Abstract
Due to advances in medical and surgical care, there are more adults than children living with congenital heart disease (CHD). Acute kidney injury (AKI) is a common complication following cardiac surgery in patients with CHD, with creatinine lacking sensitivity for early detection. Renal functional reserve (RFR), the kidney’s capacity to increase filtration under stress, has emerged as a potential predictor of AKI. Our primary study objective was to evaluate whether preoperative RFR, using both creatinine clearance (CrCl) and cystatin C estimated glomerular filtration rate (eGFR) methods, predicts AKI following cardiopulmonary bypass in young adults with CHD. As a secondary objective, we compared RFR in CHD patients to that of healthy controls. This prospective cohort study included 30 young adults (ages 18–40) with acyanotic CHD and 8 healthy controls with normal baseline kidney function by serum creatinine. Preoperative RFR was measured using CrCl and cystatin C eGFR before and after a protein load. Postoperative AKI was diagnosed using the Kidney Disease Improving Global Outcomes criteria. Twelve (40%) CHD patients developed AKI, exhibiting significantly lower RFR when compared to those without AKI (median CrCl RFR: 9.6 vs. 35.0 mL/min/1.73m2; cystatin C eGFR RFR: 5.5 vs. 11.5 mL/min/1.73m2; P < 0.01). The ROC curve area for AKI prediction was 1.0 (CrCl RFR) and 0.88 (95% CI: 0.72–1.00, cystatin C eGFR RFR). CHD patients had lower RFR than controls (median CrCl: 25.5 vs. 56.4 mL/min/1.73m2, P < 0.01; median cystatin C eGFR: 9.0 vs. 13.5 mL/min/1.73m2, P = 0.03). In conclusion, preoperative RFR accurately predicts AKI in young adults with acyanotic CHD, providing a tool for the identification of high-risk patients and potentially improving perioperative care.
Similar content being viewed by others
Introduction
Recent significant advancements in the surgical and medical care of patients with congenital heart disease (CHD) have resulted in an increase in the number of adults living with CHD1. Post-operative acute kidney injury (AKI) is common in adults with CHD and is associated with negative outcomes, including persistent kidney dysfunction, longer duration of mechanical ventilation, and increased length of intensive care unit (ICU) length of stay2,3. Although many patients with CHD have multiple cardiac surgeries beginning in infancy, leading to greater cumulative AKI risk, there is a lack of literature exploring methods to determine the risk of postoperative AKI in patients with CHD. The preoperative identification of high-risk patients could impact clinical decisions related to procedure timing, hemodynamic monitoring, and the use of medications.
The risk of AKI in patients with CHD arises from a combination of factors, including individual susceptibility and the degree of exposure to hypoxia, cardiopulmonary bypass, or nephrotoxic medications4. Among the key contributors to susceptibility, a reduction in kidney mass is a significant factor and commonly not evident based on traditional methods of assessing kidney function (e.g., serum creatinine, urinalysis, blood pressure measurements) in patients with CHD. In 1983, Bosch et al. outlined a method to quantify renal functional reserve (RFR) by quantifying baseline and stress glomerular filtration rate (GFR) after a protein load5. The kidney’s ability to maintain GFR within normal ranges despite reducing renal mass indicates the presence of RFR. Since the work of Bosch and colleagues, a lack of increase in GFR in response to a protein load has been shown in individuals with reduced nephron mass due to numerous etiologies, suggesting that a reduction in RFR may represent an increased susceptibility to the effects of kidney insults6,7,8,9,10.
As it is not yet routinely used in clinical care, work has been done to standardize the measurement of RFR using creatinine clearance (CrCl)11. We and other groups have published on using cystatin C estimated GFR (eGFR) to quantify RFR as it can be completed over a shorter time period when compared to CrCl12,13. In a prospective cohort of adults with a normal resting GFR undergoing elective cardiac surgery, Husain-Syed et al. reported that preoperative RFR calculated using CrCl highly predicted postoperative AKI with an area under the receiver operating characteristic curve of 0.8314. To our knowledge, no prior work quantifies RFR in patients with CHD or, importantly, explores its association with the risk of AKI. Therefore, the primary objective of the study was to evaluate the ability of preoperative RFR (using both CrCl and cystatin C eGFR methods) to predict AKI after cardiopulmonary bypass in young adults with CHD and a normal resting eGFR. A secondary aim of our study was to compare RFR values in young adults with CHD with those of young adults without CHD. Furthermore, many investigators have reported that urine albumin excretion is a reliable prognostic marker of chronic kidney disease progression15,16, but there is scant data on using urine albumin to predict AKI. As a result, an additional secondary aim of this study was to assess the association of preoperative albuminuria with postoperative AKI in young adults with CHD.
