Abstract
This study aimed to explore the effects of a single bout of moderate-intensity continuous exercise (MICE) and high-intensity intermittent exercise (HIIE) on sleep in inactive female healthcare workers with mild sleep impairment during continuous night-shifts. A total of 14 subjects (mean age: 31.8 ± 8.3 years) were randomized to either a MICE (70 ~ 75% of the maximum heart rate (MaxHR) for 47 min), HIIE (90 ~ 95% of the MaxHR for 4 min and 50 ~ 70% for 3 min, for 4 rounds, total 28 min), or the quiet-rest control, 14 h before undertaking a continuous night shift. Sleep quality was assessed subjectively by a single question score, and objectively by ActiGraph for one night before and 3 sleeps after exercise with a heart rate variability monitor. After the intervention, MICE resulted in a significant improvement in subjective sleep quality (P = 0.035), earlier bedtime (P < 0.001), and more total sleep time (P = 0.028) compared with the control trial (CTL). Compared with MICE, the HIIE had a greater reduction in sleep efficiency by 7.39% (P = 0.010) and had a greater increase in wake after sleep onset percentage (increase 10%, P = 0.049), sleep fragmentation index (increase 2%, P = 0.001), and a modest increase in mean activity during TIB (increase 35.49, P = 0.035). Regarding HRV, HIIE trial showed an increased LF/HF following intervention while CTL decreased. A single session of MICE preserved subjective sleep quality and promoted an earlier bedtime prior to the first night shift for female healthcare workers experiencing mild sleep disturbances during night shifts. However, compared with MICE, a single session of HIIE had a detrimental effect on sleep maintenance and increased sympathetic activity, even affecting sleep before the second night shift.
Trial registration NCT06638359. Registered 15 October 2024 Retrospectively registered, https://register.clinicaltrials.gov.
Similar content being viewed by others
Introduction
Sleep impairment is a common problem among night shift workers1. The prevalence of insomnia in shift workers ranged from 12.8 to 76.4%, higher than estimated for the general population1. Moreover, a higher prevalence was observed among women1,2. Associations between sleep impairment and deleterious health outcomes have been established, including increased risk of mortality3diabetes4metabolic syndrome5dementia6depression, and anxiety7as well as impaired quality of life8. The health risks associated with day and night-shifts for healthcare workers have been well documented9which in turn has led to a reduction in the retention rate of healthcare workers who work shifts10. Furthermore, the global demand for shift workers is increasing11and shift workers other than healthcare workers will also encounter such health problems.
In contrast, exercise has various health benefits, such as reducing the risk of cardiovascular disease and diabetes12and improving mental health13,14. Additionally, several reviews suggest that exercise effectively improves sleep quality and reduces sleep complaints15,16. A scoping review demonstrated that diverse types of physical activity were effective for improving sleep in many populations, even in the elderly and in co-morbid or perinatal populations17. However, little research has been conducted on shift worker populations.
Research has consistently shown that physical activity can positively influence sleep architecture, including increasing slow wave sleep and total sleep time, and decreasing sleep onset latency and REM sleep18. A meta-analytic review confirmed that both acute and chronic exercise produce these benefits18. Even a single session of physical activity, such as low-intensity walking, has been associated with modest improvements in sleep among older women with sleep impairment19. Similarly, one-time bouts of moderate-intensity continuous exercise (MICE) and high-intensity intermittent exercise (HIIE) have been found to enhance subjective sleep quality20.
The role of exercise intensity is particularly important. While both MICE and HIIE have potential benefits20the physiological responses they elicit differ markedly. These differences may lead to varying effects on sleep parameters, particularly in populations vulnerable to circadian disruption, such as shift workers. However, to date, no studies have directly compared the impact of a single session of MICE and HIIE on the sleep of night-shift workers.
Despite the well-established benefits of physical activity17many individuals remain inactive. The World Health Organization (WHO) recommends at least 150 min of moderate-intensity physical activity per week, yet over 70% of the global population fails to meet this guideline21. In a study conducted at a tertiary medical center, 65.02% of healthcare workers reported having no regular exercise habits, and only 9.60% met the WHO’s recommended levels22. For middle-aged adults with demanding professional and family responsibilities, especially healthcare workers who alternate between day and night-shifts, maintaining a regular exercise routine can be particularly challenging.
Given these constraints, it is reasonable to consider whether even a single exercise session might provide meaningful sleep benefits for shift workers with limited time and energy. If effective, such an approach could serve as both an immediate intervention and a stepping stone toward longer-term behavioral change to improve sleep and overall health in this population.
Additionally, night-shift work could increase the sympathetic influences on the variability between heartbeats23. Heart rate variability (HRV)is an index of autonomic function24 and is often used as a noninvasive means to assess cardiac autonomic activity in populations with sleep disturbance25. The low frequency (LF) and high frequency (HF) components of HRV are considered markers of sympathetic and parasympathetic nerve activities26. A systemic review reported that exercise therapy may improve HRV in myocardial infarction, chronic heart failure, and revascularization patients by increasing vagal tone and decreasing sympathetic activity27. Besides, a study in middle-aged and older adults found that 12-week MICE had a beneficial effect on sleep quality and cardiac autonomic function28. However, the effect of acute bout exercise on HRV and the sleep quality in night-shift workers was lacking.
This study hypothesizes that compared to no exercise, one episode of MICE and HIIE will improve the sleep of night-shift workers with sleep disturbance. Therefore, this study aimed to investigate whether a single session of MICE or HIIE would benefit the sleep of female healthcare workers with sleep disturbance on the night-shift over the following days.
Methods
Design
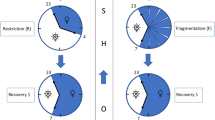
The study was conducted in a repeated-measures, three-armed crossover design, and participants were randomly assigned to the MICE trial, the HIIE trial, and the control trial (CTL) for the survey, with a minimum of 21 days between the three trials (Fig. 1). To eliminate the impact of individual physical differences, a crossover trial was conducted with the same group of subjects. This study compared MICE, HIIE, and CTL to determine the effects of different exercises on subjective, objective sleep quality, and autonomic nervous activity in workers engaged in night-shift work. The primary outcome was the results of subjective and objective sleep quality, and the secondary outcome was autonomic nervous activity. The study started on 10/12/2022 and ended on 31/08/2024.
