Abstract
Reducing neonatal mortality is a crucial part of health-care programs. We wanted to analyze the in-hospital mortality in the tertiary referral Department of Neonatology and Neonatal Intensive Care of the Medical University of Bialystok. The study was conducted on data of all neonates admitted to the Department between 2015 and 2023 (N = 19 171). During the study period the in-hospital mortality rate was 5.16 per 1000 live births and it was the highest in 2021 (8.15 per 1000 live births, p < 0.05). The leading underlying cause of death was extreme prematurity. 43.75% of the extremely preterm or extremely low birth weight neonates had a congenital/hospital acquired infection and 54.69% were not administered a full course of recommended antenatal corticosteroids. The in-hospital mortality rate in the Department was significantly higher than in Poland and other European countries due to the characteristics of the tertiary care Department and was generated mainly by deaths of premature neonates. It was the highest during the period of strict epidemiological restrictions related to the COVD-19 pandemic. Without detailed analyses of neonatal deaths in individual health care facilities and implementation of procedures to improve quality of care, it will not be possible to reduce the number of neonatal deaths.
Similar content being viewed by others
Introduction
According to World Health Organization (WHO) approximately 6500 neonates die each day. It accounts for nearly 50.00% of all deaths of children under the age of 51. In 2022 the worldwide neonatal mortality rate was 17.3 (16.2–19.3) deaths per 1000 live births2. Despite substantial global progress in reducing childhood mortality that was made in the past three decades, since 2015 survival progress has slowed significantly3. Reducing neonatal mortality is a crucial part of the 3rd Sustainable Development Goal (SDG) aimed at ensuring healthy lives and promoting well-being which is a part of the 2030 Agenda for Sustainable Development, adopted by the United Nations to provide peace and prosperity for people and our planet4. It is estimated that approximately 80% of neonatal deaths are preventable and treatable. However, adequate obstetric, antenatal and post-natal care is needed1. This aim can be achieved, among others, by understanding the causes, levels and trends in neonatal mortality5,6,7.
Despite multiple detailed studies concerning death causes of neonates and their contributing factors in developing countries, data on European countries are relatively limited. Since regional mortality patterns are crucial to plan appropriate health policy programs and ending preventable child deaths require targeted interventions, careful monitoring of neonatal deaths is important in each part of the world8.
The aim of this study was to determine the death causes, trends and risk factors of in-hospital mortality in the Department of Neonatology and Neonatal Intensive Care of The University Clinical Hospital in Bialystok, a major tertiary referral hospital in north-east Poland.
Materials and methods
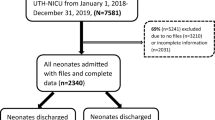
A descriptive and analytical retrospective study was conducted on the data of all neonates who were consecutively admitted to the Department of Neonatology and Neonatal Intensive Care of The University Clinical Hospital in Bialystok (UCH in Bialystok) between January 2015 and December 2023. All procedures were conducted in accordance with the ethical guidelines of the Declaration of Helsinki and approved by The Local Bioethics Committee of the Medical University of Bialystok (protocol code: APK.002.36.2024, date of approval: 18.01.2024).
Data collection methods
A structured questionnaire was used for data collection. All live births with a gestational age of 22 or more weeks were included. We analyzed data from medical records of 19 171 neonates who were admitted to the Department of Neonatology and Neonatal Intensive Care between 2015 and 2023 paying particular attention to the following variables: sex, mode of delivery, birth type, place of delivery, birth weight, body weight, gestational age, 1-min and 5-min Apgar scores, maternal age, gravidity and parity, place of residence and length of hospitalization. Special attention was paid to neonates who died in the Department before discharge. In their case full medical records were analyzed and the following variables were additionally taken into account: occurrence of a congenital malformation/deformation/chromosomal abnormality, occurrence of a congenital/hospital acquired infection, administration of antenatal corticosteroids, occurrence of prognostically significant clinical problems, underlying cause of death, time of death. The underlying causes of death were coded according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD 10).
Patient informed consent to participate in this study was waived by The Local Bioethics Committee of the Medical University of Bialystok (protocol code: APK.002.36.2024, date of approval: 18.01.2024).
Variables and measurements
The dependent variable in this study was neonatal mortality. It was dichotomized as binary outcomes. A neonate who died before discharge from the Department was recorded as ‘’1’’. A neonate who survived at discharge was recorded as ‘’0’’. The explanatory variables were: (1) neonatal related factors variables (sex, gestational age category, birth weight category and percentile, 1- and 5-min Apgar score), (2) maternal related and socio-demographic factors (place and mode of delivery, birth type, parity, maternal age, place of residence).
