Abstract
Anthropogenic underwater noise from seismic airguns is increasing, posing a potential threat to marine life. Despite their ecological importance, knowledge of the impacts of seismic surveys on zooplankton remains limited. In the North Sea, we utilized various methods to assess potential effects on zooplankton around an ongoing seismic survey. A seismic airgun array (3060 inch3) performed a survey along parallel lines, while we sampled from a research vessel positioned at the end of three of these lines. The airgun-generated noise reached a maximum broadband sound exposure level of 182 dB re 1µPa2s at the closest proximity (~50 m). Zooplankton biomass exhibited a consistent distribution in line with hydrography and chlorophyll distribution, before and after airgun exposure. Immediate mortality in Calanus spp. was uniform across sound levels, never exceeding 35.9%. Cultured Calanus finmarchicus were exposed while submerged in bags. These displayed low immediate mortality (<10%), with an increasing trend (<30%) up to 7 days post-exposure. These findings highlight the interplay between sound exposure, environmental conditions, and the impact on zooplankton in areas of seismic activity, indicating that immediate impacts of seismic surveys on zooplankton may be limited. However, delayed impacts that could lead to population effects require further investigation.
Similar content being viewed by others
Introduction
The impact of anthropogenic underwater noise on marine life has emerged as an increasing cause of concern1,2. Anthropogenic underwater noise is comprised of diverse sources, encompassing continuous noise such as boat traffic and operational wind turbines, as well as impulsive noise from activities such as pile driving, underwater explosions, and seismic surveys using airguns that produce loud sounds to map the sea bottom3. While the effects of seismic surveys on marine mammals and fish, such as acoustic masking, hearing loss, behavioral changes, and physical damage, are relatively well-explored4,5,6,7,8,9,10, our understanding of their impact on zooplankton is limited11.
Seismic surveys, involving the use of seismic airgun arrays, are globally employed for oil and gas exploration and seafloor mapping, often spanning weeks or months6. On the Norwegian Shelf, seismic exploration began in the late 1960s and now covers an area of approximately 20 000 km2 annually (as of 2021)12. During these surveys, arrays of airguns, typically organized in sets of 18 to 48 airguns, generate acoustic waves through the sudden release of high-pressure air stored in the airguns. The energy of the generated sound is mainly concentrated within low frequencies (below ~100 Hz). Although the sound pressure amplitude decreases with distance, the lower frequencies can propagate over hundreds to thousands of kilometers from the source because they are less absorbed than higher frequencies13,14. Given the extensive and ongoing utilization of seismic surveys in diverse exploration activities, it is essential to enhance our understanding of the potential impacts of these surveys on marine life.
Zooplankton play a crucial role in pelagic food webs and contribute to the biological pump15. In the North Atlantic Ocean, the large (2–4 mm) copepod Calanus spp. is pivotal in the food web, serving as the primary food for many commercially important fish species, such as mackerel (Scomber scombrus), herring (Clupea harengus) and blue whiting (Micromesistius poutassou)16,17. The consequences of seismic surveys on zooplankton are not yet fully understood, but potential impacts include physical harm caused by fluid motion18, disorientation due to effects on their sensory systems19 or altered behavior due to rapid pressure changes related to airguns20.
Despite the ecological importance of zooplankton, knowledge on the effects of seismic surveys is scarce, with existing studies presenting inconsistent results11,21,22. For example, McCauley et al. (2017) observed a ~2.5-fold increase in dead zooplankton following exposure to a relatively small airgun (150 inch3) up to 1 km away from the source19. In contrast, Parry et al. (2002) found no discernible effects on bivalve larvae mortality after exposure to a full airgun array (3542 inch3)23. Mortality caused by the propeller of the sampling vessel were not controlled for in either of these studies. Fields et al. (2019), using a different approach without a vessel and exposing zooplankton in bags, reported lower mortality (max. 15% in exposed vs. ~2% in control) in the large copepod C. finmarchicus, but only at close proximity (<5 m) to two slightly larger airguns (each 260 inch3) than those used in McCauley et al. (2017)18,19. Similarly, Vereide et al. (2023) observed higher but limited mortality (14% in exposed vs. 4% in controls) in Acartia tonsa nauplii exposed to an array of two small airguns (each 40 inch3)24, and Pearson et al. (1994) reported no mortality in Cancer magister larvae exposed to seven airguns (total 840 inch3)25. Overall, studies investigating zooplankton mortality following seismic exposure demonstrate diverse outcomes influenced by various airgun sources and distances from the source.
To the best of our knowledge, this study is the first to investigate the effects of a seismic survey on zooplankton mortality and distribution across varying distances and nearby (<50 m) an ongoing seismic survey using a full airgun array. Most experiments exploring the impact of anthropogenic noise on invertebrates have been conducted in the laboratory11,26. While laboratory experiments contribute valuable insights into the mechanisms involved, large scale field experiments near actual seismic surveys (this study) can provide direct evidence of the effects of real noise sources in a natural environment21,27,28.
To assess the influence of seismic surveys on zooplankton mortality and distribution at various distances from the seismic source, and to characterize the sound levels generated by the seismic airgun array, we utilized a diverse range of experimental methods and field sampling techniques. Our approach encompassed investigations into both natural zooplankton populations and cultured copepods, providing a comprehensive understanding of the potential impacts of seismic surveys on zooplankton.