Participants
Potential participants were recruited from six hospitals in central Taiwan through hospital and internet advertisements. Only women were recruited in this study because previous research indicates more sleep difficulties in females than in males29. Those who were interested in the study were invited to attend an orientation meeting if they met the following eligibility criteria: healthcare workers who have worked at least three consecutive eight-hour night-shifts; reporting sleep disturbed during the night-shift; age between 20 and 64 years; not taking sleep/psychotropic medications; no clinical diagnosis of sleep apnea or other sleep disorder; and not exercising regularly (i.e., below 60 min per week). Twenty-six healthcare workers participated in the preliminary meeting, at which the research team described the study purpose, procedure, exercise program, and rest condition.
The initial sleep assessment was made through the Chinese version of the self-reported 5-item Athens Insomnia Scale (AIS-5)30and sleep recording device ActiGraph (wActiSleep, Pensacola, Florida, United States) on the nondominant hand for 4 sleeps just before night-shifts. This study aimed to investigate the effects of a single session of exercise on individuals with sub-optimal sleep quality; thus, individuals were included if they scored above 5 on the AIS-530 or had a sleep efficiency below 85%31 before their first night-shift. Among the 26 potential participants, 3 did not meet the criteria and 4 decided not to attend the study.
A simple randomization method was used to randomize 19 participants into three groups using Microsoft 365-Excel (Microsoft, Redmond, Washington, United States) by the research assistant, F.W.H. They were randomly assigned to either a MICE-HIIE-CTL (n = 5), HIIE-CTL-MICE (n = 6), or CTL-MICE-HIIE (n = 3), as shown in Fig. 1. Finally, of the 19 subjects, 1 withdrew after the MICE trial, 2 withdrew after the controlled trial, and 2 withdrew after both the MICE trial and the controlled trial because they could not fit into the schedule, resulting in a sample size of 14 participants. All participants were aware of their group allocation and completed the study without harm or injury. Participants who attended and completed the study were rewarded with TWD 2500 (approximately USD 78). Previous research had indicated a minimum of 12 participants per group was able to detect a small effect size of physical activity on sleep outcome by setting beta at 20% and alpha at 5%19,32,33. The study was approved by the institutional review board of China Medical University Hospital, Taiwan (CMUH111-REC2-186) and registered in the ClinicalTrials.gov (Date: 15/10/2024, ID “NCT06638359”; https://register.clinicaltrials.gov). This study was conducted following the Declaration of Helsinki. Written and informed consent was obtained from all subjects at the beginning of the study.
Protocol
Experimental procedure
All participants were to have at least one day off before their three consecutive eight-hour night-shifts began. The subjects were arranged to receive a sleep recording device ActiGraph (non-dominant hand), heartbeat variability monitor (Polar V800, Kempele, Finland, dominant hand and chest strap) and fill in the sleep questionnaire, and also taught how to use the sleep recording device and heartbeat variability monitor, before noon on the day (Day 0, off day) before the start of the continuous night-shift (Fig. 2). The subjects used the above devices for the first time on this night to record normal sleep, as if they were not working a night-shift. After getting up, they arrived at the laboratory of the National Taiwan University of Sport at 10:00 am on the day (Day 1) when the night-shift started. The experimental process was explained first, and treadmill training (MICE, or HIIE) began at 10:30. The control group read books and newspapers. The purpose is to get the subjects to sleep naturally in the evening before they go to work. Participants were allowed to choose their sleep times freely, with no specific sleep schedules recommended. Studies have confirmed that such a sleep period is helpful for night-shift workers’ cognition, concentration, and sleep34. Each treadmill session was performed individually and supervised by a certified instructor from the National Taiwan University of Sport. In addition, sleep logs and sleep devices were completed, monitored, and evaluated using Google questionnaires from the day before the start of the intervention until the third sleep after its completion. The two devices were returned after getting up on the third post-intervention sleep, as shown in Fig. 2. The subjects were instructed not to consume alcohol or participate in organized physical activities (e.g. training and exercise) for at least 24 h before each experiment and during data collection.
A washout period of at least three weeks was required between each intervention session and measurement, as female subjects must be tested between day 1 and day 10 of the menstrual cycle. This is due to the fact that cortisol levels respond to sleep restriction, which has been demonstrated to vary with the menstrual cycle35. Therefore, all the subjects continued to work in shifts, taking breaks between the 21-day periods. However, as each person’s menstrual cycle was different, this interval might exceed 21 days.
Intervention
The following two interventional exercises have been proven by previous studies to consume equal amounts of calories36. During exercise, by adjusting the inclination and speed of the treadmill, the subject’s heart rate reached the target heart rate and target exercise intensity (Rating of Perceived Exertion, RPE) 1–10 points set according to different sensation of subjects (A score of 1 is very relaxed, while a score of 10 is exhausting and almost impossible to continue)37. If neither the target heart rate nor the target RPE could be achieved, heart rate was the preferred indicator of intensity. All exercises were performed in an air-conditioned room, with the room temperature controlled at 25 degrees Celsius and a humidity of 60%, with electric fans blowing towards the subjects. If the subjects needed to drink water during exercise, they were allowed to drink water, and the researchers provided canned mineral water.
Moderate-Intensity continuous exercise trial
Participants were asked to walk or run on a treadmill at moderate intensity for 52 min (3 min of warm-up at 50 ~ 70% of age-predicted maximal heart rate (MaxHR), 47 min of walking at 70 ~ 75% of MaxHR, and 2 min of cool-down) at 10:30 in the morning before going to work. Age-predicted MaxHR was calculated as 220-age according to the Fox Equation38. Walking speed was adjusted to maintain the heart rate (HR) at target levels. The speed of the treadmill was between 3.0 and 6 km/h and the RPE = 3/10. The mean average HR and mean MaxHR were 132.3(± 6.2), and 149.1(± 5.2), respectively.