Sample size and missing data points
The study was conducted retrospectively, using data collected from January 2015 to December 2023. Because of the nature of the research, sample size calculations were not needed. We used all available data from the medical records. A “complete case analysis” approach was used to handle missing data, therefore, cases with missing values for any of the variables were excluded. In case of 13 neonates who survived at discharge, full data was not available, therefore they were excluded from further analysis (0.07% of all children assessed in the study). In case of logistic regression to establish an adequate number of events per independent variable in order to avoid overfitting we used the 10 Events Per Variable (EPV) rule9. In the study there were 60 events. Therefore the explanatory variables were divided into two separate groups containing 6 variables: (1) neonatal related factors variables and (2) maternal related and socio-demographic factors.
Statistical analysis
The analysis was done using the Statistica 13.3 Package. The descriptive statistics were presents as frequencies with percentages for categorical variables and as median and interquartile range (Q1-Q3) for non-normally distributed continuous variables. To determine differences in outcomes between groups, we used the Chi-square test (for categorical variables; sample size = 19 171)) or the Mann-Whitney U Test (for continuous variables; due to the non-normal data distribution assessed using the Shapiro-Wilk test; sample size = 19 171)). The alpha level for all tests was set at 0.05. The results were considered significant at p < 0.05. The univariable and multivariable logistic regression model was used to analyze associations between the dependent variable and the explanatory variables (sample size = 159). For the multivariable binary logistic regression analysis we selected variables with a p-value < 0.2. Adjusted odds ratios with 95% confidence intervals (CI) were computed in the multivariable logistic regression analysis. To determine factors significantly associated with the child’s death, variables with a p-value of < 0.05 were used. To estimate the survival time of the neonates the Kaplan Meier survival curve and log rank test were used.
Data quality control
Both data collectors and supervisors were trained for 3 days. The training included: the purpose of the study, the procedures of enumeration, data collection, handling and storage. The process of data collection was monitored by the principal investigator and supervisors. We checked the completed questionnaires for data completeness and consistency. It there were any missed data, the information was sent to the collectors and the correction was done before data analysis.
Operational definitions
According to the gestational age, a neonate was classified into one of the following groups: EPT – extreme preterm: <27 6/7 weeks of gestation, VPT – very preterm: 28 0/7–31 6/7 weeks of gestation, MPT – moderate preterm: 32 0/7–33 6/7 weeks of gestation, LPT – late preterm: 34 0/7–36 6/7 weeks of gestation, term: 37 0/7–41 6/7 weeks of gestation, post term: ≥ 42 0/7 weeks of gestation.
According to the birth weight, a neonate was classified into one of the following groups: ELBW – extremely low birth weight: <1,000 g, VLBW – very low birth weight: 1,000–1,499 g, LBW – low birth weight: 1,500–2,499 g, NBW – normal birth weight: 2,500–4,000 g, HBW – high birth weight: >4,000 g.
The birth weight percentile was assessed using the Fenton Preterm Growth Charts10. According to the birth weight percentile, a neonate was classified into one of the following groups: SGA—small for gestational age: birth weight below the 10th percentile for the gestational age, LGA – large for gestational age: birth weight above the 90th percentile and AGA – appropriate for gestational age: birth weight between 10th and 90th percentile.
The 1-min Apgar scores were assessed using the International Classification of Diseases 10th Revision. According to the 1-min Apgar score, a neonate was classified into one of the following groups: severe birth asphyxia: 1-minute Apgar score 0–3, mild and moderate birth asphyxia: 1-minute Apgar score 4–7, good condition: 1-minute Apgar score 8–10. In the case of 5-min Apgar score, 2 groups were distinguished: score of 7–10 and score < 711.
The time of death was analyzed using to the following classification – very early neonatal death: 0 to < 24 h, early neonatal death: 0 to < 7 days, late neonatal death: ≥7 to < 28 days, postneonatal deaths: ≥28 days to 364 days. Moreover, according to the hour of death, we classified the neonates who died in the first day of life in the following way: <1 h, 1 to < 12 h, 12 to < 24 h. The mortality rates were calculated using the above presented formulas and expressed as number of deaths per 1000 live births.
The length of hospital stay was calculated using the time of birth and the time of death or 1st discharge from the Department. For neonates who were transferred to the Department from other hospital, we included the time spent at other hospitals.
Results
The characteristics of the neonates
During the study period 19 171 neonates were admitted to the Department of Neonatology and Neonatal Intensive Care and 99 of them died, giving an in-hospital mortality rate of 5.16 per 1000 live births. Its value in the following years was: 2015–8.79, 2016–4.07, 2017–6.32, 2018–4.77, 2019–3.94, 2020–2.34, 2021–8.15, 2022–2.90, 2023–5.79 per 1000 live births.
Both deceased children (N = 99, 0.52%) and children alive at discharge (N = 19 072, 99.48%) were characterized. Socio-demographic variables, maternal related factors and neonatal related factors were analyzed and presented in the Table 1.
