the risk of exudation have become much-debated topics in ophthalmology. Since the advent of OCTA, non-invasive identification of these lesions has become widely accessible, allowing OCT-based features to be studied for a better understanding of ne-MNV’s natural history. In this retrospective observational study, we identified 40 cases of unilateral ne-MNV among 241 patients with unilateral nAMD in the fellow eye, resulting in a prevalence of 16.6%. The conversion rate to exudation was 32.5%, with a mean time to activation of 12.6 months, and a significant association with the presence of a SIRE was demonstrated.
Identifying predictive OCT biomarkers to assess the risk of ne-MNV exudation is of utmost importance, not only for individualized patient follow-up but also for initiating treatment at the earliest stage. In our study, the presence of a SIRE was the only statistically significant predictor of subsequent exudation, although the interval to activation was not significantly shorter in eyes with SIRE compared to those without. This suggests that SIRE may function more as a binary risk indicator, its presence conferring a higher likelihood of exudation, but not necessarily as a temporal predictor for when exudation will occur. First described by Narita et al. as an extension of the DLS, SIRE has been associated with an increased likelihood of ne-MNV. 15 SIRE is characterized by a shallow, irregular elevation of the RPE and may reflect underlying neovascular tissue with immature, permeable vasculature that predisposes to leakage. In contrast, DLS without the shallow and irregular morphology may represent a more quiescent neovascular complex with intact RPE and Bruch’s membrane barriers, corresponding to a lower short-term risk of exudation. This morphological distinction suggests that SIRE may serve as an OCT correlate of an early, evolving neovascular process. Our findings suggest that patients presenting with SIRE should not only be screened for the presence of ne-MNV with OCTA or ICGA, but if confirmed, should also be followed up with strict and continuous monitoring, as exudation seems more likely in these patients. (Fig. 2) Alongside lesion growth, which is considered the most critical indicator of future exudation, the individual risk of exudative conversion can be readily assessed using structural OCT alone, allowing for personalized follow-up.6 Other potential OCT and OCTA biomarkers reported to predict exudative activity in neovascular AMD include increase in central macular thickness, increase in PED height and width, higher perfusion density at baseline, emergence of branching pattern and appearance of a hypointense halo surrounding the lesion.7,9 However, these features are much more time-consuming and complex to assess in everyday clinical routine.
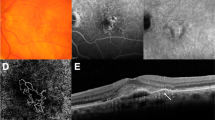
A 79-year-old male patient with non-exudative macular neovascularization (ne-MNV) in the right eye that became active within three months of initial presentation. (a) Swept-source OCT angiography (SS-OCTA) showing a 0.79 mm2 in a 4.5 × 4.5 mm scan corresponding to the color-coded B-scan with semi-automated segmentation lines (blue). The green line measures a shallow irregular retinal pigment epithelial elevation (SIRE) of 1284 μm in length. Above the SIRE a subretinal hyperreflective material (SHRM) is visible, which (b) persists throughout the follow-up. The corresponding OCT B-scan from the spectral-domain device is shown in panel B to improve visualization of the SIRE. (c) After 80 days this ne-MNV becomes active with subretinal fluid slightly inferior to the foveal umbo and was treated with anti-vascular endothelial growth factor (anti-VEGF).
In this study, 32.5% of eyes with a confirmed diagnosis of ne-MNV developed exudation, with a mean time to exudation of 12.6 months—consistent with conversion rates reported in other studies, which range from 6.6 to 80.0%.4,7,10,11,12,18,19,20,21 At one year, De Oliveira Dias et al. reported an incidence of exudation of 24.0%, which aligns with our finding of a 20% conversion within one year, when the patient cohort is analyzed in a subgroup of patients with at least on year follow-up. De Oliveira Dias et al. reported a progressive increase in exudation risk over time, with a conversion rate of 24.0% at one year and 34.5% at two years. 20 Our overall conversion rate of 32.5%, observed over a longer and variable follow-up period, is similar in magnitude. However, in contrast to their findings, most exudation events in our cohort occurred within the first year of follow-up (53.8%), while only five patients converted after one year. These findings highlight the importance of intensive monitoring particularly during the first 12 months following ne-MNV detection. Bailey et al.11 reported a two-year rate as high as 80.0%, the only one so far to report such a high conversion to exudation of ne-MNV. Interestingly, this is in complete contrast to the results of Amaresekera et al., who described 15.0% of patients progressing to an exudative form, with the longest observation period of at least 4 consecutive years. 4 Due to this wide range described in the literature, the timing of ne-MNV activation remains unclear. However the comparison of shorter (less than two years) and longer (more than two years) observation intervals suggests that longer persistence of ne-MNV reduces the risk of activation. This finding may also support the idea of arteriogenesis versus angiogenesis in the development of MNV, with a short-term activation of ne-MNV rather being driven by the process of angiogenesis and a long-term non-exudation of ne-MNV being mainly driven by arteriogenesis.6 Intensive monitoring within the first two years is therefore recommended for newly diagnosed ne-MNV, with the suggestion of extended intervals after this period.