High-Intensity intermittent exercise trial
The first 3 min, walking on the treadmill slowly speeds up until the heart rate reaches 50 ~ 70% of the MaxHR and RPE = 3/10, and then maintains the heart rate at 50 ~ 70% of the MaxHR for 10 min. Main exercise (high-intensity interval training): 4 min of exercise with the HR reaching 90 ~ 95% of the MaxHR and RPE = 5/10, followed by 3 min of cool-down exercise (50%~70% of the MaxHR). Four consecutive sets, totaling 28 min. 2 min of cool-down exercise (50%~70% of the MaxHR). Total exercise time was 43 min for the HIIE trial. The mean average HR and mean MaxHR were 135.1(± 8.4), and 178.4 (± 12.1), respectively.
Control trial
Subjects in the control trial attended at the same time on Day 0. They were instructed to sit quietly on their own during this period in a separate room where water, books, newspapers, and magazines were available and were accompanied by a research assistant.
Measure of sleep
Participants wore a wrist ActiGraph on the non-dominant hand for 4 sleep periods from the pre-exercise sleep (normal sleep as no night-shift) to the third post-exercise sleep (before the third night-shift). The sleep data were obtained from ActiGraph monitors and analyzed by ActiLife software version 6 using the Cole-Kripke algorithm, including eight sleep indices (sleep latency, total activity counts, sleep efficiency, total time in bed (TIB), total sleep time (TST), wake after sleep onset (WASO), number of awakenings, total length of awakenings)39,40. Participants also recorded their bedtime and wake-up time, enabling the researchers to coordinate these with the actigraphic data. This allowed them to determine the timing of participants’ movement in preparation for sleep (as the start point of sleep) and the timing of continuous movement upon waking (as the end point of sleep). Given the characteristics of sleep in night-shift workers, shorter sleep duration can impact other sleep indices. To mitigate the impact of shorter sleep duration, we employed mean activity during TIB (total activity counts divided by TIB)41percentage of minutes awake during TST (WASO %, WASO divided by the TST)42and sleep fragmentation index (the ratio of the number of awakenings to the TST)41,43. Daily sleep quality was measured using a question that was chosen because it was easy to conceptualize, simple to understand, and less burdensome for participants. The question asked was: On a scale of 0–10, with 0 being the worst sleep and 10 being the best, rate the quality of the previous sleep44. HRV indicators were calculated according to time (RMSSD and SDNN) and frequency (LF, HF and LF/HF ratio) domains.
Participants self-reported age, marital status, exercise habits, smoking, caffeine and alcohol consumption, and chronic diseases. Height, weight, and body fat percentage were measured from which body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). These participants completed the Satisfaction with Life Scale (SWLS) and initial sleep assessments. The SWLS is a simple self-administered psychometric properties questionnaire to examine the mental health of individuals and the possible risk of depression or other psychiatric diseases45.
Statistical analysis
Data descriptive statistics were expressed as means ± standard deviation or as the median with interquartile range according to the statistical distribution (the assumption of normality was assessed with the Shapiro–Wilk test) to describe the distribution of continuous variables.
The between-exercise factor as intervention (HIIE, MICE, and CTL) was analyzed. The Wilcoxon signed-rank test was used to compare data between two groups. This test is a non-parametric method for comparing the median difference between two related samples or pairs of data that can be analyzed without assuming that the data conform to a normal distribution46,47.
The effect size is evaluated using the effect value that is commonly employed in nonparametric statistics namely the success rate difference (SRD), which is also known as Cliff’s delta48. This value ranges from − 1 to 1, with 0 indicating no difference between the pre- and post-test results, and closer to 1 or -1 indicating a greater difference between the pre- and post-test results. Kraemer et al. attempted to convert SRD and Cohen’s d, and according to the criteria for the size of the effect of Cohen’s d (> 0.8 is large; 0.5–0.8 is moderate; 0.2–0.5 is small), suggested that an SRD value of > 0.43 represents a large change; 0.28–0.43 is moderate; 0.11–0.27 is small49.
For HRV during sleep, we used 4-hour data 30 min after bedtime33. If the participant had data shorter than 4 h, the longest available data was used. Kubios software 4.1.0 was used with medium-beat correction50. All HRV variables were log-transformed. Repeated measure ANOVA was employed to examine the difference of HRV variables between 3 level of exercise conditions. The effect size was calculated by Eta-squared (η2 = 0.14 is large; 0.06 is moderate; 0.01 is small)51,52.
All statistical tests were performed using the two-tailed test with a significance level (α) of 0.05 which were performed with SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
Demographic
Table 1 shows the baseline characteristics of participants. Briefly, participants had a mean age of 31.8(± 8.3), a mean body fat of 33.5(± 6.6) %, and a mean BMI of 23.9 (± 3.8) kg/m2. The Satisfaction with Life Scale (SWLS) score was 18.9 ± 6.7, scores from 15 to 19 are interpreted as falling in the slightly dissatisfied range53. The AIS-5 score was 7.1 ± 3 in the night-shifts, 3.6 ± 2.6 in the evening shifts, and 3.9 ± 2.3 in the morning shifts (P < 0.001), indicating that the subjects subjectively experienced significant sleep disturbance during the night-shifts.
Sleep status in night-shifts in CTL trial
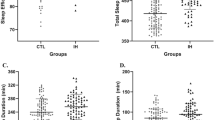
Figure 3 and Table S1 present the sleep status of the control trial. Mean sleep efficiency in the sleep before the first night-shift in CTL was 74.76 ± 27.4% placing this sample in the poor sleep range since a sleep efficiency of 85% or more is regarded as normal [32, 50]. Compared to the sleep before the intervention (normal sleep as no night-shift), the sleep before the first night-shift in CTL showed a significantly lower sleep efficiency (median = 86.25(interquartile range[IQR] = 77.88–87.62) % vs. 89.85(84.34–92.34) %, P = 0.035, SRD = 0.36, moderate effect); a shorter TIB (295(93–316) minutes vs. 487.5(420–599) minutes, P < 0.001, SRD = − 0.78, large effect), and a shorter TST (258.5(81–278) minutes vs. 437.5(367–567) minutes, P < 0.001, SRD = − 0.79, large effect). Although the total counts of movement during sleep and the number of awakenings were apparently reduced, this should be related to the relatively short sleep duration in the sleep before the first night-shift. There were no significant differences in mean activity during TIB (P = 0.684), and sleep fragmentation index (P = 0.190). However, compared to the sleep before the intervention, WASO % was significantly increased before the first night-shift (from 10(7–19) % to 16(14–23) %, P = 0.026, SRD = 0.25, small effect). The value considered normal in adults is < 10% of total sleep minutes or 42 min for a person who sleeps 7 h/night42. As often seen in this population, sleep maintenance was more problematic than sleep initiation54. The sleep before the second night-shift was also significantly reduced in TIB (from 487.5(420–599) minutes to 308.5(247–396) minutes, P = 0.006, SRD=-0.64, large effect), and TST (from 437.5(367–567) to 274(233–356) minutes, P = 0.004, SRD=-0.67, large effect). The sleep before the third night-shift was also significantly reduced in sleep efficiency (from 89.85 (84.34–92.34)% to 86.25 (79.46–89.38)%, P = 0.006, SRD=-0.31, moderate effect) and increased in WASO % (from 10(7–19)% to 15(8–22)%, P = 0.020, SRD = 0.24, small effect). All of the above objective observations suggest that night-shift work leads to sleep disturbance in these subjects.