Materials and methods
Study design and participants
Patients with acyanotic CHD 18–40 years of age with a documented eGFR ≥ 60 (based on serum creatinine within the prior two years) and plans for cardiac surgery requiring cardiopulmonary bypass in the following four weeks were approached for study participation. Patients were excluded if they had a history of urologic diseases, dialysis, or kidney transplant. Individuals 18–40 years of age with no known history of cardiac disease, kidney disease, transplantation or urologic disease were recruited from the University of Pittsburgh campus. Notably, we chose the young adult age range of 18–40, given that human studies have demonstrated an annual decline in GFR of about 0.8 mL/min/1.73m2 per year after the age of 30 years17. The Institutional Review Board approved the study at the University of Pittsburgh (Study Number 18110108). Informed consent was obtained from all study participants. All procedures followed the ethical standards of the Institutional Research Committee and the 1964 Helsinki Declaration.
Baseline variables
Information regarding the CHD diagnosis and percent ejection fraction from an echocardiogram done in the preceding month was obtained from the medical record. For all study participants, height and weight were recorded at the start of the study, and body surface area was calculated using the DuBois and DuBois formula18. Blood pressure stages for systolic and diastolic values were determined using automatic readings based on the 2017 guidelines from the American College of Cardiology and the American Heart Association19. Baseline eGFR was quantified from the first serum creatinine and cystatin value upon arrival to the study visit. For individuals > 25 years of age, we defined baseline eGFR using the 2021 Chronic Kidney Disease Epidemiology Collaborative (CKD-EPI) equation, including both serum creatinine and cystatin C20. The Chronic Kidney Disease in Children under age 25 (CKiD U25) equation was used to define baseline eGFR for study participants 18–25 years of age, also including both serum creatinine and cystatin C21.
All subjects collected urine specimens immediately after awakening on the day of RFR quantification of total protein and creatinine. Clinically significant proteinuria was defined as a protein-to-creatinine ratio of ≥ 0.2 mg/mg22. Urine albumin was also determined from the first-morning specimen, and albuminuria categories were classified by a ratio of albumin to creatinine as previously defined23. Using a modified Jaffe technique, serum and urine creatinine were run in the clinical laboratory at UPMC Children’s Hospital of Pittsburgh (CHP). Also, in the clinical laboratory at CHP, cystatin C was run using the turbidimetric method, enlisting the most up-to-date material from the International Federation of Clinical Chemistry.
Quantification of renal functional reserve and glomerular filtration rate
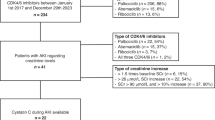
Figure 1 shows the overall study flow. Before study participation, individuals were mailed instructions on a low protein diet (< 15 g/meal) for the night before and the morning of the study and to discontinue the use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker medications 48 h before the study24. A urine specimen container was also mailed to the subjects, and they were instructed to bring a first-morning urine specimen on the day of the study, which was sent for albumin, total protein, and creatinine, as described above. To ensure normal hydration status, all subjects were asked to drink eight ounces of water before study arrival. See Supplementary Fig. 1 for the study data collection form. The protocol was constructed based on prior published studies using CrCl and cystatin C eGFR to quantify RFR12,13,14. The study occurred at CHP’s Pediatric Clinical and Translational Research Center (PCTRC). For participant convenience, the study day corresponded with the subject’s pre-operative cardiac surgery clinic visit in an office next to the PCTRC, and the protein load was ingested during the clinic visit. In addition, pre-operative labs were obtained with one of the study blood draws.