Results
Sound levels
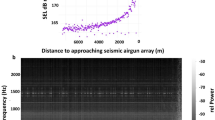
Sound levels were measured with a hydrophone deployed from the research vessel during all three seismic approaches. From a distance of approximately 6 km from the seismic source, airgun-generated sound exceeded the noise from the research vessel of 158 ± 0.5 dB re 1 µPa² s (broadband SEL; Fig. 1). The sound exposure level showed a consistent relation with distance across all three seismic approaches (1–3), reaching its maximum output of 182 dB re 1 µPa² s in the broadband spectrum closest to the hydrophone (seismic approach 3). For the peak-to-peak pressure levels (Pa), the maximum was 35 kPa (seismic approach 3; Fig. 1).
Bottom-mounted echosounders (WBAT)—zooplankton vertical movement
Zooplankton vertical movement was recorded using acoustic data from three bottom-mounted, upward-facing echosounders positioned at fixed locations during the seismic approaches. The dispersion of zooplankton (inertia; I) increased with distance from the seismic airguns for the two approaches included in the analyses (GLM, Approach 1; p = 0.008, Approach 3; p <0.001; Fig. 2). In contrast, there was no significant effect of the decreasing distance with the seismic source on the center of mass of zooplankton along the entire seismic approach (GLM, Approach 1; p = 0.178, Approach 3; p = 0.603; Fig. 2), indicating no or limited changes in vertical movement in zooplankton due to seismic shooting. The variability between the seismic approaches exceeded the fluctuations observed along a single seismic approach (Fig. 2) but was smaller than the changes in distribution governed by the diel vertical migration of the dominating zooplankton.
Overview of the WBAT data from the seismic approaches that was included in the analysis (1, 3) during exposure. A Approach 1 (1st of May), B Approach 3 (5th of May), C), Centre of mass (CM; m) and inertia (I; m− 2) during seismic approach 1 and 3 for distance intervals up to 9 km away from the seismic source. All echograms from the specific exposure dates from each WBAT can be found in Supplementary Material.
Zooplankton biomass—vertical distribution
The vertical distribution of zooplankton biomass was assessed with a Multinet, which sampled zooplankton at discrete depth intervals at the start and end of each seismic approach. The 1000–2000 μm size fraction, comprised primarily of C. finmarchicus (~90%) and Calanus helgolandicus (~10%), dominated the zooplankton biomass across the various depth intervals and stations (Fig. 3). During daytime, much of the zooplankton was distributed around the deep chlorophyll-a maximum (Fig. 3, see Supplementary Material for environmental conditions). Overall, the zooplankton vertical distribution appeared relatively similar before and after seismic shooting activity (Fig. 3). However, in seismic approach 1, the biomass peak shifted from 20–30 m depth to 10–20 m depth post-shooting. The peak abundance of zooplankton was deeper (20–30 m) in seismic approach 3 compared to 1 and 2, in correspondence with a wind-induced deepening of the distribution of chlorophyll-a. During the night (control), zooplankton migrated upwards, with a biomass peak of 0.8 g DW m− 3 in the upper 10 m (Fig. 3).
Zooplankton biomass (g DW m− 3) within different size fractions (≤ 1000 μm, 1000–2000 μm, ≥ 2000 μm) and animal categories; fish, krill, and chaetognaths, for depth intervals 0–10 m, 10–20 m, 20–30 m, 30–45 m, 45–60 m. A complete overview of sampled taxa from seismic approach 1 (before), seismic approach 3, and night control can be found in Supplementary Material.
In terms of abundance, small echinoderm larvae dominated across all samples in the surface layers. A comprehensive overview of all represented taxa and the distribution of Calanus spp. developmental stages from seismic approach 1 and 3 is given in Supplementary Material.
Immediate mortality of field caught Calanus spp.
To assess the immediate mortality of in situ zooplankton, WP2 nets were deployed multiple times during each seismic approach, and the proportion of live zooplankton was determined using Neutral Red Stain. The highest recorded mortality occurred at a distance of 6 km from the seismic vessel (SEL ~163 dB re 1 µPa2 s) where 35.9% of the copepods were dead. Aside from this particular instance, the mortality fraction consistently remained below 30% (Fig. 4), with no consistent patterns along the transects. In other words, there was no significant correlation between the immediate mortality of field caught Calanus spp. and distance from the seismic source during exposure (Spearman correlation test, nWP2 nets=27, p = 0.27) (Fig. 4). Additionally, there was no difference in mortality between Calanus spp. sampled during and after exposure (Mann Whitney, nWP2 nets=37, p = 0.56).
Mortality in large copepods from seismic approach 1–3, sampled at different distances from the seismic source. The squared shapes were sampled during shooting as the seismic vessel was approaching, and the circular shapes were sampled after the seismic vessel had passed the research vessel and stopped shooting.
Immediate and delayed mortality of cultured Calanus
The immediate and delayed mortality (up to 7 days after exposure) were measured in cultured C. finmarchicus using two main approaches; (i) with bags of 10 individuals introduced at different distances to the seismic airgun source during all three seismic approaches, and (ii) with bags containing 50 individuals introduced close to the seismic airgun source during one seismic approach.
In the bags with 10 individuals, the cumulative mortality in the bags exposed from close distance was significantly higher than the controls (GLM, nbags=12 (control), 18 (handling control), 9 (far exposure), 36 (close exposure), p = 0.007; Fig. 5). After 7 days, the mortality was 25.1 ± 15.3% (mean ± SD) in the airgun exposure from close distance, 22.3 ± 14.2% from far distance (>6 km), and 12.7 ± 14.2% and the 11.7 ± 7.1% in the regular and handling controls. However, the immediate mortality was not significantly different from the controls at close (5.4 ± 8.2%) or far (2.3 ± 4.7%) distance from the seismic source.