In the study group the following variables did not differ between the boys and the girls: gestational age (p = 0.87), birth weight (p = 0.55), body length (p = 0.62), 1-min Apgar score (p = 0.80) and 5-min Apgar score (p = 0.21) (Mann-Whitney U Test). Contrary, in the reference group the boys had significantly higher birth weight and body length than girls (median (Q1-Q3): 3450 (3040–3800) vs. 3300 (2930–3640) g and 56 (53–58) vs. 55 (53–57) cm, respectively) (p < 0.001 in each case, Mann-Whitney U Test).
No differences were found between groups in the following variables: mode of delivery (p = 0.99), maternal age (p = 0.69), gravidity (p = 0.70) and parity (p = 0.64) (Chi-squared test). Compared to the control group, the study group was characterized by a significantly higher proportion of boys (p = 0.01), multiple pregnancies (p < 0.001), outborn neonates (p < 0.001), preterm children (p < 0.001), low birth weight neonates (p < 0.001), children with 1-min Apgar score 0–3 (p < 0.001) and 5-min Apgar score < 7 (p < 0.001) (Chi-squared test). Median birth weight, gestational age, body length, 1-min and 5-min Apgar score of the deceased children were significantly lower than in the alive children (p > 0.001 in each case, Mann-Whitney U Test).
In the next stage of our analysis, the neonates were divided into 4 groups according to their main diagnosis: group E: extremely immature neonates and neonates with extremely low birth weight, group P: premature neonates aged ≥ 28 weeks of gestation and with body weight ≥ 1500 g, group T: term neonates not burdened with genetic disorders, group C: neonates with a severe congenital malformation, deformation, chromosomal abnormality.
The groups were analyzed separately and the results were presented in the Table 2.
Analysis of the clinical problems
In the Group E, 23.44% of the neonates needed resuscitation with epinephrine use in delivery room (N = 15), 4 of them were born with no signs of life. 25.00% of the children (N = 16) had a congenital sepsis/infection and 18.76% (N = 12) had a hospital acquired infection/sepsis. Antenatal corticosteroids were given in nearly 75% of cases requiring this action (N = 44, 74.58%). However, only 54.55% of children were administered a full course of therapy (N = 24).
In the group P, 37.50% of the neonates needed resuscitation with epinephrine use in delivery room (N = 3), 2 of them were born with no signs of life. 25.00% of children (N = 2) had a congenital sepsis. In 87.50% of cases that required it (N = 7), antenatal corticosteroids were not given. In the remaining part, only one dose was administered (N = 1, 12.50%).
In the group C, there were 13 neonates (59.09%) with a lethal congenital malformation/deformation (including: brain defects, kidney defects, thanatophoric short stature and conjoined twins), 5 neonates (22.73%) with a lethal chromosomal abnormality (Edwards’ syndrome, Patau’s syndrome) and 4 (18.18%) children with a malformation syndrome.
The neonates from the group T were briefly characterized.
Patient 1: A neonate admitted from another hospital, undergoing hypothermia therapy, and then receiving palliative care (underlying cause of death – severe birth asphyxia (P21.0); day of death – 36).
Patient 2: A neonate admitted from another hospital in agonal state, died within 24 h of admission (underlying cause of death – neonatal aspiration of meconium (P24.0); day of death – 1).
Patient 3: A neonate treated with nitric oxide, mutation of the EDN1 (Endothelin 1) gene (underlying cause of death – persistent fetal circulation; day of death – 5).
Patient 4: A neonate with trisomy 21, due to intestinal perinatal perforation peritoneal drainage was introduced (underlying cause of death – perinatal intestinal perforation; day of death – 4).
Patient 5: A neonate of a mother without rectal group B streptococcus (GBS) colonization assessment and without perinatal antibiotic prophylaxis (underlying cause of death – sepsis due to Streptococcus, group B; day of death – 7).
Factors associated with the in-hospital mortality of neonates with severe congenital anomalies
The medical records of the children from the group C were analyzed in search for risk factors associated with congenital anomalies in children. In case of 86.36% of the children (N = 19) the mother’s age was < 35 years. All fathers were < 45 years old. No type of mother’s or father’s occupation dominated. 86.36% of the neonates (N = 19) were born from singleton pregnancies. The mothers of 63.64% of the children (N = 19) were multigravida and multiparous. In 13.64% of the cases (N = 3), maternal diabetes was recorded, and in 13.64% (N = 3) – pregnancy-induced hypertension. None of the mothers were diagnosed with pregestational diabetes mellitus or pre-eclampsia. Regarding the history of abortions (spontaneous and induced) and deaths of previous children, they occurred in the case of 5 (22.73%) and 1 (4.55%) children, respectively. All mothers denied the occurrence of the following problems during pregnancy: fever, infectious contact, maternal smoking active or passive, drinking alcohol, taking drugs (medicines), contact with pollutants. We did not find any data regarding periconceptional folic acid, consanguinity and maternal BMI before pregnancy in the patient’s medical records.