Flow chart of the renal functional reserve test. This figure depicts the structured timeline and procedures for preparing and acquiring blood and urine samples to assess renal functional reserve. Three urine and blood specimens were obtained before and after participants ate a 78-g protein-containing burger.
After emptying their bladders at Time 0, participants completed three spontaneous urine collections with blood draws immediately following. Since the majority of prior studies quantifying RFR have used a 60–80 g oral red meat protein load, and red meat has been shown to induce the greatest change in GFR, the protein load used for this study was three beef burgers with protein dosed at 78 g5,6. The protein load was administered after the third urine collection and blood draw over 15–20 min. Participants then completed three more timed spontaneous urine collections with blood draws to follow. CrCl was calculated and adjusted for 1.73m2 of body surface area: CrCl = (urine creatinine (mg/dL) x urine volume (mL))/(time (minutes)*serum creatinine (mg/dL)) × 1.73/body surface area (kg/m2). The mean value of the three CrCl values obtained before the protein load was considered the resting CrCl GFR. The stress CrCl was defined as the highest value reached after the protein load14. Renal functional reserve using CrCl was calculated from the difference between the stress and resting CrCl. Blood for cystatin C was obtained at baseline (prior to the protein load) and then 1, 2 and 3 h after the protein load and eGFR was calculated using the CKD-EPI and CKiD U25 equations as described above20,21. The baseline cystatin C based eGFR was subtracted from the nadir cystatin C based eGFR after the protein load to calculate the cystatin C eGFR determined RFR.
Variables obtained during hospital admission
The type of cardiac surgery, cardiopulmonary bypass time, and cross clamp time were collected. Hourly doses of all vasoactive medications were recorded for the first 24 h after admission to the ICU, and the maximum vasoactive-inotropic score was calculated25. Hourly urine output and daily serum creatinine values were obtained from patient charts throughout their hospital admission in order to diagnose AKI using the Kidney Disease Improving Global Outcomes (KDIGO) criteria26. Baseline serum creatinine was the value obtained at the start of the study visit quantifying RFR. The time window for defining AKI began on postoperative day zero and included the entire hospital stay. All medications given throughout the hospital admission were recorded. Data regarding length of stay and mortality was also obtained.
Statistical analysis
Continuous variables were summarized as medians with interquartile ranges (IQR), and categorical data were displayed as counts (percentages). Normality was evaluated through histograms and the Shapiro–Wilk test. Continuous and categorical variables were compared using the Kruskal–Wallis test, Pearson’s Chi-square test, or Fisher’s exact test where appropriate. Receiver-operator (ROC) curves were generated with 95% confidence intervals (CI) to evaluate the sensitivity and specificity of RFR based on CrCl, RFR based on cystatin C eGFR, baseline serum creatinine, and baseline serum cystatin C for predicting AKI. Two-sided p-values of less than or equal to 0.05 were deemed statistically significant. Analyses were performed using STATA 16.1 (Stata Corp, College Station, TX, USA).
Results
Baseline characteristics of the young adults with congenital heart disease
Table 1 shows the baseline characteristics of the 30 patients with CHD who were enrolled in the study. No participants met clinical criteria for hypertension or proteinuria at the time of study enrollment19,22. The most common diagnoses were Ebstein Anomaly and aortic stenosis (40% of the total cohort). All CHD participants underwent cardiac surgery with cardiopulmonary bypass within two weeks of completing the RFR study. As shown in Table 2, the most common surgeries done were pulmonary valve replacement and Ross and Cone procedures, each occurring in 20% of patients. The median baseline eGFR based on cystatin C and creatinine was 111.5 (100.0–124.0) ml/min/1.73m2 among all patients with CHD.
The association of characteristics and exposures with acute kidney injury in patients with congenital heart disease
Twelve (40%) of patients experienced AKI, five stage 1 and seven stage 2. Of the twelve patients with AKI, the diagnosis was made by serum creatinine in six patients, urine output in three patients, and both serum creatinine and urine output criteria in three patients. The majority of AKI was diagnosed on postoperative days 0 or 1 (83.3%), with only two patients meeting AKI criteria for the first time on postoperative days 2 or 3. Of the ten patients where AKI was diagnosed on days 0 or 1, five were diagnosed by a rise in serum creatinine, three were diagnosed based on urine output, and two were diagnosed by meeting both criteria. The median duration of AKI during the hospitalization was 116 (IQR 75–139) hours. In 9 patients (75%), AKI persisted beyond 48 h. Additionally, five patients meeting AKI criteria (41.7%) did not have documented creatinine and/or urine output evidence of AKI recovery at the time of hospital discharge.