The proportion of dead individuals (C. finmarchicus) immediately after treatment (day 0) and up to 7 days after. Upper panel) Bags of 10 individuals (exposure from close distance; 89–1192 m from the seismic vessel; n = 36, exposure from far distance; >6 km from the seismic vessel; n = 9, normal control; n = 12, and handling control; n = 18. Lower panel) Bags of 50 individuals (exposure from close distance; 89–178 m from the seismic vessel; n = 4, and handling control; n = 4.
In the bags with 50 individuals, the cumulative mortality in the airgun exposed C. finmarchicus from a close distance (89–178 m) was lower than in the bags with 10 individuals from close distance (89–1192 m). In the bags with 50 individuals, there were no dead individuals in either the airgun exposed treatment (89–178 m from the airgun source) or the handling control immediately after treatment (Fig. 5). There was no significant effect on the cumulative mortality up to 7 days after treatments in the airgun exposed C. finmarchicus (GLM, nbags=4 (per treatment), p = 0.63). On day 7, the mortality in the airgun exposed copepods was 13.3 ± 5.8% in comparison to 8.8 ± 6.2% in the control.
Discussion
The results of this study showed that discharge from seismic airguns (a maximum of 182 dB re 1 µPa2 s) caused no detectable change in in situ zooplankton immediate mortality or vertical distribution (center of mass). However, zooplankton spread (inertia) increased closer to the seismic source, and the cultured copepods near the source exhibited increased mortality up to 7 days after exposure.
In our study, the immediate mortality of wild Calanus spp. surpasses that previously reported in C. finmarchicus18, where only two small airguns were used. On the other hand, the mortality was considerably lower (<30%) than the levels reported in McCauley et al. (2017) (~45%) who used lower sound levels (156 dB re 1 µPa² s-1 from 509 to 658 m)19. They also found high mortality in samples primarily dominated by krill larvae and smaller copepods like Acartia spp. In contrast, Pearson et al. (1994)25 and Parry et al. (2002)23 found limited to no effects of airgun blasts on zooplankton, albeit with other species than those studied here. These disparities suggest that effects on zooplankton may be influenced by the number of airguns, directionality of the exposure or be species specific. For example, our study showed no immediate effects on Calanus spp. yet smaller copepod species or developmental stages might respond differently to seismic exposure. For example, Vereide et al. (2023)24 reported increased mortality and decreased development in response to seismic exposure on Acartia tonsa nauplii and Vereide et al. (2024)20 reported a higher mortality in Acartia than in Calanus when comparing the effect of a pressure drop.
Accounting for background mortality is essential for accurately assessing the effects of anthropogenic disturbances on zooplankton. In areas without seismic activities, natural mortalities range from 11.6 to 59.8% (reviewed by29), reflecting high natural non-predatory mortality due to factors like senescence, turbulence, temperature, or parasitism. The high variation in natural mortality mirrors the observations in our study, where the mortality varies along the entire seismic approach (Fig. 4). In fact, background noise levels from our research vessel (158 ± 0.5 dB re 1 µPa² s; broadband SEL) exceeded reported levels of airguns from similar studies19.
Our bag experiments provided a controlled setup eliminating sources of potential damage by vessels, e.g., propeller turbulence30. Overall, the exposure of C. finmarchicus in bags did not show a discernible pattern in immediate mortality, with consistent rates observed up to 6 km from the seismic source. Nonetheless, initial sub-lethal or delayed effects may still be present, as indicated by the delayed mortality in the copepods that were exposed to the seismic source nearby. Although not measured in this study for in situ zooplankton, delayed effects are a critical aspect of the overall impact and should be considered when assessing the broader implications. It is also important to note that increased predation, caused by e.g., physical harm or less activity, is excluded when using bags. Therefore, additional indirect causes of mortality directly due to the airguns should be considered in future investigations.
The biomass distribution showed that zooplankton congregating around the pycnocline and subsurface chlorophyll maximum, as is often the case31,32,33,34. While the center of mass (CM) of the zooplankton layer remained constant with distance from the seismic source, the spread (inertia) varied along the transect, indicating shifts in the distribution of individuals within the layer. This suggests that certain taxa might be more affected than others or move asynchronously within the layer19.
While both copepods and echinoderm larvae are common in similar areas35, the community composition may be influenced by offshore constructions and activities. For example, the installation of wind turbine foundations can create new hard substrates, potentially leading to an increase in the population of hard-bottom species and thereby also meroplankton36. Possibly, the large presence of echinoderm larvae may be partially attributed to offshore constructions, fostering alterations in the local food web dynamics37.
In conclusion, the data show limited direct impacts of seismic activity on zooplankton mortality and distribution, and a potential for a delayed impact due to delayed mortality. The natural variation in mortality and vertical distribution exceeded the effect of seismic exposure on in situ zooplankton, indicating that direct effects of seismic surveys on zooplankton are limited and species-specific. Whether the delayed effect on mortality we found can lead to population-level impacts needs to be further investigated.