Comparison of sleep before and after intervention in CTL. Compared to the sleep before the intervention (normal sleep as no night shift), the sleep before the first night shift in CTL showed a significantly lower sleep efficiency (P = 0.035, SRD = 0.36, moderate effect); a shorter TST (P < 0.001, SRD=-0.79, large effect).; and an increased WASO % (P = 0.026, SRD = 0.25, small effect). The sleep before the second night shift was also significantly reduced in TST (P = 0.004, SRD=-0.67, large effect). The sleep before the third night shift also significantly reduced sleep efficiency (P = 0.006, SRD=-0.31, moderate effect) and increased WASO % (P = 0.020, SRD = 0.24, small effect). *: P < 0.05; MICE: moderate intensity continuous exercise; HIIE: high intensity intermittent exercise; CTL: control trial; IQR: interquartile range; TST: total sleep time; SRD: success rate difference; WASO: wake after sleep onset.
The effect of HIIE and MICE on sleep before the first night-shift
Based on the actigraphy monitoring before the intervention, there were no significant differences in any sleep indices between the interventions and control trial at baseline, as shown in Table 2.
As shown in Table 3, sleep metrics before and after the intervention were compared. Sleep efficiency significantly declined in both the HIIE group (86.82% [80.99–90.33%] to 80.45% [69.61–86.93%], p = 0.021) and the CTL group (89.85% [84.34–92.34%] to 86.25% [77.88–87.62%], p = 0.035). In contrast, no significant change was observed in the MICE group. Self-reported sleep quality also declined significantly in the HIIE group (7 [5–8] to 5 [4–7], p = 0.029), while no significant differences were found between the MICE and CTL groups. These findings suggest that the MICE intervention effectively mitigates the negative impact of going to bed early before the first night shift, preserving both objective and subjective sleep quality.
As shown in Table 4, in the first sleep after exercise (before the first night-shift), both TIB in HIIE (304 (222–339) minutes), and in MICE (302.5 (247–374) minutes) were increased compared to CTL (295(93–316) minutes), with P = 0.024, SRD = 0.23, small effect; P = 0.047, SRD = 0.32, moderate effect, respectively. However, there was no improvement in sleep efficiency in HIIE compared to CTL, with P = 1.00. HIIE even had lower sleep efficiency than MICE (80.45 (69.61–86.93) % vs. 87.84 (82.26–91.95) %, P = 0.001, SRD= -0.45, large effect). Compared to MICE, mean activity during TIB, sleep fragmentation index and WASO% were significantly increased in HIIE (110.68(74.08-210.85) vs. 75.19(49.12-124.86), P = 0.035, SRD = 0.33, moderate effect; 6(4–8) % vs. 4(3–6) %, p = 0.010, SRD = 0.48, large effect; 24(15–37) % vs. 14(7–19) %, P = 0.049, SRD = 0.44, large effect, respectively), as Fig. 4. These results suggest that a single session of HIIE had an adverse effect on sleep maintenance before the first night-shift in non-exercising female healthcare workers with mild sleep disturbance. There was no significant improvement in sleep efficiency in MICE compared to CTL (P = 0.067). However, self-reported daily sleep quality improved significantly in MICE compared to CTL (6 ± 1.57 vs. 4.36 ± 2.17, P = 0.035, SRD = 0.45, large effect).
Effect of HIIE and MICE on the sleep before the first night shift. In the first sleep after exercise (before the first night shift), HIIE had lower sleep efficiency than MICE (P = 0.001, SRD= -0.45, large effect). Compared to MICE, mean activity during TIB, sleep fragmentation index, and WASO% were significantly increased in HIIE (P = 0.035, SRD = 0.33, moderate effect; P = 0.010, SRD = 0.48, large effect; P = 0.049, SRD = 0.44, large effect, respectively). *: P < 0.05; MICE: moderate intensity continuous exercise; HIIE: high intensity intermittent exercise; CTL: control trial; IQR: interquartile range; TIB: total time in bed; SRD: success rate difference; WASO: wake after sleep onset.
Furthermore, the interval between the intervention and bedtime in both the HIIE and MICE was markedly shorter than that observed in the CTL (408.5(248–510) minutes vs. 454(394–664 min), P = 0.035, SRD = 0.28, moderate effect; 326 (211–416) minutes vs. 454 (394–664) minutes, P < 0.001, SRD = 0.61, large effect, respectively)(Table 4). It may represent that the subjects experienced increased fatigue, which may have resulted in an earlier bedtime, and longer TIB, however, more fragmented in their sleep after HIIE.
The effect of HIIE and MICE on sleep before the 2nd night-shift
In the second sleep after exercise (before the second night-shift), the HIIE trial had more total counts movements in sleep than CTL (9441 (19208–39169) vs. 17670.5 (12105–24652), P < 0.001, SRD = 0.62, large effect). HIIE had a longer WASO than CTL and MICE, 51.5 (33–62) minutes vs. 33 (14–46) minutes, P = 0.008, SRD = 0.47, large effect; 51.5 (33–62) minutes vs. 25 (17–54) minutes, P = 0.033, SRD = 0.46, large effect, respectively. Compared to CTL, WASO % was significantly increased in HIIE (15(11–26) % vs. 11(8–15) % P = 0.049, SRD = 0.36, moderate effect, as shown in Table S2.