There were no differences in any baseline or surgical characteristics when comparing patients with and without AKI (Tables 1 and 2). Supplementary Table 1 shows potentially nephrotoxic medications administered to study participants perioperatively26,27. There was no difference in AKI incidence based on any of the medications given during the hospital admission. The majority of patients (66.7%) received no vasoactive medications after admission to the ICU. The maximum vasoactive-infusion score in the first 24 h after surgery was not different between patients who developed AKI and those who did not (median [IQR] 0 [0–1] vs. 0 [0–2], p = 0.70). The median (IQR) ICU and hospital length of stays were 2 (2–3) and 5 (4–6) days, respectively. There was no difference in length of stay when comparing those with versus without AKI. No patients died during their hospital admission.
All patients completed the 78-g protein load within 15–20 min at study hour 3.5. The peak increase in CrCl and cystatin C eGFR occurred two hours after the protein load in 25 (83.3%) and three hours after the protein load in 5 (16.7%) participants. Supplementary Fig. 2 shows the median values of cystatin C eGFR, and CrCl GFR prior to the protein load, and at 1, 2, and 3 h following protein ingestion. As shown in Table 3, the median (IQR) RFR based on CrCl was 25.5 (10.1–36.0) mL/min/1.73m2. The median (IQR) cystatin C eGFR RFR was 9.0 (6.0–12.0) mL/min/1.73m2. The median (IQR) RFR based on CrCl for patients with AKI was 9.6 (8.0–11.6) mL/min/1.73m2. The median (IQR) cystatin C eGFR RFR for patients with AKI was 5.5 (5.0–7.4) mL/min/1.73m2. The median (IQR) RFR based on CrCl for patients without AKI was 35.0 (27.1–43.8). The median (IQR) cystatin C eGFR for patients without AKI was 11.5 (9.0–14.0). The RFR quantified by both CrCl and cystatin C eGFR was significantly lower in patients who met the criteria for AKI than in those who did not (P < 0.001; Table 3 and Fig. 2). As shown in Fig. 3, the RFR based on CrCl perfectly predicted AKI with a cutoff between 12.3 and 20.0 mL/min/1.73m2, achieving 100% sensitivity and specificity (AUC = 1.0). The AUC for predicting AKI for cystatin C eGFR RFR was 0.88 (95% CI: 0.72–1.00). In contrast, the AUC for creatine was 0.50 (95% CI: 0.29–0.74), and baseline cystatin C was 0.52 (95% CI: 0.30–0.72).
Preoperative renal functional reserve in patients with acute kidney injury as compared to patients without acute kidney injury. Median (IQR) renal functional reserve quantified by both creatinine clearance (CrCl) and cystatin C estimated glomerular filtration rate (cysC eGFR) was significantly greater in young adults with congenital heart disease without AKI (CrCl: 35.0 (27.1–43.8) ml/min/1.73m2; cysC eGFR: 11.5 (9.0–14.0) ml/min/1.73m2) as compared to young adults with congenital heart disease with AKI (CrCl: 9.6 (8.0–11.6) ml/min/1.73m2; cysC eGFR: 5.5 (5.0–7.4) ml/min/1.73m2).
The areas under the receiver operating characteristic curve (AUC) for four kidney function assessment methods to predict acute kidney injury. Each AUC is shown with associated 95% confidence intervals for: (a) renal functional reserve quantified by cystatin C estimated glomerular filtration rate (GFR), (b) renal functional reserve quantified by creatinine clearance (CrCl), (c) baseline serum creatinine, and (d) baseline serum cystatin C. *confidence interval cannot be calculated due to perfect separation.
The median (IQR) preoperative albumin to creatinine ratio was 90.1 (59.3–219.5) mg/g and there was no difference in median values when comparing patients with and without AKI (Table 3). Eighty-seven percent of patients (n = 26) had an albumin-to-creatinine ratio of 30–300 mg/dl.