Factors associated with the in-hospital mortality of extremely preterm neonates
Univariate and multivariate logistic regression was used to identify which factors are associated with in-hospital mortality of extremely preterm neonates. In the final model of multivariable logistic regression analysis the following predictor variables were associated with the mortality of extremely preterm neonates: gestational age, parity, birth weight percentile, 1-min and 5-min Apgar scores. The detailed data were presented in the Table 3.
Mortality rate and death time analysis
During the study period 99 neonates died (0.52%). There were: 77 early neonatal deaths (77.78%) (of which 42 (54.55%) occurred within the first day of life), 18 late neonatal deaths (18.18%) and 4 postneonatal deaths (4.04%). In the group of children who died in the first 24 h of life 6 deaths occurred under 1 h of life (13.95%), 26 deaths occurred between 1 and 11 h of life (60.47%) and 11 deaths occurred between 12 and 23 h of life (25.58%). The in-hospital mortality rate of preterm neonates was 3.55% (83/2337) and was about 37 times higher than the mortality rate of term neonates (0.10%, 16/16834).
The mortality rates depending on the day of death were: neonatal mortality rate = 4.96 (early neonatal morality rate = 3.81, late neonatal mortality rate = 1.15) and postneonatal mortality rate = 0.21 per 1000 live births.
The mortality rates depending on the gestational age were: 22–23 weeks of gestation = 642.86, 24–25 weeks = 466.6, 26–27 weeks = 197.18, 28–31 weeks = 61.59, 32–36 weeks = 3.68, 37–41 weeks = 0.95 per 1000 live births.
In the group E, 95.31% (N = 61) of all deaths occurred within the first 28 days of life, of which 73.77% (N = 45) occurred within 7 days after birth and 39.34% (N = 24) within 1 day after birth (very early neonatal death). The majority of very early neonatal deaths occurred between 1 and 11 h (N = 18, 75.00%). All deaths from the Group P (N = 8) occurred within the first 28 days of life, of which 75.00% (N = 6) occurred within 7 days after birth and 37.50% (N = 3) within 1 day after birth. 80.00% (N = 4) of neonates from the group T died within the first 28 days of life. All neonates from the Group C died within 7 days after birth (N = 22), of which 68.18% (N = 15) died within the first day of life.
The mortality rate of EPT neonates was 38.36% (61/159). 96.72% (N = 59) of all deaths occurred within the first 28 days of life, of which 71.19% (N = 42) occurred within 7 days after birth and 38.98 (N = 23) within 1 day after birth. In the EPT group, the mortality rates for subsequent weeks of gestation were as follows: 22 weeks – 75.00%, 23 weeks – 60.00%, 24 weeks – 58.06%, 25 weeks – 34.48%, 26 weeks – 25.81%, 27 weeks – 15.00%. Additionally, the mortality rates calculated for VPT and MPT + LPT neonates were: 6.14% (17/277) and 0.32% (5/1901), respectively.
The in-hospital mortality rate of LBW neonates (< 2 500 g) was 4.36% (85/1950) and was about 54 times higher than the mortality rate of neonates with body weight ≥ 2,500 g (0.08%, 12/15191). The mortality rate of ELBW neonates was 33.53% (58/173). 94.83% (N = 55) of all deaths occurred within the first 28 days of life, of which 74.55% (N = 41) occurred within 7 days after birth and 40.00% (N = 22) within 1 day after birth. Additionally, the mortality rates were calculated for VLBW and LBW neonates and were: 6.11% (14/229) and 0.84% (13/1535), respectively.
Survival analysis
For the preterm neonates admitted to the Department, we generated a Kaplan-Meier survival curves to determine their probability of surviving (Fig. 1). Log-rank test was used to compare survival curves for neonates with different characteristics.
In case of the following factors: week of gestation, birth weight, 1-min and 5-min Apgar scores, significant differences in survival probability were observed (p < 0.001 in each case, log rank survival estimate). Significantly lower survival probability was observed in younger children with lower birth weight and lower 1- and 5-min Apgar scores. Maternal age (p = 0.45), gravidity (p = 0.89), parity (p = 0.74), sex (p = 0.77), birth weight percentile (p = 0.42) and place of residence (p = 0.85) had no effect on the probability of survival (log rank survival estimate).
We generated the Kaplan-Meyer curves for groups separated from the EPT neonates according to the gestational age, body weight, 1-min Apgar score and 5-min Apgar score (Figs. 2 and 3).