The effect of HIIE and MICE on sleep before the 3rd night-shift
There were no significant differences in any sleep indices between the interventions and CTL in the sleep before the third night-shift, as shown in Table S3. This means that the detrimental effects of a single session of HIIE on sleep persisted into the second sleep after HIIE, while the third sleep was unaffected.
The effect of HIIE and MICE on HRV in sleep before the first night-shift
The mean duration of HRV data used during sleep was 3.39 h (± 1.19) before intervention and 3.17 h (± 1.29) after intervention. Pre-post differences showed a significant increase in LF/HF in sleep after HIIE and a decrease in LF/HF in sleep after CTL (P = 0.043, η2 = 0.178, large effect). This result suggests that HIIE leads to a shift in sympathetic activity (as well as poor sleep), as shown in Table 5.
Discussion
This study was designed to investigate the effects of a single session of HIIE and MICE on sleep outcomes in non-exercising female healthcare workers with mild sleep disturbance during night-shifts. The results demonstrated that an acute bout of MICE has a large effect on improving subjective sleep quality and a small effect on increasing TST in this population. However, HIIE has a detrimental effect on sleep before the first night-shift, resulting in a greater reduction in sleep efficiency, a modest increase in mean activity during TIB, and a greater increase in the WASO % and sleep fragmentation index, and an HRV shift toward sympathetic activity.
The effect of HIIE and MICE on sleep before the first night-shift
These findings are not consistent with previous two studies that have used a single session of HIIE and MICE, one with 11 overweight, inactive men, and the other with 8 endurance-trained male runners, both of which suggested that HIIE appeared to be beneficial for improving sleep quality20,55. Exercise habits and physical tolerance to exercise were therefore not the main factors contributing to the deterioration of sleep quality in this study. However, these male subjects did not experience sleep disturbance due to night-shift work before the intervention, as did the subjects in this study. A systematic review and meta-analysis revealed that acute evening HIIE performed 2–4 h before bedtime does not disrupt nighttime sleep in healthy, well-sleeping adults56. Another study also demonstrated that increased exercise intensity or duration does not disrupt sleep quality in 14 healthy male adults33. However, few studies have investigated the effects of HIIE on sleep in night-shift workers. Therefore, we conclude that HIIE may not be helpful for sleep in people with sleep impairment during the night-shift and may even be detrimental.
Possible factor in the adverse effect of HIIE on sleep – time interval between exercise and bedtime
Some researchers believe the adverse effect of HIIE on sleep quality may be related to the short interval between exercise and sleep. A systematic review and meta-analysis found that people who did HIIE within an hour of bedtime took longer to fall asleep and had poorer sleep quality57. Besides, another systematic review and meta-analysis also found that HIIE was more likely to disrupt sleep in elite athletes than moderate-intensity exercise, especially when done three hours before bedtime58. In this study, the interval between HIIE and bedtime was 408.5 (248–510) minutes. This interval should be long enough compared to other studies and should not be a major factor in reduced sleep quality in this study.
Possible factor in the adverse effect of HIIE on sleep-cardiac autonomic activity
Previous studies have shown that higher physical activity, cardiorespiratory fitness, and favorable body composition were associated with a lower level of HRV-based stress59and better recovery during sleep60. However, the subjects in this study were inactive female night-shift healthcare workers with mild sleep disturbance with a mean body fat of 33.5(± 6.6) %, and a mean BMI of 23.9 (± 3.8) kg/m2, which means they had higher HRV stress levels. Another study also revealed that a significantly better nocturnal HRV profile (QTc, SDNN, pNN50, RMSSD) was observed following the low activity day compared to the days where the high-intensity exercise took place61. Besides, sleep disorders and insufficiencies are often characterized by sympatho-excitation or sympathetic/baroreflex dysfunction, with several studies suggesting women may be at heightened risk62. Therefore, subjects in this study showed higher sympathetic activity after HIIE, which may explain the fragmented sleep and increased WASO%.
The characteristics of sleep in night-shifts in CTL trial
In this study, the main sleep problems of the subjects during the night-shift were shortened TST and increased WASO %, resulting in decreased sleep efficiency. Sleep latency was relatively brief during night-shifts and almost the same as that observed prior to the intervention. The worst sleep occurred before the first night-shift. The above objective observations are consistent with previous research that night-shift workers are more likely to report shorter sleep duration63which is characterized by increased subjective difficulty in maintaining sleep, but also by greater ease of sleep onset54. However, a study in the US found that night-shift nurses reported poorer sleep quality, which may be related to difficulties in falling asleep64. In another study from South Korea, nurses also demonstrated shorter sleep hours, lower sleep efficiency, and longer sleep latency before night-shifts65. In conclusion, the sleep of night-shift workers is characterized by shorter sleep duration and less sleep efficiency. However, whether these sleep disturbances are related to poor sleep maintenance or initiation is still inconclusive and may depend on individuals17. Nevertheless, the subjects in this study had a short sleep latency, which allowed this study to assess the effects of HIIE and MICE on sleep maintenance. The results revealed that MICE subjectively improved sleep quality, but HIIE objectively had a detrimental effect on sleep maintenance.
Subjective and objective measurements of sleep
We administer a single subjective question about sleep quality. Previous studies found a low correlation between actigraphy sleep parameters and subjective sleep quality, suggesting that the two methods of measurement capture different dimensions of sleep66,67. Likewise, Hughes et al. found no agreement between subjective and objective sleep assessment; compared with objective measures, half of participants reported worse sleep efficiency on questionnaires68. However, Fabbri et al. found that the self-report questionnaires assessing sleep quality from different perspectives have high internal consistency and test-retest reliability69. In the present study, MICE showed a significant improvement in subjective sleep quality compared to CTL, but there was only a trend toward improvement in objective sleep efficiency (P = 0.067), which was not statistically significant. However, HIIE had a significant adverse effect on the objective sleep indices compared to MICE (Fig. 3), but there was no difference in subjective sleep quality.