Renal functional reserve in healthy young adults
Eight healthy young adults were enrolled and completed the study. The median (IQR) age was 23.5 (21.5–24.5) years and 62.5% of participants were female. All study participants had no clinically significant albuminuria or proteinuria based on a first-morning urine specimen22,23. Blood pressure values were obtained at the beginning of the study, and no participants met the criteria for hypertension19. The median baseline eGFR based on cystatin C and creatinine was 122.5 (112.5–126.5) ml/min/1.73m2. Similarly to the patients with CHD, all participants completed the 78-g protein meal within 15–20 min at study hour 3.5. The peak increase in CrCl and cystatin C eGFR occurred two hours after the protein load in 6 (75%) participants and three hours after the protein load in 2 (25%) participants. The median (IQR) RFR based on CrCl was 56.4 (42.5–70.7) mL/min/1.73m2. The median (IQR) cystatin C eGFR RFR was 13.5 (11.5–19.0) mL/min/1.73m2.
Renal functional reserve in young adults with congenital heart disease as compared to healthy young adults
Subjects with CHD were older (29.0 (25–34) vs. 23.5 (21.5–24.5) years, P = 0.01) with a higher BMI (28.0 (24.5–30.1) vs 23.9 (22.3–26.4) kg/m2, P = 0.03) kg/m2 when compared to those without. There was no difference in sex or baseline eGFR when comparing those with and without CHD. Renal functional reserve quantified by both CrCl and cystatin C was significantly less in young adults with CHD when compared to those without (Fig. 4).
Renal functional reserve in young adults with congenital heart disease as compared to young adults without congenital heart disease. Median (IQR) renal functional reserve quantified by both creatinine clearance (CrCl) and cystatin C estimated glomerular filtration rate (cysC eGFR) was significantly greater in young adults without congenital heart disease (CrCl: 56.4 (42.5–70.7) ml/min/1.73m2; cysC eGFR: 13.5 (11.5–19.0) ml/min/1.73m2) as compared to young adults with congenital heart disease (CrCl: 25.5 (10.1-36.0) ml/min/1.73m2; cysC eGFR: 9.0 (6.0-12.0) ml/min/1.73m2).
Discussion
Our study findings show that RFR strongly predicts AKI after cardiac surgery in young adults with acyanotic CHD with normal baseline eGFR, using either a CrCl or a cystatin C eGFR method. Notably, RFR based on CrCl showed perfect predictive ability for AKI, while baseline renal function markers such as creatinine and cystatin C were poor predictors. Preoperative risk assessment for AKI is essential for optimizing patient management and potentially improving outcomes. Stratifying patients by AKI risk before surgery can aid clinical decision-making, patient education and identify high-risk patients for trial enrollment.
Prior study results have demonstrated that the risk of AKI during cardiac surgeries can be reduced by identifying high-risk patients and applying a comprehensive care bundle accordingly28. However, these studies stratified patients based on biomarker evidence of kidney stress. Patients with reduced RFR can be identified well before receiving surgery or other planned stressors and, therefore, benefit from a proactive management strategy, including minimizing nephrotoxic medications, reducing exposure to radiocontrast, adjusting fluid intake, nephrology consultation upon hospital admission, and possibly even changes to the surgical and anesthetic plans28,29. Medications like ACE inhibitors, ARBs, and sodium-glucose cotransporter 2 inhibitors have shown promise in mitigating kidney damage in conditions like diabetes and hypertension30,31,32. Furthermore, this strategy could also be combined with biomarker measurements following surgery. Future work is needed to explore if patients with reduced RFR may benefit from these medications to help reduce intraglomerular pressure and GFR decline.
Importantly, patients with CHD in our study had significantly lower RFR values, whether measured by CrCl or cystatin C eGFR, compared to those without CHD despite all participants having a similarly normal baseline eGFR. This finding suggests that a lower RFR could indicate compromised "kidney fitness” or the kidney’s ability to adapt to physiological stressors, such as cardiac surgery33. A kidney experiencing reduced fitness might be operating at or near its maximum filtration capacity, thus limiting its ability to increase filtration under stress and heightening the risk of AKI. Interestingly, although not statistically significant, baseline eGFR was higher in the AKI group when compared to those who did not experience AKI, suggesting possible hyperfiltration in which the remaining functional nephrons in the kidney increase their filtration rate to compensate for lost renal mass, potentially masking early kidney damage34.