Materials and methods
This study was conducted in the North Sea (3°20 E, 56°55 N; Fig. 6), between the 1st and 5th of May 2022. The experiments (Table 1) were carried out aboard the Institute of Marine Research (IMR) research vessel RV Kristine Bonnevie during a seismic survey, where a seismic airgun array surveyed along parallel lines. To examine zooplankton layers acoustically before, during and after an approach of a seismic vessel, bottom-mounted upward-looking Wide Band Autonomous Transceivers (WBAT; EK80), were located at the end of three of the seismic lines. Prior to each seismic line being surveyed, the research vessel was positioned at the end of the seismic line, where net sampling and exposure experiments were conducted to assess zooplankton mortality at varying distances from the seismic source. The seismic vessel then approached the stationary research vessel and echosounder at 4.5 knots, firing along the seismic line. This procedure - referred to as seismic approaches 1–3 throughout the paper - was repeated three times. No seismic activity occurred within 2 nautical miles of the observation station for at least 48 h before each approach.
Map of the study site and overview of the positions of the two vessels during experimental stations. Left upper panel) location of the survey area in the North Sea. Right upper panel) Overview of the position of the stationary research vessel (grey square), the WBAT (bottom mounted echosounders) (black diamond) and the position of the approaching seismic vessel when WP2 plankton net (blue circle) or line with bags (red triangle) were deployed by the research vessel (in the grey square position). The three panels show seismic approaches 1, 2 and 3. Lower panels) Close-up view of each of the seismic approaches near the WBAT and the research vessel. The three seismic approaches were conducted 1st of May (approach 1), 2nd of May (approach 2), and 5th of May (approach 3).
At the observation site, multiple parameters were measured before, during, and after each seismic approach. Environmental conditions were assessed using CTD casts, along with measurements of chlorophyll-a, turbidity, and light intensity. Sound levels were recorded at 10 m depth with a hydrophone during each seismic approach. Zooplankton species composition at discrete depths was sampled using Multinets before and after each approach. Continuous WP2 net tows from the research vessel allowed for immediate mortality assessment using Neutral Red Stain. Finally, to assess the effects of the seismic sound in the absence of propeller damage or possible previous seismic exposure, cultured C. finmarchicus (CV/CVI stages) were exposed to seismic airguns in bags placed at various distances and times during each seismic approach. Detailed descriptions of each experimental method are provided below.
Study site
The field experiments took place in the southern part of the Norwegian sector in the North Sea (3°20 E, 56°55 N) across a uniform depth of ~70 m (Fig. 6). Seismic surveys have been conducted annually in this area since 1971. In 2022, the seismic survey began on 18th of April, 13 days before the field experiments.
To ensure that the zooplankton drifted in the direction of the research vessel after exposure, the research vessel was positioned downstream from the approaching seismic vessel. To guide this positioning, Copernicus Marine Data Store provided predictions from the Northwest Shelf (NWS) oceanographic model. A custom Python script, utilizing the MOTU APU, was employed to access and download daily predicted speeds and directions in the specific area of experiments. A comparison between model predictions and measurements from a vessel-mounted ADCP (RDI 150 kHz ADCP) was conducted.
Environmental data
The conductivity, temperature, and depth were measured with a CTD (SBE 911plus). The CTD was equipped with a fluorometer (Chelsea Aqua 3), transmissometer (LI-COR Biospherical), and oxygen sensor (SBE 43). Chlorophyll concentrations were measured in water samples collected with NISKIN bottles (5 L) at different depths (~5, ~10, ~20, ~30, ~50, and ~65 m). The water samples were filtered, the filters extracted in 90% acetone, and the extracts centrifuged and measured fluorometrically using Turner Design model 10 AU38.
Seismic airgun source and survey operations
An airgun array of 24 (12 clusters × 2 airguns) Sercel G-Gun II airguns (operating pressure 2000 psi, total volume 3060 inch3) was used as the seismic source during an ongoing seismic survey. The array consists of three sub-arrays (6 m apart) with a length of 8.55 m and 12 m width. The depth of the airguns was 5 m ± 0.5 m below the surface. The airgun source was towed 50 m behind the seismic vessel and discharged every 50 m (every ~10.7 s), while the vessel traveled at 4.5 knots.
Sound measurements
Ambient sound and the sound from the seismic survey was recorded with an Ocean Sonics Eth-X2 hydrophone (sensitivity 205 dB re 1 µPa) deployed at 10 m depth midship at the port side of the research vessel. The hydrophone recorded at varying time intervals during each of the three seismic approaches. During the approaches, the seismic ship was approaching from ~10 km until it passed the research vessel, whereupon shooting stopped at the end of a seismic line. In addition, we recorded ambient sound without seismic shooting.
In our acoustic analysis, we measured the broadband Sound Exposure Level (SEL) across the frequency range of 10 Hz to 20 kHz over 10 s intervals. In addition, we reported the maximum peak-to-peak pressure (Pa) in each 10 s interval. Given that the intervals (10 s) used in the sound analyses were shorter than the actual airgun shot intervals (~10.7 s), some measurements did not include seismic shots.
Zooplankton vertical movement
Bottom-mounted echosounders (WBAT)
Three bottom-mounted upward-looking echosounders, WBAT EK80, were deployed at fixed positions prior to the cruise (12th of April 2022; Fig. 6). The positions were set at the end of the three seismic lines (Fig. 6). Each WBAT continuously recorded acoustic data from 5 m above the seafloor at 200 kHz.
To assess the distribution of zooplankton, we utilized the center of mass and inertia as our primary indicators39. The center of mass (CM), determined through volume backscattering coefficient values (sv; dB re 1 m− 1), served as key metrics for depicting the spatial arrangement and variability of population densities40. For the associated equations, see Supplementary Material. The inertia (I) represented the dispersion (spread) of zooplankton along the water column39. Active acoustic data was analyzed using LSSS (Large Scale Survey System, version 2.14.0) software. The surface layer (depth varying between 5 and 20 m depending on weather conditions) was excluded from the analysis due to its susceptibility to disturbances from the surface (e.g., wind, turbulence). The echogram analysis involved distinguishing acoustic signals from different animals, focusing particularly on backscatter from zooplankton41,42,43. Reports were generated in the form of nautical area scattering coefficients (sA) gridded with a resolution of 2 m on the depth-axis and 2 s on the time-axis of the echogram.