Strengths and Limitations
This study is the first to assess the effects of an acute bout of HIIE and MICE on sleep quality in inactive female healthcare workers with sleep disturbance on the night-shift. It used a rigorous randomized design, a carefully monitored exercise session, and subjective and objective measurement of sleep. It was also conducted between days 1 and 10 of the women’s menstrual cycle when cortisol levels were relatively stable. Nonetheless, some limitations should be acknowledged. First, this study only included female healthcare workers. Caution is needed therefore when interpreting these findings, which may not generalize to other populations. Second, wrist actigraphy is useful in the estimation of several sleep parameters (e.g., TST, WASO), but is less reliable in terms of estimating sleep latency70. However, we asked participants to record the time they went to bed to assist actigraphy scoring, which might mitigate the effects. Third, the time and duration of the participants’ daily naps were not reported. Naps of different durations and times throughout the day may have different effects on sleep71. Fourth, five of our participants dropped out, all before HIIE and none after HIIE. A possible reason may be that they were intimidated by the name “High-Intensity” Intermittent Exercise, however, this protocol is for heart failure patients36. Another possible reason may be that night-shift nurses are already tired enough and have to do the exercise in the first ten days of the menstrual cycle. The physical discomfort combined with the psychological exhaustion will make the HIIE even more rejected. Furthermore, we did not control for light exposure or recommend light avoidance, which could also result in disrupted sleep72. In addition, other potential factors, such as physical activity during the shift, fatigue, and family responsibilities, may also affect sleep between night-shifts73. Finally, we did not control sleep behavior well; we allowed the subjects to sleep naturally before the first night-shift. In fact, only one person in the control group naturally gave up sleep before their first night-shift. None of the participants in the HIIE or MICE groups skipped sleep before their first night shift. Therefore, there were no differences in sleep behaviors between the HIIE and MICE groups before the first night shift.
Conclusion
In conclusion, this study suggests that a single session of MICE can preserve subjective sleep quality and promote an earlier bedtime after intervention before the first night-shift in female healthcare workers with mild sleep disturbance on the night-shift. However, a single session of HIIE has a detrimental effect on sleep maintenance, and results in a greater reduction in sleep efficiency, a modest increase in mean activity during TIB, a greater increase in WASO % and sleep fragmentation index, and an autonomic function shift toward sympathetic activity. These findings imply that although HIIE can significantly increase EPOC and energy expenditure74it may also worsen sleep in night-shift workers. More studies are needed on this topic to investigate how to optimize the potential improvements in sleep in this population to increase the public health reach.
Data availability
The raw data supporting the conclusions of this article will be made available by the author, Shih-Hao Wu, without undue reservation. E-mail address: ambertwu@gmail. com.
Abbreviations
- MICE:
-
Moderate-intensity continuous exercise
- HIIE:
-
High-intensity intermittent exercise
- MaxHR:
-
Maximum heart rate
- CTL:
-
Control trial
- TIB:
-
Total time in bed
- HRV:
-
Heart rate variability
- LF:
-
Low frequency
- HF:
-
High frequency
- WHO:
-
World Health Organization
- REM:
-
Rapid eye movement
- AIS-5:
-
5-item Athens insomnia scale
- RPE:
-
Rating of perceived exertion
- HR:
-
Heart rate
- TST:
-
Total sleep time
- WASO:
-
Wake after sleep onset
- WASO%:
-
Percentage of minutes awake during TST
- RMSSD:
-
The root mean square of successive differences between adjacent normal cycles
- SDNN:
-
Standard deviation of normal to normal
- BMI:
-
Body mass index
- SWLS:
-
The satisfaction with life scale
- SRD:
-
Success rate difference
- η2:
-
Eta-squared
- IQR:
-
Interquartile range
References
Brito, R. S., Dias, C., Afonso Filho, A. & Salles, C. Prevalence of insomnia in shift workers: A systematic review. Sleep. Sci. 14, 47–54 (2021).
Lecca, R. et al. Gender and nightshift work: A cross sectional study on sleep quality and daytime somnolence. Brain Sci. 13, 607 (2023).
Huang, B. H. et al. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br. J. Sports Med. 56, 718–724 (2022).
Schipper, S. B. et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: A review of the literature. Diabetologia 64, 2367–2377 (2021).
Che, T. et al. The association between sleep and metabolic syndrome: A systematic review and meta-analysis. Front. Endocrinol. 12, 773646 (2021).
Fernandez-Mendoza, J. et al. Objective short sleep duration increases the risk of all-cause mortality associated with possible vascular cognitive impairment. Sleep. Health 6, 71–78 (2020).
Seow, L. S. E. et al. Independent and combined associations of sleep duration and sleep quality with common physical and mental disorders: Results from a multi-ethnic population-based study. PLoS One 15, e0235816 (2020).
Lee, S., Kim, J. H. & Chung, J. H. The association between sleep quality and quality of life: A population-based study. Sleep Med. 84, 121–126 (2021).
Kecklund, G. & Axelsson, J. Health consequences of shift work and insufficient sleep. Bmj 355 (2016).
Yong, E. Why health-care workers are quitting in droves. The Atlantic 16 (2021).
Wickwire, E. M., Geiger-Brown, J., Scharf, S. M. & Drake, C. L. Shift work and shift work sleep disorder clinical and organizational perspectives. Chest 151, 1156–1172. https://doi.org/10.1016/j.chest.2016.12.007 (2017).
Kanaley, J. A. et al. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the American college of sports medicine. Med. Sci. Sports. Exerc. 54, 353 (2022).
Herbert, C. Enhancing mental health, well-being and active lifestyles of university students by means of physical activity and exercise research programs. Front. Public. Health 10, 849093 (2022).
Martland, R., Mondelli, V., Gaughran, F. & Stubbs, B. Can high-intensity interval training improve physical and mental health outcomes? A meta-review of 33 systematic reviews across the lifespan. J. Sports Sci. 38, 430–469 (2020).
Wang, F. & Boros, S. The effect of physical activity on sleep quality: A systematic review. Eur. J. Physiotherapy 23, 11–18 (2021).
Solis-Navarro, L. et al. Effects on sleep quality of physical exercise programs in older adults: A systematic review and meta-analysis. Clocks Sleep. 5, 152–166 (2023).
Huang, H. H. et al. The effect of physical activity on sleep disturbance in various populations: A scoping review of randomized clinical trials. Int. J. Behav. Nutr. Phys. Activity. 20, 44 (2023).