The underlying causes of deaths
The underlying causes of neonatal deaths fell into two main categories: certain conditions originating in the perinatal period (P00-P96) (N = 77, 77.78%) and congenital malformations, deformations and chromosomal abnormalities (Q00-Q99) (N = 22, 22.22%). The following causes of death were found in the studied groups of children:
Group E: disorders related to length of gestation and fetal growth (N = 61, 95.31%), respiratory and cardiovascular disorders specific to the perinatal period (N = 2, 3.12%) and infections specific to the perinatal period (N = 1, 1.56%);
Group P: respiratory and cardiovascular disorders specific to the perinatal period infections specific to the perinatal period (N = 2, 25.00%), infections specific to the perinatal period (N = 2, 25.00%), conditions involving the integument and temperature regulation of newborn (N = 2, 25.00%), hemorrhagic and hematological disorders of newborn (N = 1, 12.50%) and congenital malformations of the circulatory system (N = 1, 12.50%);
Group T: respiratory and cardiovascular disorders specific to the perinatal period (N = 3, 60.00%), infections specific to the perinatal period (N = 1, 20.00%) and digestive system disorders of newborn (N = 1, 20.00%).
Group C: congenital malformations of the urinary system (N = 7, 31.81%), congenital malformations and deformations of the musculoskeletal system (N = 5, 22.73%), chromosomal abnormalities (N = 5, 22.73%), congenital malformations of the nervous system (N = 2, 9.09%) and other congenital malformations (N = 3, 13.64%).
Discussion
This study analyzed data of 19 171 children admitted to the tertiary referral Department of Neonatology and Neonatal Intensive Care Unit of the UCH in Bialystok between 2015 and 2023 and showed the infant mortality rate of 5.16 per 1000 live births and the neonatal mortality rate of 4.96 per 1000 live births. According to the Polish Central Statistical Office in Poland the above mentioned indicators in 2022 were: 3.79 (3.65–3.94) and 2.69 (2.49–2.83), respectively12,13. Noticeably higher mortality rates are most likely due to the characteristics of the tertiary referral Department of Neonatology and Neonatal Intensive Care, which is intended for children requiring intensive medical care and advanced treatment. In their comparative study covering European countries Sartorius et al., estimated the neonatal mortality rate in Poland at 2.74 per 1000. However, for countries with the lowest neonatal mortality rate (Finland, Norway, Sweden) it was only 1.44 per 100014. The neonatal mortality rates in the individual age groups for our Department, for Poland and for Scandinavian countries were respectively: 22–23 weeks of gestation = 642.86, 807.28 and 491.85; 24–25 weeks = 466.6, 456.04 and 174.53; 26–27 weeks = 197.18, 198.23 and 71.73; 28–31 weeks = 61.59, 59.84 and 30.89; 32–36 weeks = 3.68, 7.74 and 5.21; 37–41 weeks = 1.0, 0.65 and 0.52; ≥42 weeks = 0.00, 0.85 and 0.4214. On the scale of our country we have significantly lower mortality rates for neonates born at 22–23 and 32–36 weeks of gestation and comparable for other age groups. However, when compared to Scandinavian countries, we have significantly higher mortality rates for neonates born at 22–31 weeks, lower for children born at 32–36 weeks and comparable for neonates born at ≥ 37 weeks of gestation.
Analyzing the in-hospital mortality rates in individual years, we found that in 2021 the neonatal mortality rate was significantly higher than in other years (2021 vs. 2020 vs. 2022: 8.15 vs. 2.34 vs. 2.90 per 1000 live births, p < 0.05). No differences were found between the remaining years (p > 0.05). This phenomenon is consistent with the Polish Central Statistical Office data. According to the nationwide data the neonatal mortality rate in Poland in 2021 was 2.9 per 1000 live births (2020 and 2022: 2.6 per 1000 live births). The reason for the increase in mortality rates may be the epidemiological situation related to the COVID-19 (coronavirus disease 2019) pandemic. The pandemic led to reductions in both maternity healthcare-seeking and healthcare provision, which has contributed significantly to worse pregnancy outcomes at that time15. Moreover, during the COVID-19 pandemic increased levels of stress, anxiety and depression were observed amongst pregnant women, which increase the risk of adverse pregnancy outcomes16,17.
The most common underlying causes of early neonatal deaths (81.05% of neonatal deaths) were: disorders related to length of gestation and fetal growth (55.84%) (including extreme prematurity (95.35%), congenital malformations, deformations and chromosomal abnormalities (28.57%), respiratory and cardiovascular disorders specific to the perinatal period (7.79%) and infections specific to the perinatal period (3.90%). These results seem consistent with the literature8,18,19.
