Abstract
The epidermal patterning factor-like (EPFL) gene family, encoding cysteine-rich polypeptides (CRPs), regulates diverse developmental processes in plants, including the control of stomatal density and the guidance of pollen tube elongation. To investigate its potential association with male sterility in Brassica rapa, we performed a comprehensive genome-wide identification using bioinformatics approaches. Our analysis revealed 14 BrEPFL members distributed across 8 of the 10 chromosomes. Phylogenetic analysis of EPFL proteins from 15 Brassicaceae species classified 204 orthologous genes into six distinct subfamilies, revealing their evolutionary conservation across crucifers. Syntenic collinearity and nonsynonymous/synonymous (Ka/Ks) analyses identified 63 orthologous gene pairs between B. rapa and three other species, all of which have undergone strong purifying selection during evolution. Tissue-specific expression profiling through RNA-seq and RT-qPCR demonstrated that most BrEPFL genes exhibit predominant expression in reproductive tissues. Co-expression network analysis implicated three BrEPFL coexpressed genes (BraA07g025640.3 C, BraA09g052700.3 C, and BraA10g002620.3 C) in pollen development pathways. These findings may provide valuable information for the further understanding of BrEPFL functions in male sterility in B. rapa.
Similar content being viewed by others
Introduction
Cysteine-rich polypeptides (CRPs) have a wide range of biological functions, such as defense, protease inhibition, and heavy metal detoxification1,2,3. Secreted CRPs are also involved in a series of plant growth, development and reproductive processes4,5, including regulation of inflorescence structure, guidance of pollen tube elongation, and control of stomatal density and formation2,6.
Members of the epidermal patterning factor-like (EPF/EPFL) gene family encode small proteins predicted to be processed into secreted CRPs of approximately 45 ~ 75 amino acids in length7,8. These proteins are involved in regulating stomatal density and spatial distribution and function as ligands of TOO MANY MOUTH (TMM) and ERECTA (ER) family receptors7,8,9. EPFL proteins typically possess an N-terminus signal sequence for secretion and 6 ~ 8 conserved cysteine residues at the C-terminus, which form intramolecular disulfide bonds9,10.
In Arabidopsis, the EPF/EPFL gene family comprises 11 members, including 9 genes encoding EPFL-secreted peptides and two additional genes, EPF1 and EPF2, which regulate various developmental processes1,11,12. Among these, AtEPFL9/STOMAGEN antagonizes AtEPF1 and AtEPF2, positively regulating stomatal formation, patterning and density12,13,14,15; EPF2 primarily influences early asymmetric cell division, while EPF1 mainly regulates guard mother cell (GMC) differentiation and stomatal spacing9,16,17. Functional roles have been reported in maize18,19, tomato20 and poplar21. Furthermore, EPF1 and EPF2 genes are responsive to drought stress. Ectopic overexpression of the AtEPF2 and HvEPF1 genes reduced stomatal density and improved water use efficiency and drought tolerance, respectively22,23.
Beyond their roles in stomatal density regulation, EPFL genes are also implicated in reproductive development, seed germination, and responses to abiotic stress24,25. The EPFL4 and EPFL6 genes act as ligands in signaling transduction pathways and serve as partially redundant upstream components of ER-mediated inflorescence growth13,26. Knockout of AtEPFL4-6 genes results in shortened filaments and male sterility12. The CHAL genes (EPFL5/CLL, EPFL4/CLL2, and EPFL6) function as ligands for the ERECTA gene family and regulate inflorescence structure, pedicel length, and stomatal development26,27,28,29. Expression patterns of EPFL1 (At5G10310) and EPFL2 (At4G37810) genes were similar to those of CHAL genes and showed CHAL-like functions in overexpression detection27. In Brassica napus, BnEPFL6 is essential for filament elongation, and its overexpression in Arabidopsis plants resulted in male sterility7. In rice (Oryza sativa), OsEPFL6-9 and OsEPFL5 are critical for panicle morphogenesis25,30, while in wheat (Triticum aestivum), TaEPFL1 plays an important role in stamen development, with overexpression resulting in filament degradation and sterility in Arabidopsis1.
The stamen is an important reproductive organ, and its development significantly affects plant fertility and crop yield1. Male sterility has been widely exploited in hybrid breeding to enhance crop vigor and seed production31,32. Therefore, studying the developmental process of stamens and identifying key genes involved in male sterility are essential for improving plant traits and increasing crop yield. EPFL genes are widely conserved among land plants, and have been identified genome-wide in a few plants11. However, functional studies related to male sterility have been studied only in B. napus and wheat1,7. Little information is available on the EPFL family and its biological function in Chinese cabbage (B. rapa L.), which is one of the most important Brassicaceae vegetables cultivated across China33,34,35,36.