To select the appropriate frequency for zooplankton detection, factors such as size, shape, and density must be considered. While 333 kHz would likely provide better resolution for copepods, particularly Calanus species42, it is limited to a range of about 50 m, whereas 200 kHz extends to 100–200 m, covering a larger portion of the water column. In this study, all three WBATs offered 200 kHz, while only one supported 333 kHz. We therefore used the 200 kHz in our analyses and used the 333 kHz to confirm that the 200 kHz reflected mainly zooplankton (Supplementary Material).
Multinets
Zooplankton vertical distribution was examined with a 0.25 m2 HydroBios Multinet (180 μm mesh size) and sampled at discrete depth intervals during a vertical haul (60 − 45, 45 − 30, 30 − 20, 20 − 10, 10 – 0 m). Each haul was conducted at a mean retrieval speed of 0.176 m− s. The Multinet was deployed at the beginning (4–6 km from the seismic vessel) and end of each seismic approach (~1 h after passing). In addition, one Multinet was deployed as a night control (3rd of May, 21:19 UTC). The Multinet zooplankton samples from each depth interval were split using a Motodo plankton splitter and half of the final sample was further split into three size fractions (<2 mm, 1–2 mm, 1–180 μm), rinsed in freshwater, and then dried. From the 2 mm fraction, chaetognaths, fish larvae, krill, and amphipods were identified, enumerated, and dried. The remaining half of the final sample was fixed in 4% borax-buffered formalin for later identification and counting. A complete overview of species distribution is provided in the Supplementary Material. The Multinet samples were also used for ground truthing the acoustic data.
Zooplankton mortality
Immediate mortality of field caught zooplankton
To determine the immediate mortality of in situ zooplankton, WP2 nets (180 μm mesh size) were deployed multiple times as the seismic vessel approached (Fig. 6). This procedure was replicated three times (seismic approaches 1–3). The net was hauled from near the bottom (~2 m) to the surface. We used WP2 nets instead of Multinets, for this purpose, because these allowed for a higher sampling frequency as the seismic vessel approached.
Staining
To quantify the proportion of live and dead copepods, the samples from the WP2 nets were stained with Neutral Red Stain (Table 1), following Elliot and Tang (2009)44. After rinsing, the samples were split, and ½ were preserved in formalin (4% buffered), and the other ½ split again. The two ¼ fractions were photographed in Petri dishes using a Sony α7R IV 35 mm full-frame camera (ILCE-7RM4) equipped with a Sony FE 90 mm f/2,8 Macro G OSS lens with manually fixed settings. All images included a scale bar for size calibration. Light was provided from below by a Reflecta light Table (10319), and the entire setup was covered by a black tent. The images were stored as 60.1 Mpixel raw files. Finally, the samples were filtered through a 100 μm mesh (⌀=4.5 cm) and stored in Petri dishes (⌀=5 cm) at -20 °C.
At all staining times, we also stained, photographed, and stored batches of dead zooplankton to control for the diffusive uptake of pigment in dead zooplankton44. As the animals in our study appeared lighter than the images in Elliot and Tang (2009)44 and Daase and Søreide (2021)45, we conducted an ad hoc test of the effect of staining time. While increased staining time may facilitate visual inspection, our automated image analysis correctly classified animals as alive or dead at all staining times.
Image analysis
To ensure objectivity within the subtle staining gradients, we developed macros within the open-source image analysis software ImageJ (version 1.53e;46). These macros enabled the application of color thresholding to highlight areas that had reached specific staining levels. Pixels meeting these criteria were marked in a vivid green color. We then analyzed color-thresholded control images of live and dead plankton samples. Plankton displaying green marking, as determined through visual observation, were classified as live (see Supplementary Material for examples). Our images were then systematically categorized into taxa and dead/live using an ObjectJ macro that allowed manual counting and marking into categories.
Immediate and delayed zooplankton mortality—cultured C. finmarchicus
To assess delayed and immediate mortality, we used cultured C. finmarchicus of known origin and exposure history. The animals were sourced from the running culture at NTNU SeaLab in Trondheim47. Prior to departure, approximately 3000 copepodites in stage V were shipped as air freight in insulated containers at a density of 75 individuals L− 1. C. finmarchicus (CV) was maintained at 10 °C in a climate container on deck on a light: dark cycle of 12:12 h, in 20 L buckets (375 individuals in each bucket) filled with 0.2 μm filtered seawater (FSW) with continuous aeration.
Pre-exposure
Before each experiment, 10 or 50 individuals (Table 2) were transferred to transparent plastic bags (Lamizip®, 1 L, internal measures 144 × 227 mm, thickness 0.16 mm, PET/PE) filled with FSW (10 °C). We enclosed the bags with no air inside. The bags were attached to a set of lines, in random order but at a given distance (Table 2). Bags with 10 individuals were placed 0.8 m apart with three bags per line (Table 2). Bags with 50 individuals had 0.4–0.8 m between bags with four bags per line.