There is a lack of methods to determine AKI risk in patients without kidney disease recognizable by serum creatinine, proteinuria, or blood pressure findings. Cooper et al. highlighted the persistent elevation of biomarkers such as IL-18 and liver-type fatty acid–binding protein seven years after cardiopulmonary bypass-induced AKI, suggesting subclinical kidney damage despite normal kidney function by conventional measures35. Volovelsky and colleagues demonstrated the predictive value of preoperative fibroblast growth factor 23 for severe AKI post-cardiac surgery36. This concept of kidney frailty or fitness emphasizes the importance of early risk stratification and intervention, particularly for vulnerable populations like those with CHD where the six-year mortality rate is five-fold higher for adults with CHD with a moderate or severe reduction in GFR when compared to patients with a normal GFR37.
Our results highlight the utility of cystatin C for estimating GFR, eliminating the need for timed urine collections required by CrCl. The cystatin C method offers a faster and more practical alternative for clinical use. Our data also indicates that creatinine-based estimates of RFR exceed those derived from cystatin C. This discrepancy is likely due to the increased tubular secretion of creatinine following a protein-rich meal, which prior studies have shown raises serum creatinine concentrations through non-steady state conditions induced by the conversion of creatine to creatinine during cooking12. Our results also provide important insights into the timing of a GFR increase following a protein load. The peak increase in CrCl and cystatin C eGFR occurred two hours after the protein load in 82% of our study participants. This is consistent with previous studies that show a peak GFR increase within three hours after a protein load in subjects with a normal baseline eGFR11,12,13. Notably, Christiadi et al. demonstrated that in patients with CKD, plasma cystatin C levels reached their lowest point approximately four hours after a protein load in more than 40% of CKD stage 3 patients and 52% of those with CKD stage 438. This delayed response suggests that individuals with reduced baseline kidney function may require a longer sampling period to fully capture the peak GFR increase compared to those with a normal baseline GFR.
The albumin-to-creatinine ratio is a well-established predictor of CKD progression, with evidence linking elevated ACR levels to worse outcomes, such as accelerated declines in GFR39. However, its use in predicting AKI is less extensively studied. We found no statistically significant difference in the preoperative albumin to creatinine ratio between patients with AKI and those without, although values were numerically greater in those with AKI.
Our study has several strengths, including its prospective design and the use of both CrCl and cystatin C-based eGFR methods to assess RFR in patient cohorts at increased risk for kidney disease progression. However, our study also has limitations, such as the small sample size and the focus on a younger, predominantly acyanotic CHD population, which may limit the generalizability of our results to older or more diverse cohorts. In larger cohorts, it is plausible that the observed perfect prediction may not hold as precisely, given the potential for increased variability. We did not have data available regarding a history of prior AKI episodes or detailed data on prior surgical history, including the number and type of previous cardiac surgeries. In patients with congenital heart disease, multiple staged surgical interventions are common and could influence the risk of kidney injury and long-term renal outcomes. In addition, we did not assess urinary retention with bladder ultrasound or urinary catheterization, which could have resulted in measurement error. Our clinical laboratory used a modified Jaffe technique for reporting serum creatinine, and the CKiD25 equation was validated using an enzymatic method. Additionally, the discrepancy between creatinine-based and cystatin C-derived estimates of renal function points to the need for more precise methods to assess RFR. An important consideration in interpreting our findings is the time-dependent nature of serum biomarker responses to acute changes in GFR. Cystatin C may require time to reach a new steady-state concentration following changes in filtration rate, potentially limiting its ability to capture rapid, transient increases in GFR fully. This may partly explain the smaller magnitude of RFR observed with cystatin C compared to creatinine clearance. Nonetheless, the statistically significant changes observed with cystatin C suggest that it remains sensitive to detecting RFR, albeit potentially with reduced sensitivity to very short-lived fluctuations. Real-time GFR monitoring using fluorescent molecules and transdermal sensors shows significant potential for accurately and efficiently assessing RFR in future investigations40.