In this study, we performed a comprehensive analysis of the BrEPFL family. The biophysical characteristics of the BrEPFL gene were determined. In addition, RNA-Seq data from seven male-sterile B. rapa plants were analyzed to determine tissue-specific expression patterns, allowing the identification of EPFL genes potentially involved in male sterility. These findings contribute to a better understanding of the molecular functions of BrEPFL genes in the reproductive development of B. rapa.
Results
Identification and phylogenetic analysis of EPFL gene families
Based on the AtEPFL proteins, we identified a total of 193 EPFL members in 14 Brassicaceae species (Table S2). In particular, 14 BrEPFL members were identified in both versions V1.5 and V3.0 of the Brassica rapa genome (Table S2). Across the 15 Brassicaceae species analyzed, the number of EPFL members varied from 5 in Aethionema arabicum to 33 in Camelina sativa, suggesting a lineage-specific expansion of the EPFL gene family within Brassicaceae (Table S2).
To examine the evolutionary relationships among these EPFL members, a maximum likelihood (ML) phylogenetic tree was generated, which classified all EPFLs into six subgroups (Groups I-VI; Fig. 1A). Group I was the largest, followed by Groups II, V, IV and III, comprising 78, 54, 33, 23, and 13 members, respectively. However, Group VI contained only 3 EPFL members from B. napus, Aethionema arabicum and Thellungiella halophila (Fig. 1A), suggesting the possible acquisition of specialized functions in these remaining genes during long-term evolution.
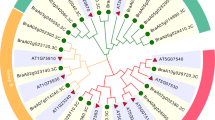
Phylogenetic trees of EPFL proteins. (A), Phylogenetic tree of EPFL proteins from 15 Brassicaceae species. (B), Phylogenetic tree of EPFL proteins from three Brassica species and A. thaliana. Percent bootstrap values (1000 iterations) are indicated in every branch. The different-colored arcs indicate different groups of EPFLs.
Among B. rapa, B. nigra, B. oleracea, and B. napus, 14, 15, 9, and 29 EPFL members were identified, respectively (Table S2). The phylogenetic tree constructed using EPFLs from these Brassica species and A. thaliana clustered 78 members into five distinct groups (Fig. 1B). Notably, B. rapa and B. oleracea had nearly equal numbers of EPFL genes in each group (Fig. S1), suggesting a closer evolutionary relationship between these two species compared to B. rapa and B. nigra.
Physicochemical analysis of BrEPFLs
Physicochemical property analysis revealed that all BrEPFL proteins were small secreted proteins, and contained CRP domains at the C-terminus. Their molecular weights (MWs) ranged from 7.091 to 23.356 kDa (Table S3; Fig. S2). However, the majority of BrEPFLs were alkaline proteins, and their MW ranged from 11 ~ 15 kDa (Table S3; Fig. S3). Furthermore, approximately half of the BrEPFLs were predicted to have 1 ~ 2 transmembrane domains, and all of them except BrA04g021610.3 C possess a signal peptides (Figs. S4 and S5). These features suggest that BrEPFL proteins might have diverse functions associated with different subcellular localization and may play broad biological roles as secreted proteins.
Gene structure, conserved sequence, and cis-element analyses of BrEPFLs
To obtain more insights into the evolution of BrEPFL genes in B. rapa, the exon/intron structures were assessed in conjunction with a phylogenetic tree (Fig. 2A). All BrEPFL genes possessed conserved cysteine residues in their C-terminus (PF17181; EPF domain) regions, and exhibited 0 to 3 introns. Genes with similar exon-intron structures tended to cluster together within the same phylogenetic groups (Fig. 2B). Conserved motifs were identified, and found to be similarly distributed across BrEPFL members, indicating evolutionary conservation within the gene family (Figs. 2C and S6). Furthermore, predicted three-dimensional structural models revealed comparable protein conformations across all BrEPFLs (Fig. 3). These results suggest that the structural similarities among BrEPFL genes was positively associated with their evolutionary relationship.
Analysis of cis-acting elements in the promoters of BrEPFL family genes showed enrichment in elements related to growth and development, light response, and multiple hormone signaling (Figs. 4 and S7). These specific cis-elements may contribute to the functional diversification of BrEPFLs by mediating distinct biological processes and environmental responses.