Exposure
The bags containing copepods (Table 2) were lowered into the water by a crane (at the same speed for all treatments, ~0.3 m− s) to a depth of 10 m (based on the middle bag). The bags were maintained at 10 m for 15 s before they were lifted back into the vessel. The 15 s window was chosen to allow multiple exposures to the seismic source from varying distances, rather than a single prolonged exposure.
With the bags of 10 individuals, this procedure was conducted with five sets of lines for each seismic approach (1–3). The first line of bags was deployed at the beginning of each seismic approach when the seismic vessel was 6–12 km away from the research vessel (dependent on the distance of the seismic line; Fig. 6). Thereafter, four lines of bags were deployed when the seismic vessel was near the research vessel (Fig. 6). Once the first line was onboard, we deployed the second, third, and fourth lines of bags, following the same procedure (Fig. 6). In addition to the three seismic approaches, there was a control treatment in which the same procedure was conducted without any seismic shooting (Table 2). We also kept three bags of 10 individuals in the climate container during exposure to control for mortality due to handling (handling control). With the four bags of 50 individuals, the procedure was only conducted at the closest distance to the seismic vessel (Table 2). Here, four additional bags were kept in the climate container for handling control.
Post-exposure.
Upon retrieval to deck, the bags were immediately placed in a climate container at 10 °C. We examined all individuals by visual inspection and noted whether they were dead or alive. Live copepods were transferred to individual bottles (50 mL, cell culture flask) prefilled with FSW (10 °C). Bottles were kept at 10 °C for 7 days, during which they were examined by visual inspection daily for mortality. Animals were not fed during the study period.
Data analysis
All data analyses were conducted in the R statistical software (version 4.2.2;48). For all analyses, a significance threshold of 5% was used.
The effect of the approaching seismic airgun array on the vertical distribution of zooplankton was assessed using a General Linear Model (GLM) separately for each seismic approach. Due to differences in transducer configurations, the WBATs collected varying amounts of data. WBAT 2, equipped with two split-beam transducers, had to transmit sequentially on each channel, resulting in less than half the data collected on the 200 kHz channel compared to WBAT 1 (one split-beam and one single-beam) and WBAT 3 (one split-beam), which could transmit simultaneously. As seismic approach 2 (~ 5690 m) relied on WBAT 2 which was substantially shorter than approaches 1 (~ 7530 m) and 3 (~ 8820 m), and with fewer measurements, echosounder data analysis was limited to seismic approaches 1 and 3. In these models, the center of mass (CM) was used as the response variable and distance as the continuous variable. Similarly, inertia (I) was used as the response variable, with distance maintained as the continuous variable.
To test the effect of seismic exposure on the mortality of field-caught Calanus spp., we tested the correlation between proportion of dead individuals and distance from the seismic source using a Spearman correlation test. Additionally, we tested the difference in mortality between the Calanus spp. sampled during exposure and the Calanus spp. sampled after exposure using a Mann Whitney test.
We tested the effect of treatment on mortality in C. finmarchicus after exposure in bags in different ways. Firstly, to test the impact of treatment on cumulative mortality (up to 7 days after treatment) in the bags containing 10 individuals, we applied a binomial GLM. We used the proportion of dead individuals as a response variable. We included the treatment (seismic exposure close (89–1192 m), seismic exposure far (>6 km), control, handling control) and time after treatment (day 0 to 7) as fixed effects, as well as the interaction between time and treatment. Furthermore, to investigate the immediate mortality effects of treatment in the bags of 10 individuals, a separate binomial GLM was implemented. This model focused solely on mortality immediately after treatment and utilized the proportion of dead individuals as a response variable, and treatment as a fixed effect. Secondly, for the bags of 50 individuals, the effect of treatment on mortality was tested using a binomial general linear model (GLM). Similarly, we used the proportion of dead individuals as a response variable. We included treatment (seismic exposure (89–178 m from the seismic source) and handling control), and time after treatment (day 0 to 7) as fixed factors, as well as the interaction between time and treatment.
Data availability
The datasets generated during and/or analysed during the current study are available in the Amazon S3 service (Institute of Marine Research repository); this repository contains the raw data collected during the cruise. https://ftp.imr.no/nmdc/IMR/Doc/S3-Cruise.pdf.
References
Duarte, C. M. et al. The soundscape of the anthropocene ocean. Science 371, eaba4658 (2021).
Kok, A. et al. How chronic anthropogenic noise can affect wildlife communities. Front. Ecol. Evol. 11, 1130075 (2023).
Hildebrand, J. A. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 395, 5–20 (2009).
Gordon, J. et al. A review of the effects of seismic surveys on marine mammals. Mar. Technol. Soc. J. 37, 16–34 (2003).
Popper, A. N. & Hawkins, A. D. An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. J. Fish Biol. 94, 692–713 (2019).
Slabbekoorn, H. et al. Population-level consequences of seismic surveys on fishes: an interdisciplinary challenge. Fish Fish. 20, 653–685 (2019).
Affatati, A. & Camerlenghi, A. Effects of marine seismic surveys on free-ranging fauna: a systematic literature review. Front. Mar. Sci. 10, 2296–7745 (2023).
McCauley, R. D., Fewtrell, J. & Popper, A. N. High intensity anthropogenic sound damages fish ears. J. Acoust. Soc. Am. 113, 638–642 (2003).
Handegard, N. O., Tronstad, T. V., Hovem, J. M. & Jech, J. M. Evaluating the effect of seismic surveys on fish — the efficacy of different exposure metrics to explain disturbance. Can. J. Fish. Aquat. Sci. 70, 1271–1277 (2013).