In this study, we didn’t classify the presence of SHRM as a sign of exudation, limiting exudation indicators to intra- or subretinal hyporeflective fluid on OCT. Sacconi et al. described SHRM as exudative in their review of ne-MNV, but did not define further characteristics and whether all cited studies also considered SHRM as exudation. 6 In fact, Shah et al. described different types of SHRM and described that it could be a sign of active nAMD, but could also be neovascular tissue, subretinal fibrosis or lipid, among others.22 Dansingani et al. proposed that OCTA could distinguish vascular from avascular components of SHRM, but acknowledged that their findings need to be confirmed in a reading centre setting.23 In our study, SHRM was seen in three patients with ne-MNV, but only one subsequently developed exudation in the form of intraretinal fluid after 2 months (Fig. 2). The other two patients, including the case depicted in Fig. 3, showed no exudation during follow-up periods of 18 months and over 3 years, respectively. Notably, this case exhibited SHRM in the absence of a SIRE, highlighting that SHRM can occur in ne-MNV without associated SIRE. These findings underscore the need for further studies investigating SHRM subtypes, as early initiation of anti-VEGF treatment is known to improve visual outcomes.24.
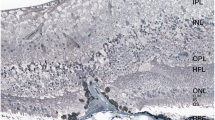
A patient with non-exudative macular neovascularization (ne-MNV) and subretinal hyperreflective material (SHRM) showing no activation during more than three years of follow-up. (a) Early phase of a fluorescence angiography (FA) with a spectral-domain (SD)-OCT scan through the foveal umbo, showing the SHRM. (b) The ne-MNV is visible in the indocyanine green (ICG) intermediate phase, (c) but was not detected with SS-OCTA (d) Follow-up SD-OCT of the SRHM, shows no fluid (e) but increases over the course of a three-year follow-up.
The strengths of this study include the relatively large sample size, given that nonexudative MNV is a relatively rare condition. Our cohort of 40 confirmed cases represents one of the largest single-center series described to date, comparable to or exceeding the sample sizes reported in prior studies. 10,11,20 Furthermore, all patients underwent both OCTA and/or ICGA at baseline, ensuring robust multimodal imaging confirmation. In addition, we deliberately focused on imaging biomarkers that are simple, practical, and quick for clinicians to assess in everyday clinical settings, allowing longitudinal monitoring with structural OCT alone at regular follow-up visits. Most patients were followed for more than one year, supporting an evaluation of mid- to long-term conversion rates. Short-term exudation, defined as occurring within six months, was observed in only one patient, and apart from two patients with a follow-up of three months, all patients had at least six months of follow-up.
A key limitation of our study is its retrospective nature, including the assessment of imaging biomarkers only at baseline and the absence of a prespecified minimum follow-up period. Furthermore, once exudation was detected on OCT, anti-VEGF treatment was promptly initiated according to clinical guidelines; thus, our study was not designed to evaluate fluid resolution over time or treatment response outcomes. We acknowledge that additional biomarkers, such as vascular density, and branching pattern on OCTA or late-phase ICGA features could further inform progression risk. However, these parameters were not assessed in this study, as ICGA was not routinely repeated during follow-up and quantitative OCTA metrics were not consistently available retrospectively. Our study focused instead on readily accessible structural OCT features applicable in routine clinical settings. Another potential limitation is that all fellow eyes were under ongoing anti-VEGF treatment, raising the possibility of a systemic contralateral effect. While the clinical relevance of such an effect remains debated, it may have influenced the natural history of ne-MNV in the study eyes and could partially account for the moderate conversion rate observed. 25 Future prospective studies incorporating a broader set of biomarkers and standardized imaging intervals are warranted to validate and expand upon these findings.
In conclusion, the prevalence of ne-MNV in this study was 16.6%, of which 32.5% progressed to an exudative state. The presence of a SIRE in patients with ne-MNV was statistically significantly associated with an increased risk of exudation and can be considered a predictive marker. Although it remains challenging to predict if and when ne-MNV will become active over time, the identification of reliable biomarkers may enhance patient management. Prophylactic anti-VEGF treatment is not recommended for ne-MNV, however treatment should be initiated as soon as exudation occurs. 26 Therefore, large multicenter studies, including all ne-MNV subtypes, focusing on the biomarkers described to date are warranted.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Spaide, R. F. et al. Consensus nomenclature for reporting neovascu lar age-related macular degeneration data: Consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology 127, 616–636 (2020).
Sarks, S. H. New vessel formation beneath the retinal pigment epithelium in senile eyes. Br. J. Ophthalmol. 57, 951–965 (1973).