In conclusion, our study highlights the strong predictive value of RFR for identifying AKI risk after cardiac surgery in young adults with acyanotic CHD and normal baseline eGFR. Using cystatin C as an alternative to creatinine clearance offers a faster, more practical method for quantifying RFR. Future work should determine the relationship between RFR and long-term outcomes, such as CKD progression, and explore the use of real-time monitoring technologies to improve early risk stratification and intervention.
Data availability
The data supporting the findings of this study are not publicly available due to restrictions imposed by the University of Pittsburgh Institutional Review Board. The IRB determined that sharing the dataset could potentially compromise participant confidentiality due to the small sample size and identifiable nature of the data. However, the corresponding author (DYF) can provide the data to the editors, reviewers, or readers upon request.
References
Dellborg, M. et al. Adults with congenital heart disease: trends in event-free survival past middle age. Circulation 147, 930–938 (2023).
Fuhrman, D. Y. et al. Outcomes of adults with congenital heart disease that experience acute kidney injury in the intensive care unit. Cardiol. Young. 31, 274–278 (2021).
Fuhrman, D. Y. et al. Postoperative Acute Kidney Injury in Young Adults With Congenital Heart Disease. Ann. Thorac. Surg. 107, 1416–1420 (2019).
Roshanravan, B. et al. A prospective study of frailty in nephrology-referred patients with CKD. Am. J. Kidney Dis. 60, 912–921 (2012).
Bosch, J. P. et al. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med. 75, 943–950 (1983).
Bosch, J. P. et al. Renal hemodynamic changes in humans. Response to protein loading in normal and diseased kidneys. Am. J. Med. 81, 809–815 (1986).
Raes, A. et al. Renal hemodynamic changes and renal functional reserve in children with type I diabetes mellitus. Pediatr. Nephrol. 22, 1903–1909 (2007).
Tufro, A., Arrizurieta, E. E. & Repetto, H. Renal functional reserve in children with a previous episode of haemolytic-uraemic syndrome. Pediatr. Nephrol. 5, 184–188 (1991).
Pecly, I. M., Genelhu, V. & Francischetti, E. A. Renal functional reserve in obesity hypertension. Int. J. Clin. Pract. 60, 1198–1203 (2006).
Zitta, S. et al. Dynamic renal function testing by compartmental analysis: assessment of renal functional reserve in essential hypertension. Nephrol. Dial. Transplant. 15, 1162–1169 (2000).
Sharma, A. et al. Optimizing a kidney stress test to evaluate renal functional reserve. Clin. Nephrol. 86, 18–26 (2016).
Rodenbach, K. E. et al. Renal Response to a Protein Load in Healthy Young Adults as Determined by Iohexol Infusion Clearance, Cimetidine-Inhibited Creatinine Clearance, and Cystatin C Estimated Glomerular Filtration Rate. J. Ren. Nutr. 27, 275–281 (2017).
Fuhrman, D. Y., Maier, P. S. & Schwartz, G. J. Rapid assessment of renal reserve in young adults by cystatin C. Scand. J. Clin. Lab. Invest. 73, 265–268 (2013).
Husain-Syed, F. et al. Preoperative Renal Functional Reserve Predicts Risk of Acute Kidney Injury After Cardiac Operation. Ann. Thorac. Surg. 105, 1094–1101 (2018).
Hemmelgarn, B. R. et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303, 423–429 (2018).
Tonelli, M. et al. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann. Intern. Med. 154, 12–21 (2011).
Delanaye, P. et al. Normal reference values for glomerular filtration rate: what do we really know?. Nephrol. Dial Transplant. 27, 2664–2672 (2012).
Burton, R. F. Estimating body surface area from mass and height: theory and the formula of Du Bois and Du Bois. Ann. Hum. Biol. 35, 170–184 (2018).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138, e426–e483 (2018).
Inker, L. A. et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 385, 1737–1749 (2021).
Pierce, C. B. et al. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 99, 948–956 (2021).