Kavanagh, A. S., Nykänen, M., Hunt, W., Richardson, N. & Jessopp, M. J. Seismic surveys reduce cetacean sightings across a large marine ecosystem. Sci. Rep. 9, 19164 (2019).
Vereide, E. H. & Kühn, S. Effects of anthropogenic noise on marine zooplankton. The Effects of Noise on Aquatic Life: Principles and Practical Considerations (eds Popper, A. N., Sisneros, J., Hawkins, A. D. & Thomsen, F.) Cham: Springer International Publishing. 1–24 (2023).
Seismic, N. D. P. Norwegian Petroleum Directory edited by N. P. Directorate. ndp.no (2021).
Gavrilov, A. Propagation of underwater noise from an offshore seismic survey in Australia to antarctica: measurements and modelling. Acoust. Australia. 46, 143–149 (2018).
Erbe, C. et al. Managing the effects of noise from ship traffic, seismic surveying and construction on marine mammals in Antarctica. Front. Mar. Sci. 6, 647 (2019).
Pinti, J. et al. Model estimates of metazoans’ contributions to the biological carbon pump. Biogeosciences 20, 997–1009 (2023).
Dalpadado, P., Ellertsen, B., Melle, W. & Dommasnes, A. Food and feeding conditions of Norwegian spring-spawning herring (Clupea harengus) through its feeding migrations. ICES J. Mar. Sci. 57, 843–857 (2000).
Prokopchuk, I. & Sentyabov, E. Diets of herring, mackerel, and blue whiting in the Norwegian sea in relation to Calanus finmarchicus distribution and temperature conditions. ICES J. Mar. Sci. 63, 117–127 (2006).
Fields, D. M. et al. Airgun blasts used in marine seismic surveys have limited effects on mortality, and no sublethal effects on behaviour or gene expression, in the copepod Calanus finmarchicus. ICES J. Mar. Sci. 76, 2033–2044 (2019).
McCauley, R. D. et al. Widely used marine seismic survey air gun operations negatively impact zooplankton. Nat. Ecol. Evol. 1, 195 (2017).
Vereide, E. H., Khodabandeloo, B. & de Jong, K. The copepod Acartia Sp. is more sensitive to a rapid pressure drop associated with seismic airguns than Calanus Sp. Mar. Ecol. Prog. Ser. 730, 15–30 (2024).
Carroll, A. G., Przeslawski, R., Duncan, A., Gunning, M. & Bruce, B. A critical review of the potential impacts of marine seismic surveys on fish & invertebrates. Mar. Pollut. Bull. 114, 9–24 (2017).
Solé, M. et al. Marine invertebrates and noise. Front. Mar. Sci. 10, 1129057 (2023).
Parry, G. D., Heislers, S., Werner, G. F., Asplin, M. D. & Gason, A. Assessment of Environmental Effects of Seismic Testing on Scallop Fisheries in Bass Strait (Marine and Freshwater Resources Institute, 2002).
Vereide, E. H. et al. Effects of airgun discharges used in seismic surveys on development and mortality in nauplii of the copepod Acartia tonsa. Environ. Pollut. 327, 121469 (2023).
Pearson, W. H., Skalski, J. R., Sulkin, S. D. & Malme, C. I. Effects of seismic energy releases on the survival and development of Zoeal-Larvae of Dungeness-Crab (Cancer-Magister). Mar. Environ. Res. 38, 93–113 (1994).
Wale, M., Briers, R. & Diele, K. Marine invertebrate anthropogenic noise research—trends in methods and future directions. Mar. Pollut. Bull. 173, 112958 (2021).
Slabbekoorn, H. Aiming for progress in understanding underwater noise impact on fish: complementary need for indoor and outdoor studies. In: The Effects of Noise on Aquatic Life II (eds. A. N. Popper & A. Hawkins) (2016).
Hawkins, A. D. &Popper A. N. A sound approach to assessing the impact of underwater noise on marine fishes and invertebrates. ICES J. Mar. Sci. 74, 635–651 (2017).
Tang, K. W., Gladyshev, M. I., Dubovskaya, O. P., Kirillin, G. & Grossart, H. P. Zooplankton carcasses and non-predatory mortality in freshwater and inland sea environments. J. Plankton Res. 36, 597–612 (2014).
Bickel, S. L., Hammond, M., Tang, K. W. & J. D. & Boat-generated turbulence as a potential source of mortality among copepods. J. Exp. Mar. Biol. Ecol. 401, 105–109 (2011).
Nielsen, T. G., Løkkegaard, B., Richardson, K., Pedersen, F. B. & Hansen, L. Structure of plankton communities in the Dogger bank area (North Sea) during a stratified situation. Mar. Ecol. Prog. Ser. 95, 115–131 (1993).
Möller, K. et al. Marine snow, zooplankton and thin layers: indications of a trophic link from small-scale sampling with the video plankton recorder. Mar. Ecol. Prog. Ser. 468, 57–69 (2012).
Trudnowska, E., Sagan, S. & Błachowiak-Samołyk, K. Spatial variability and size structure of particles and plankton in the Fram Strait. Prog. Oceanogr. 168, 1–12 (2018).
Zampollo, A. et al. The bottom mixed layer depth as an indicator of subsurface chlorophyll a distribution. Biogeosciences 20, 3593–3611 (2023).
Marques, R. et al. Response of the meso- and macro-zooplankton community to long-term environmental changes in the Southern North sea. ICES J. Mar. Sci. 81, 526–539 (2024).