Kubitz, K. A., Landers, D. M., Petruzzello, S. J. & Han, N. W. The effects of acute and chronic exercise on sleep—a meta-analytic review. Sports Med. 21, 277–291. https://doi.org/10.2165/00007256-199621040-00004 (1996).
Chen, L. J., Stevinson, C., Fang, S. H., Taun, C. Y. & Ku, P. W. Effects of an acute bout of Light-Intensity walking on sleep in older women with sleep impairment: A randomized controlled trial. J. Clin. Sleep. Med. 15, 581–586. https://doi.org/10.5664/jcsm.7718 (2019).
Larsen, P. et al. High-intensity interval exercise induces greater acute changes in sleep, appetite-related hormones, and free-living energy intake than does moderate-intensity continuous exercise. Appl. Physiol. Nutr. Metab. 44, 557–566. https://doi.org/10.1139/apnm-2018-0503 (2019).
Hyde, E. T., Whitfield, G. P., Omura, J. D., Fulton, J. E. & Carlson, S. A. Trends in meeting the physical activity guidelines: Muscle-strengthening alone and combined with aerobic activity, united states, 1998–2018. J. Phys. Activity Health 18, S37–S44 (2021).
Chou, F. Y. et al. The effect of exercise on the risk of metabolic syndrome associated with sleep insufficiency: A cross-sectional study. Front. Cardiovasc. Med. 10 https://doi.org/10.3389/fcvm.2023.1192241 (2023).
Chung, M. H. et al. Sleep and autonomic nervous system changes—enhanced cardiac sympathetic modulations during sleep in permanent night shift nurses. Scand. J. Work Environ. Health 180–187 (2009).
Bigger, J. T. Jr et al. RR variability in healthy, middle-aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation 91, 1936–1943 (1995).
Stein, P. K. & Pu, Y. Heart rate variability, sleep and sleep disorders. Sleep Med. Rev. 16, 47–66 (2012).
Camm, A. J. et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Circulation 93, 1043–1065 (1996).
Routledge, F. S., Campbell, T. S., McFetridge-Durdle, J. A. & Bacon, S. L. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 26, 303–312 (2010).
Tseng, T. H., Chen, H. C., Wang, L. Y. & Chien, M. Y. Effects of exercise training on sleep quality and heart rate variability in middle-aged and older adults with poor sleep quality: A randomized controlled trial. J. Clin. Sleep Med. 16, 1483–1492 (2020).
McArdle, N. et al. Prevalence of common sleep disorders in a middle-aged community sample. J. Clin. Sleep Med. 18, 1503–1514 (2022).
Chiang, H., Chen, H. & Bai, C.
Reed, D. L. & Sacco, W. P. Measuring sleep efficiency: what should the denominator be? J. Clin. Sleep Med. 12, 263–266 (2016).
Hartescu, I., Morgan, K. & Stevinson, C. D. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: A randomized controlled trial. J. Sleep. Res. 24, 526–534. https://doi.org/10.1111/jsr.12297 (2015).
Myllymäki, T. et al. Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. Eur. J. Appl. Physiol. 112, 801–809. https://doi.org/10.1007/s00421-011-2034-9 (2012).
Cheng, W. J., Hang, L. W., Kubo, T., Vanttola, P. & Huang, S. C. Impact of sleep timing on attention, sleepiness, and sleep quality among real-life night shift workers with shift work disorder: A cross-over clinical trial. Sleep 45, zsac034 (2022).
LeRoux, A., Wright, L., Perrot, T. & Rusak, B. Impact of menstrual cycle phase on endocrine effects of partial sleep restriction in healthy women. Psychoneuroendocrinology 49, 34–46 (2014).
Wisløff, U. et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. Circulation 115, 3086–3094. https://doi.org/10.1161/CIRCULATIONAHA.106.675041 (2007).
Borg, G. A. Psychophysical bases of perceived exertion. Med. Sci. Sports. Exerc. 14, 377–381 (1982).
Shookster, D., Lindsey, B., Cortes, N. & Martin, J. R. Accuracy of commonly used age-predicted maximal heart rate equations. Int. J. Exerc. Sci. 13, 1242 (2020).
Chen, L. J., Fox, K. R., Ku, P. W. & Chang, Y. W. Effects of aquatic exercise on sleep in older adults with mild sleep impairment: A randomized controlled trial. Int. J. Behav. Med. 23, 501–506 (2016).
Actigraph, L. ActiLife 6 users manual. (ActiGraph, LLC, Pensacola, FL, USA, 2012).
Fekedulegn, D. et al. Actigraphy-based assessment of sleep parameters. Ann. Work Exposures Health 64, 350–367 (2020).
Berger, A. M. et al. in Oncology nursing forum.
Natale, V., Léger, D., Martoni, M., Bayon, V. & Erbacci, A. The role of actigraphy in the assessment of primary insomnia: A retrospective study. Sleep Med. 15, 111–115 (2014).
Sullivan Bisson, A. N., Robinson, S. A. & Lachman, M. E. Walk to a better night of sleep: Testing the relationship between physical activity and sleep. Sleep. Health 5, 487–494. https://doi.org/10.1016/j.sleh.2019.06.003 (2019).
Diener, E., Emmons, R. A., Larsen, R. J. & Griffin, S. The satisfaction with life scale. J. Pers. Assess. 49, 71–75. https://doi.org/10.1207/s15327752jpa4901_13 (1985).
Taheri, S. & Hesamian, G. A generalization of the Wilcoxon signed-rank test and its applications. Stat. Pap. 54, 457–470 (2013).
Rosner, B., Glynn, R. J. & Lee, M. L. T. The Wilcoxon signed rank test for paired comparisons of clustered data. Biometrics 62, 185–192 (2006).
Cliff, N. Dominance statistics: Ordinal analyses to answer ordinal questions. Psychol. Bull. 114, 494 (1993).
Kraemer, H. C. & Kupfer, D. J. Size of treatment effects and their importance to clinical research and practice. Biol. Psychiatry 59, 990–996 (2006).
Alcantara, J. M. A. et al. Impact of using different levels of Threshold-Based artefact correction on the quantification of heart rate variability in three independent human cohorts. J. Clin. Med. 9 https://doi.org/10.3390/jcm9020325 (2020).