Ginsberg, J. M. et al. Use of single voided urine samples to estimate quantitative proteinuria. N. Engl. J. Med. 309, 1543–1546 (1983).
Levey, A. S. et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 67, 2089–2100 (2005).
Tietze, I. N. et al. Impaired renal haemodynamic response to amino acid infusion in essential hypertension during angiotensin converting enzyme inhibitor treatment. J. Hypertens. 15, 551–560 (1997).
Gaies, M. G. et al. Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr. Crit. Care Med. 11, 234–238 (2010).
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2, 1–138 (2012).
Clifford, K. M. & Selby, A. R. Reveles KR The risk and clinical implications of antibiotic-associated acute kidney injury: a review of the clinical data for agents with signals from the Food and Drug Administration’s Adverse Event Reporting System (FAERS) database. Antibiotics 11, 1367 (2022).
Meersch, M. et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intens. Care Med. 43, 1551–1561 (2017).
Zarbock, A. et al. Prevention of Cardiac Surgery-Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: The PrevAKI-Multicenter Randomized Controlled Trial. Anesth. Analg. 133, 292–302 (2021).
Ruilope, L. M. et al. Influence of a low sodium diet on the renal response to amino acid infusions in humans. Kidney Int. 31, 992–999 (1987).
De Nicola, L., Blantz, R. C. & Gabbai, F. B. Renal functional reserve in treated and untreated hypertensive rats. Kidney Int. 40, 406–412 (1991).
Perkovic, V. et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Goldstein, S. L. et al. Consensus-Based Recommendations on Priority Activities to Address Acute Kidney Injury in Children: A Modified Delphi Consensus Statement. JAMA Netw. Open. 5, e2229442 (2022).
Helal, I. et al. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 8, 293–300 (2012).
Cooper, D. S. et al. Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury (FRAIL-AKI). Clin. J. Am. Soc. Nephrol. 11, 21–29 (2016).
Volovelsky, O. et al. Pre-operative level of FGF23 predicts severe acute kidney injury after heart surgery in children. Pediatr. Nephrol. 33, 2363–2370 (2018).
Dimopoulos, K. et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation 117, 2320–2328 (2008).
Christiadi, D. et al. Cystatin C kidney functional reserve: a simple method to predict outcome in chronic kidney disease. Nephrol. Dial. Transplant. 37, 1118–1124 (2022).
Levey, A. S. et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am. J. Kidney Dis. 75, 84–104 (2020).
Solomon, R. & Goldstein, S. Real-time measurement of glomerular filtration rate. Curr. Opin. Crit Care. 23, 470–474 (2017).
Acknowledgements
We would like to thank Ms. Morgan Hindes for her help in coordinating this study. We are also indebted to the Pediatric Clinical and Translational Research Center at UPMC Children’s Hospital of Pittsburgh for completing the renal function reserve portion of the study. Above all, we are grateful to the study participants for their cooperation and enthusiasm in helping us complete this work.
Funding
This study was supported by a National Institutes of Health (NIH) Career Development Award (K23) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), grant number K23DK116973 (DYF). The funding provided through this award supported the development and implementation of the study protocol, data collection, and analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NIDDK.
Author information
Authors and Affiliations
Contributions
Conception and Design (DYF, GJS, JAK), Methodology (DYF, GJS, DSC, AKH, JAK), Data Collection (DYF), Data Analysis and Interpretation (DYF, VBT), Original Draft Preparation (DYF), Reviewing and Editing of the manuscript (DYF, GJS, DSC. VBT, AKH, JAK).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fuhrman, D.Y., Schwartz, G.J., Cooper, D.S. et al. Preoperative renal functional reserve as a predictor of acute kidney injury in young adults with congenital heart disease. Sci Rep 15, 23690 (2025). https://doi.org/10.1038/s41598-025-09461-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09461-6
Keywords
This article is cited by
-
The promise of biomarkers: precision medicine will pave a roadmap for pediatric acute kidney injury management in critically ill children
Intensive Care Medicine – Paediatric and Neonatal (2025)
-
Each nephron is worth every heartbeat: navigating acute kidney injury in children post-cardiac surgery
Intensive Care Medicine – Paediatric and Neonatal (2025)