Krone, R., Gutow, L., Joschko, T. J. & Schröder, A. Epifauna dynamics at an offshore foundation – Implications of future wind power farming in the North sea. Mar. Environ. Res. 85, 1–12 (2013).
Abramic, A., Cordero-Penin, V. & Haroun, R. Environmental impact assessment framework for offshore wind energy developments based on the marine good environmental status. Environ. Impact Assess. Rev. 97, 106862 (2022).
Welschmeyer, N. A. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 39, 1985–1992 (1994).
Urmy, S. S., Horne, J. K. & Barbee, D. H. Measuring the vertical distributional variability of pelagic fauna in Monterey Bay. ICES J. Mar. Sci. 69, 184–196 (2012).
Foote, K. G. Linearity of fisheries acoustics, with addition theorems. J. Acoust. Soc. Am. 73, 1932–1940 (1983).
Macaulay, M. C. A generalized target strength model for euphausiids, with applications to other zooplankton. J. Acoust. Soc. Am. 95, 2452–2466 (1994).
Stanton, T. K., Chu, D. & &Wiebe, P. H. Acoustic scattering characteristics of several zooplankton groups. ICES J. Mar. Sci. 53, 289–295 (1996).
Lavery, A. C. et al. Determining dominant scatterers of sound in mixed zooplankton populations. J. Acoust. Soc. Am. 122 (6), 3304–3326 (2007).
Elliott, D. T. & Tang, K. W. Simple staining method for differentiating live and dead marine zooplankton in field samples. Limnol. Oceanography-Methods. 7, 585–594 (2009).
Daase, M. & Søreide, J. E. Seasonal variability in non-consumptive mortality of Arctic zooplankton. J. Plankton Res. 43, 565–585 (2021).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to imageJ: 25 years of image analysis. Nat. Methods. 9, 671–675 (2012).
Hansen, B. H., Altin, D., Nordtug, T. & Olsen, A. J. Suppression subtractive hybridization library prepared from the copepod Calanus finmarchicus exposed to a sublethal mixture of environmental stressors. Comp. Biochem. Physiol. Part. D Genomics Proteom. 2, 250–256 (2007).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022).
Acknowledgements
This study was a part of the project ZoopSeis (#302675), funded by the Research Council of Norway. KdJ, ACUP and SK were additionally funded through Orchestra (#339519, #03F0932B), funded by the Research Council of Norway and the Federal Ministry of Education and Research (BMBF), Germany. We particularly thank Akvaplan-niva and the project Glider II, funded by ConocoPhillips, for excellent collaboration before, during, and after field experiments, and for the opportunity to conduct field experiments around the seismic survey. We also thank ConocoPhillips and Magseis Fairfield for accommodating industrial operations to allow research activity to take place. The authors thank the crew of the research vessel Kristine Bonnevie RV, and Sigurd Hannaas for helping out with the experiments. Thanks to the EMBRC-ERIC Laboratory for Low Level Trophic Interactions at NTNU Sealab for providing laboratory reared C. finmarchicus. We would like to honor our late colleague Thor Klevjer, who contributed greatly to the experimental design of this field study as well as plankton sampling and handling.
Funding
Open access funding provided by Institute Of Marine Research
Author information
Authors and Affiliations
Contributions
Vereide, Emilie Hernes (EHV; corresponding author), Utne-Palm, Anne Christine (ACUP), Titelman, Josefin (JT), Pedersen, Geir (GP), Strand, Espen (ES), Mihaljevic, Marina (MM), Kühn, Saskia (SK), Altin, Dag (DA), Thorsen, Anders (AT), Campillo, Lucie (LC), Fields, David M. (DMF), Khodabandeloo, Babak (BK), de Jong, Karen (KdJ). EHV was the lead author of the manuscript, with extensive contributions from ACUP, JT, and KdJ. All authors reviewed and approved the manuscript. The concept of the study and preparations for the field experiments were carried out collaboratively by EHV, ACUP, JT, GP, ES, SK, DMF, and KdJ. All authors participated in discussions regarding data analysis and interpretation following fieldwork. Fieldwork and experiments were conducted by the following team members: EHV: Collection of field-caught Calanus and experimental work with Calanus finmarchicus. ACUP: Cruise leader. JT: Collection of field-caught Calanus and following pictures. GP: Operation of WBAT (Wideband Acoustic Technology). ES: Collection of field-caught Calanus. MM: Collection of field-caught Calanus and help with experimental work. SK: Help with experimental work with Calanus finmarchicus. KdJ: Sound measurements and help with experimental work with Calanus finmarchicus. Preparation of experimental animals (Calanus finmarchicus) was carried out by DA. Data analysis was performed by EHV, with support from KdJ. The method for color analysis of live and dead Calanus finmarchicus was developed by AT and implemented by EHV. Specific data preparation and figure contributions included: Sound data (Fig. 1): Prepared by KdJ, with figures created by EHV. WBAT data (raw data and Fig. 2): Prepared by GP, LC, and BK. Biomass and hydrography data (Fig. 3 and Supplementary Material): Prepared by ES, with figures created by EHV. Mortality figures (Fig. 5, based on in situ Calanus and Calanus finmarchicus): Created by EHV. Map (Fig. 6): Created by EHV.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vereide, E.H., Utne-Palm, A.C., Titelman, J. et al. Zooplankton mortality and distribution around a seismic survey. Sci Rep 15, 33907 (2025). https://doi.org/10.1038/s41598-025-09465-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09465-2
Keywords
This article is cited by
-
In situ behavioral responses of crustacean zooplankton to an approaching seismic survey
Scientific Reports (2025)