It is estimated that serious birth defects are responsible for around 7% of neonatal mortality20. In our study the percentage was higher (22.22%), most likely due to the characteristic of the tertiary care facility. The awareness of the importance of birth defects in mortality of the youngest children is very important as about 60% of them can be prevented21. Socioeconomic, environmental and health factors of parents are considered as risk factors for congenital anomalies22. It was suspected that the incidence of these problems would be significant in the group C, however, we did not observe such phenomenon. This is probably due to the small size of the analyzed group. Therefore, it would be beneficial to extend the study group in the following years.
Neonatal sepsis is estimated to contribute annually to 203,000 deaths worldwide23. In our study sepsis was considered the main cause of 4 deaths. Moreover, in more than 40% of the extremely preterm and extremely low birth weight neonates had a congenital or hospital acquired infection, which was a significant prognostic factor. At this point it is necessary to refer to the death of a term neonate due to group B streptococcal (GBS) sepsis. The child’s mother was not assessed for rectal GBS colonization and no intrapartum antibiotic prophylaxis was administered. The group B streptococcal infections can be prevented by administration of prophylactic intrapartum antibiotics. According to Schrag et al., after introducing prophylactic antibiotic therapy, the incidence of early-onset neonatal infections in the United States decreased by 65% between 1993 and 1998. It prevented 200 neonatal deaths and 3900 early-onset infections in the United States in 199824.
We tried to identify factors that may predispose to death in extremely premature neonates. Our results showed that gestational age, parity, birth weight percentile, 1-min and 5-min Apgar scores were independently associated with the in-hospital mortality of extremely preterm neonates in the Department of Neonatology. Among the variables analyzed, similarly to the literature, 5-min Apgar score of < 7 was the strongest determinant of in-hospital mortality22,25,26. We found that the risk of death increased 3 times in neonates aged < 24 weeks of gestation compared to more mature children. Gestational age has a significant impact on the baby’s chances of survival; at 22 weeks the risk of death is almost 1,000 per 1,000 live births and at 40 weeks – less than 1 per 1000 live births27,28. We found that small for gestational age neonates were over 3.5 times more likely to die during hospitalization compared to appropriate for gestational age children. This was consistent with other studies29,30. According to McEwen each 1-percentile increase of birth percentile decreases the risk of perinatal mortality31. We found that the risk of child’s death is significantly lower in multiparous women. Similarly Devabhaktuni et al. and Garces et al. showed that perinatal mortality is higher in nulliparous women21,32. This phenomenon can be partially explained by immaturity of the mother expressed through small size of the uterus, incomplete growth and fetal competition for nutrients21.
Administration of antenatal steroids is associated with decreased neonatal mortality33. According to Polish recommendations, the use of antenatal steroids in case of threatened preterm birth should be considered between 23 0/7 and 23 6/7 weeks of pregnancy, is recommended between 24 0/7 and 33 6/7 weeks of pregnancy, and is not recommended between 34 0/7 and 36 6/7 weeks of pregnancy34. In our study, the mothers of 23.44% (N = 15) of the EPT neonates were not administered recommended antenatal corticosteroids. In case of 31.25% of the children, the mothers received only one dose. Failure to use steroids as recommended in all cases was associated with rapid progression of labor. It seems that earlier recognition of threatened preterm labour and use of antenatal corticosteroids could have reduced the number of deaths.
Limitations
Our study has the advantage of reviewing all neonates admitted to the Department from 2015 to 2023. This procedure eliminated potential sampling error. However, several limitations should be taken into account when interpreting the results. Firstly, a relatively small sample size should be mentioned. Moreover, we have to emphasize that our results should not be generalized to the general population of neonates. We showed the trends and determinants of in-hospital mortality in a major tertiary referral hospital in the north-east Poland. It was a single-center study, which included a tertiary care facility where most at-risk neonates are refereed from surrounding facilities. Moreover, this study focused on the in-hospital mortality of children hospitalized in the Department. We did not follow up on the child after discharge or transfer to another hospital/ward. For this reason, the obtained results may be underestimated by the number of children who died in facilities other than our ward. It would be important, with the help of the mentioned centers, to follow the further history of the children included in the study to fully illustrate mortality in the region. As the study was based on medical record data, despite all efforts, potential data collection errors may have occurred. In addition, the number of variables studied that could have an impact on child mortality was limited by missing data in the documentation. The study was limited to the information documented in the medical records.
Conclusions
The neonatal mortality rate in the tertiary referral Department of Neonatology and Neonatal Intensive Care of the Medical University of Bialystok significantly exceeded its values in Poland and other European countries. This is due to the characteristics of the highest reference Department, which provides care to neonates requiring intensive medical care and advanced treatment. The in-hospital mortality rate was the highest in 2021, during the period of strict epidemiological restrictions related to the COVD-19 pandemic.
The in hospital mortality rate was generated mainly by deaths of premature neonates. The risk of in-hospital death of extremely premature children increased among small for gestational age babies born before 24 weeks of pregnancy from primiparous mothers and with both 1-minute and 5-minute Apgar scores < 7. More than half of the extremely premature neonates did not receive full prenatal steroid therapy and almost half were diagnosed with congenital or hospital acquired infection.