Levine, T. R. & Hullett, C. R. Eta squared, partial Eta squared, and misreporting of effect size in communication research. Hum. Commun. Res. 28, 612–625 (2002).
Martín, E. L. & Martinez, D. A. The effect size in scientific publication. Educación XX. 1 26, 9–17 (2023).
Pavot, W. & Diener, E. The satisfaction with life scale and the emerging construct of life satisfaction. J. Posit. Psychol. 3, 137–152. https://doi.org/10.1080/17439760701756946 (2008).
Akerstedt, T., Kecklund, G. & Knutsson, A. Spectral analysis of sleep electroencephalography in rotating three-shift work. Scand. J. Work Environ. Health 17, 330–336. https://doi.org/10.5271/sjweh.1694 (1991).
Thomas, C., Jones, H., Whitworth-Turner, C. & Louis, J. High-intensity exercise in the evening does not disrupt sleep in endurance runners. Eur. J. Appl. Physiol. 120, 359–368 (2020).
Frimpong, E., Mograss, M., Zvionow, T. & Dang-Vu, T. T. The effects of evening high-intensity exercise on sleep in healthy adults: A systematic review and meta-analysis. Sleep. Med. Rev. 60, 101535. https://doi.org/10.1016/j.smrv.2021.101535 (2021).
Stutz, J., Eiholzer, R. & Spengler, C. M. Effects of evening exercise on sleep in healthy participants: A systematic review and meta-analysis. Sports Med. 49, 269–287. https://doi.org/10.1007/s40279-018-1015-0 (2019).
Roberts, S. S. H., Teo, W. P. & Warmington, S. A. Effects of training and competition on the sleep of elite athletes: A systematic review and meta-analysis. Br. J. Sports Med. 53, 513–522 (2019).
Teisala, T. et al. Associations of physical activity, fitness, and body composition with heart rate variability–based indicators of stress and recovery on workdays: a cross-sectional study. J. Occup. Med. Toxicol. 9, 1–9 (2014).
Föhr, T. The Relationship between leisure-time Physical Activity and Stress on Workdays with Special Reference To Heart Rate Variability Analyses (University of Jyväskylä, 2016).
Miadovnik, L. A. Effects of sport-specific, intermittent high-intensity exercise on nocturnal heart rate variability and glycemia in elite athletes with type 1 diabetes. (2013).
Greenlund, I. M. & Carter, J. R. Sympathetic neural responses to sleep disorders and insufficiencies. Am. J. Physiol. Heart Circ. Physiol. 322, H337–H349 (2022).
Boersma, G. J., Mijnster, T., Vantyghem, P., Kerkhof, G. A. & Lancel, M. Shift work is associated with extensively disordered sleep, especially when working nights. Front. Psychiatry 14 https://doi.org/10.3389/fpsyt.2023.1233640 (2023).
Feng, T., Booth, B. M., Baldwin-Rodríguez, B., Osorno, F. & Narayanan, S. A multimodal analysis of physical activity, sleep, and work shift in nurses with wearable sensor data. Sci. Rep. 11, 8693 (2021).
Min, A., Hong, H. C., Son, S. & Lee, T. Sleep, fatigue and alertness during working hours among rotating-shift nurses in Korea: An observational study. J. Nurs. Adm. Manag. 29, 2647–2657 (2021).
Landry, G. J., Best, J. R. & Liu-Ambrose, T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front. Aging Neurosci. 7, 166 (2015).
Aili, K., Åström-Paulsson, S., Stoetzer, U., Svartengren, M. & Hillert, L. Reliability of actigraphy and subjective sleep measurements in adults: the design of sleep assessments. J. Clin. Sleep Med. 13, 39–47 (2017).
Hughes, J. M. et al. Measuring sleep in vulnerable older adults: A comparison of subjective and objective sleep measures. Clin. Gerontol. 41, 145–157 (2018).
Fabbri, M. et al. Measuring subjective sleep quality: A review. Int. J. Environ. Res. Public Health 18, 1082 (2021).
Martin, J. L. & Hakim, A. D. Wrist actigraphy. Chest 139, 1514–1527 (2011).
Milner, C. E. & Cote, K. A. Benefits of napping in healthy adults: Impact of nap length, time of day, age, and experience with napping. J. Sleep Res. 18, 272–281 (2009).
Brown, T. M. et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 20, e3001571 (2022).
Roskoden, F. C. et al. Physical activity, energy expenditure, nutritional habits, quality of sleep and stress levels in shift-working health care personnel. PloS One 12, e0169983 (2017).
Panissa, V. L., Fukuda, D. H., Staibano, V., Marques, M. & Franchini, E. Magnitude and duration of excess of post-exercise oxygen consumption between high‐intensity interval and moderate‐intensity continuous exercise: A systematic review. Obes. Rev. 22, e13099 (2021).
Acknowledgements
Thanks to the National Taiwan University of Sport, and China Medical University Hospital for providing the equipment and necessary assistance for this study.The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and statement that results of the present study do not constitute endorsement by ACSM.
Funding
This work was supported by the Clinical Trial Center, China Medical University Hospital (DMR-112–068).
Author information
Authors and Affiliations
Contributions
Conceptualization, S.H.W., W.J.C., L.J.C., P.W.K., and C.H.C.; methodology, S.H.W., L.J.C., P.W.K., and C.H.C.; formal analysis, S.H.W., W.J.C., F.W.H., and L.J.C.; writing—original draft preparation, S.H.W., and W.J.C.; writing—review and editing, S.H.W., W.J.C., B.S., L.J.C., P.W.K., and C.H.C.; carried out the experiment, data collection, and assisted the manuscript preparation, C.W.T., Y.H.C., and S.H.W. All authors read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The project was approved by the Institutional Review Board of China Medical University Hospital (CMUH111-REC2-186), and all participants provided written informed consent for publication of the data collected from them before enrolment. The trials were conducted in accordance with local laws and institutional requirements.
Consent for publication
This study does not contain any individual person’s data in any form (including any individual details, images or videos).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, SH., Cheng, WJ., Chen, LJ. et al. Moderate-intensity continuous exercise preserves sleep quality, while high-intensity intermittent exercise disrupts it in female night-shift healthcare workers. Sci Rep 15, 24428 (2025). https://doi.org/10.1038/s41598-025-09404-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-09404-1