Hanutsaha, P. et al. Indocyanine-green videoangiography of drusen as a possible predictive indicator of exudative maculopathy. Ophthalmology 105, 1632–1636 (1998).
Amissah-Arthur, K. N., Panneerselvam, S., Narendran, N. & Yang, Y. C. Optical coherence tomography changes before the development of choroidal neovascularization in second eyes of patients with bilateral wet macular degeneration. Eye (Lond) 26, 394–399 (2012).
Querques, G. et al. Functional characterization and multimodal imaging of treatment-naive ‘quiescent’ choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 54, 6886–6892 (2013).
Sacconi, R. et al. Towards a better understanding of non-exudative choroidal and macular neovascularization. Prog. Retin. Eye Res. 92, 101113 (2023).
Querques, G. et al. Treatment-naïve quiescent macular neovascularization secondary to AMD: The 2019 young investigator lecture of macula society. Eur. J. Ophthalmol. 31, 3164–3176 (2021).
Teo, K. Y. C., Yanagi, Y., Wong, T. Y. & Charkaravarty, U. Gemmy Cheung CM morphologic predictors and temporal characteristics of conversion from nonexudative to exudative age-related macular degeneration in the fellow eye. Ophthalmol. Retina 5, 126–140 (2021).
Solecki, L. et al. Predictive factors for exudation of quiescent choroidal neovessels detected by OCT angiography in the fellow eyes of eyes treated for a neovascular age-related macular degeneration. Eye (Lond) 35, 644–650 (2021).
de Oliveira Dias, J. R. et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology 125, 255–266 (2018).
Bailey, S. T. et al. Detection of nonexudative choroidal neovascularization and progression to exudative choroidal neovascularization using OCT angiography. Ophthalmol. Retina 3, 629–636 (2019).
Laiginhas, R., Yang, J., Rosenfeld, P. J. & Falcão, M. Nonexudative macular neovascularization—A systematic review of prevalence, natural history, and recent insights from OCT angiography. Ophthalmol. Retina 4, 651–661 (2020).
Spaide, R. F., Ooto, S. & Curcio, C. A. Subretinal drusenoid deposits AKA pseudodrusen. Surv. Ophthalmol. 63, 782–815 (2018).
Guymer, R. H. et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: Classification of atrophy meeting report 4. Ophthalmology 127, 394–409 (2020).
Narita, C. et al. Structural OCT signs suggestive of subclinical nonexudative macular neovascularization in eyes with large drusen. Ophthalmology 127, 637–647 (2020).
Metrangolo, C. et al. OCT biomarkers in neovascular age-related macular degeneration: A narrative review. J. Ophthalmol. 2021, 9994098 (2021).
Coscas, G. et al. Hyperreflective dots: A new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica 229, 32–37 (2013).
Heiferman, M. J. & Fawzi, A. A. Progression of subclinical choroidal neovascularization in age-related macular degeneration. PLoS ONE 14, e0217805 (2019).
Yanagi, Y. et al. Incidence of fellow eye involvement in patients with unilateral exudative age-related macular degeneration. JAMA Ophthalmol. 136, 905–911 (2018).
Yang, J. et al. Two-year risk of exudation in eyes with nonexudative age-related macular degeneration and subclinical neovascularization detected with swept source optical coherence tomography angiography. Am. J. Ophthalmol. 208, 1–11 (2019).
Stattin, M. et al. Optical coherence tomography and OCT angiography characteristics of indocyanine green angiographic plaques in nonexudative age-related macular degeneration. Retina 43, 16–24 (2023).
Shah, V. P., Shah, S. A., Mrejen, S. & Freund, K. B. Subretinal hyperreflective exudation associated with neovascular age-related macular degeneration. Retina 34, 1281–1288 (2014).
Dansingani, K. K. et al. Subretinal hyperreflective material imaged with optical coherence tomography angiography. Am. J. Ophthalmol. 169, 235–248 (2016).
Monés, J., Bandello, F., Souied, E., Liu, X. & Gale, R. Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world: The need for a change of mindset. Ophthalmologica 243, 1–8 (2020).
Avery, R. L. et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 98, 1636–1641 (2014).
Lim, J. H. et al. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am. J. Ophthalmol. 153(678–686), 686.e1–2 (2012).
Author information
Authors and Affiliations
Contributions
AMH: Conceptualization; Data curation; Formal analysis, Investigation; Methodology, Writing—original draft. SK: Data curation, Writing—review & editing. MS: Conceptualization; Validation, Writing—review & editing. DAB: Validation. MJ: Validation. KK: Supervision. SAS: Conceptualization, Supervision, Writing—review & editing.
All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Haas, AM., Kickinger, S., Stattin, M. et al. Short-term natural history of macular neovascularization in nonexudative age-related macular degeneration using multimodal imaging. Sci Rep 15, 24070 (2025). https://doi.org/10.1038/s41598-025-09348-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-09348-6