The results of this study have important clinical implications for the development of effective strategies for reducing in-hospital mortality of neonates. The greatest emphasis should be placed on obstetric procedures (including primarily prevention of preterm births and congenital infections and strategies for optimizing prenatal corticosteroid administration) and neonatological procedures (including primarily the improvement of neonatal care in the delivery room and prevention of hospital acquired infections).
The obtained results are valuable from a public health perspective for the development of effective global strategies for reducing neonatal mortality, including maternal and neonatal care, also in case of an epidemiological threat.
Further research, extended to the analysis of deaths of the neonates born in the Department and then transferred to other medical facilities, is necessary to verify the introduced changes in procedures and their further updates. Conducting analyses of neonatal deaths by neonatology departments around the world and sharing their results through publication seem crucial to reduce neonatal mortality.
Without detailed analyses of neonatal deaths in individual health care facilities and implementation of procedures to improve quality of care, it will not be possible to reduce the number of neonatal deaths.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
UNICEF Europe and Central Asia Regional Office. Ensuring the survival and health of newborns in Europe and Central Asia. (2018). https://www.unicef.org/eca/media/3701/file/in%20focus%20-%20health.pdf
WHO. Neonatal mortality rate (per 1000 live births). Official estimate. (2024). https://data.who.int/indicators/i/E3CAF2B/A4C49D3
World Health Organization. Newborn mortality. Geneva: World health organization. (2020). https://www.who.int/news-room/fact-sheets/detail/newborn-mortality
World Health Organization. Targets of Sustainable Development Goal 3. (2015). https://www.who.int/europe/about-us/our-work/sustainable-development-goals/targets-of-sustainable-development-goal-3
Hug, L., Alexander, M., You, D., Alkema, L. & UN Inter-agency Group for Child Mortality Estimation. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: A systematic analysis. Lancet Glob. Health. 7 (6), e710–e720. https://doi.org/10.1016/S2214-109X(19)30163-9 (2019).
Flaxman, S. et al. Assessment of COVID-19 as the underlying cause of death among children and young people aged 0 to 19 years in the US. JAMA Netw. Open. 6 (1), e2253590. https://doi.org/10.1001/jamanetworkopen.2022.53590 (2023).
World Health Organization (WHO). Improving the quality of paediatric care. Operational guide for facility-based audit and review of paediatric mortality. (2018). https://www.who.int/publications/i/item/9789241515184
Kamianowska, M., Kamianowska, A. & Wasilewska, A. Causes of death in neonates, infants, children, and adolescents at the university children’s clinical hospital of Białystok between 2018 and 2021. Med. Sci. Monit. 29, e939915. https://doi.org/10.12659/MSM.939915 (2023).
Bujang, M. A., Sa’at, N., Sidik, T. M., I. T. A., B. & Joo, L. C. Sample size guidelines for logistic regression from observational studies with large population: Emphasis on the accuracy between statistics and parameters based on real life clinical data. Malays. J. Med. Sci. 25(4), 122–130 (2018). https://doi.org/10.21315/mjms2018.25.4.12 (2018).
Chou, J. H., Roumiantsev, S. & Singh, R. PediTools electronic growth chart calculators: Applications in clinical care, research, and quality improvement. J. Med. Internet Res. 22 (1), e16204. https://doi.org/10.2196/16204 (2020).
Executive summary. Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 123(4), 896–901 (2014). https://doi.org/10.1097/01.AOG.0000445580.65983.d2
UN Inter-agency Group for Child Mortality Estimation. (2023). https://childmortality.org/all-cause-mortality/data
Statistics Poland. Indicator 3.2.2 – Neonatal mortality rate https://sdg.gov.pl/statistics_glob/3-2-2/
Sartorius, V. et al. Neonatal mortality disparities by gestational age in European countries. JAMA Netw. Open. 7 (8), e2424226. https://doi.org/10.1001/jamanetworkopen.2024.24226 (2024).
Townsend, R. et al. Global changes in maternity care provision during the COVID-19 pandemic: A systematic review and meta-analysis. EClinicalMedicine 37, 100947. https://doi.org/10.1016/j.eclinm.2021.100947 (2021).
Campos-Garzón, C. et al. Psychological impact of the COVID-19 pandemic on pregnant women: A scoping review. Behav. Sci. (Basel). 11 (12), 181. https://doi.org/10.3390/bs11120181 (2021).
Coussons-Read, M. E. Effects of prenatal stress on pregnancy and human development: Mechanisms and pathways. Obstet. Med. 6 (2), 52–57. https://doi.org/10.1177/1753495X12473751 (2013).
World Health Organization. Children: Improving survival and well-being Geneva: World health organization. (2020). https://www.who.int/news-room/fact-sheets/detail/child-mortality-under-5-years
Strong, K. L. et al. Patterns and trends in causes of child and adolescent mortality 2000–2016: Setting the scene for child health redesign. BMJ Glob. Health. 6 (3), e004760. https://doi.org/10.1136/bmjgh-2020-004760 (2021).
Tiwari, P. & Gupta, M. M. Study of lethal congenital malformations at a Tertiary-Care referral centre in North India. Cureus 12 (4), e7502. https://doi.org/10.7759/cureus.7502 (2020).
Garces, A. et al. Association of parity with birthweight and neonatal death in five sites: The global network’s maternal newborn health registry study. Reprod. Health. 17 (Suppl 3), 182. https://doi.org/10.1186/s12978-020-01025-3 (2020).
Razaz, N., Cnattingius, S. & Joseph, K. S. Association between Apgar scores of 7 to 9 and neonatal mortality and morbidity: Population based cohort study of term infants in Sweden. BMJ 365, l1656–. https://doi.org/10.1136/bmj.l1656 (2019).
GBD 2017 Causes of Death Collaborators. Global, regional, and National age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet 392 (10159), 1736–1788. https://doi.org/10.1016/S0140-6736(18)32203-7 (2018).
Schrag, S. J. et al. Group B Streptococcal disease in the era of intrapartum antibiotic prophylaxis. N. Engl. J. Med. 342 (1), 15–20. https://doi.org/10.1056/NEJM200001063420103 (2000).
Hong, J., Crawford, K., Jarrett, K., Triggs, T. & Kumar, S. Five-minute Apgar score and risk of neonatal mortality, severe neurological morbidity and severe non-neurological morbidity in term infants—an Australian population-based cohort study. Lancet Reg. Health West. Pac. 44, 101011. https://doi.org/10.1016/j.lanwpc.2024.101011 (2024).
Iliodromiti, S., Mackay, D. F., Smith, G. C., Pell, J. P. & Nelson, S. M. Apgar score and the risk of cause-specific infant mortality: A population-based cohort study. Lancet 384 (9956), 1749–1755. https://doi.org/10.1016/S0140-6736(14)61135-1 (2014).
Lawn, J. E., Wilczynska-Ketende, K. & Cousens, S. N. Estimating the causes of 4 million neonatal deaths in the year 2000. Int. J. Epidemiol. 35 (3), 706–718. https://doi.org/10.1093/ije/dyl043 (2006).
Mohangoo, A. D. et al. Gestational age patterns of fetal and neonatal mortality in europe: results from the Euro-Peristat project. PLoS One. 6 (11), e24727. https://doi.org/10.1371/journal.pone.0024727 (2011).
King, V. J. et al. Fetal growth restriction and stillbirth: Biomarkers for identifying at risk fetuses. Front. Physiol. 13, 959750. https://doi.org/10.3389/fphys.2022.959750 (2022).
Triggs, T., Crawford, K., Hong, J., Clifton, V. & Kumar, S. The influence of birthweight on mortality and severe neonatal morbidity in late preterm and term infants: An Australian cohort study. Lancet Reg. Health West. Pac. 45, 101054. https://doi.org/10.1016/j.lanwpc.2024.101054 (2024).
McEwen, E. C. et al. What birthweight percentile is associated with optimal perinatal mortality and childhood education outcomes? Am. J. Obstet. Gynecol. 218 (2S), S712–S724. https://doi.org/10.1016/j.ajog.2017.11.574 (2018).
Devabhaktuni, A., Pilliod, R. A., Caughey, A. B. & Valent, A. M. The risk of perinatal mortality in nulliparous women compared to primiparous women at term. Am. J. Perinatol. 41 (3), 270–275. https://doi.org/10.1055/a-1673-0527 (2024).
Fee, E. L., Stock, S. J. & Kemp, M. W. Antenatal steroids: Benefits, risks, and new insights. J. Endocrinol. 258 (2), e220306. https://doi.org/10.1530/JOE-22-0306 (2023).
Sieroszewski, P. et al. Guidelines of the Polish society of gynecologists and obstetricians on the diagnosis and management of pregnancies complicated by prelabor rupture of the membranes. Ginekol. Pol. https://doi.org/10.5603/gpl.98339 (2024).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. C.K., G.S and A.J. acquired the data. A.K. analyzed and interpreted the data. C.K and A.K wrote the main manuscript text. M.K. and A.W. reviewed the manuscript. All authors read, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kamianowski, A., Kamianowski, C., Szpica, G. et al. Analysis of the in-hospital mortality in the tertiary referral department of neonatology and neonatal intensive care. Sci Rep 15, 24849 (2025). https://doi.org/10.1038/s41598-025-09500-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09500-2