Chromosomal distribution and synteny analyses of BrEPFLs
Mapping of BrEPFL genes onto chromosomes showed that all 14 members were unevenly distributed on the 8 of 10 B. rapa chromosomes, with each chromosome containing 1 to 3 members (Fig. S8). Analysis of segmental duplication events identified four paralogous gene pairs within the BrEPFL family (Table S4), suggesting that gene duplication was a primary mechanism driving the expansion of this gene family.
To further assay the evolution and divergence of EPFL genes among three Brassica species and Arabidopsis, a total of 63 orthologous gene pairs were identified between B. rapa and the other three plants (Table S4). All identified paralogous and orthologous gene pairs are displayed in Fig. 5. Notably, several BrEPFL genes were involved in three collinear gene pairs shared among B. rapa and B. oleracea, B. nigra, and A. thaliana, indicating that these genes may represent evolutionarily conserved, ancestral EPFL genes (Table S4). The Ka/Ks values for all these gene pairs were calculated, revealing that most values were below 1, except for three gene pairs (Fig. S9; Table S4), demonstrating that the majority of EPFL genes have undergone strong purifying selection during evolution.
EPFL gene duplication analysis in B. rapa (AA), B. nigra (BB), B. oleracea (CC), and A. thaliana. Colored lines show paralogous pairs. Chr1-Chr5 (yellow blocks) indicate chromosomes of A. thaliana; A1-A10 (red blocks), B1-B8 (blue blocks), and C1-C9 (green blocks) represent subgenomes of B. rapa (AA), B. nigra (BB), and B. oleracea (CC), respectively.
Tissue-specific expression profiling of BrEPFLs
To determine the tissue-specific expression patterns of BrEPFL genes, transcriptome data from different tissues of B. rapa were analyzed. As shown in Fig. 6A, the BrEPFL gene family members exhibited variable expression patterns, with genes in the same cluster generally displaying similar expression patterns. Most BrEPFL genes were highly expressed in stems and flowers, suggesting potential roles in the development of these tissues. Notably, three genes (BraA10g027570.3 C, BraA02g003420.3 C, and BraA08g006830.3 C) were broadly expressed across all examined tissues (Fig. 6A), indicating that they may play fundamental roles in the growth and development of B. rapa. However, some BrEPFL genes exhibited tissue-specific expression, with BraA01g001190.3 C, BraA01g021960.3 C showing elevated expression specifically in floral tissues, indicating their possible involvement in flower development.
Expression pattern analysis of BrEPFL genes. (A), Heatmap of BrEPFL genes expression in different tissues of B. rapa based on RNA-seq (log2 FPKM). (B), UpSet plot showing differentially expressed BrEPFL genes across seven male-sterile lines. (C), Heatmap of BrEPFL DEGs in these male-sterile lines, the color bar on the top represents fold change values; all DEGs except BraA02g026150.3 C were upregulated.
Comparative transcriptome analysis of BrEPFLs in male-sterile lines
To further analyze the potential roles of BrEPFL family genes in reproductive growth, transcriptome data were analyzed from nine different male-sterile lines, including five cytoplasmic male-sterile (CMS; four ogura and one polymer) lines, one genic male-sterile (GMS; AB01) line, and three male-sterile mutant (ftms; fsm; msm)31,33,37,38,39,40,41. Differentially expression analysis revealed that 7 of 14 BrEPFL genes were differentially expressed in floral buds of seven male-sterile lines (Fig. 6B). Among them, all genes except BraA02g003420.3 C and BraA09g055980.3 C were found in multiple transcriptomes. Notably, all genes except BraA02g026150.3 C, which was greatly downregulated in floral buds of four male-sterile lines, were upregulated in the floral buds of the seven male-sterile lines (Fig. 6C). However, only three BrEPFL genes (BraA09g031060.3 C, BraA01g001190.3 C, and BraA04g021610.3 C) were differentially expressed in both fsm and msm male-sterile lines compared to their respective wild type (Fig. S10). These results indicated that these genes may be involved in anther development, and their abnormal expression could be associated with male sterility in B. rapa. The reliability of the RNA-Seq data was further validated by qRT-PCR experiments in floral buds at different developmental stages in the ogu-CMS line, in which anther abortion initiates at the tetrad period (Fig. 7A and B), characterized by microspore degeneration and vacuolation of the tapetum. Most BrEPFL genes were upregulated during anther development in ogu-CMS buds (Fig. 7C), further supporting their potential roles in anther formation and fertility regulation. Furthermore, we analyzed the expression of genes in the anther development regulatory network, including TPD1, DYT1, TDF1, AMS, and MS1/MYB80, and found that these genes were significantly downregulated in the male-sterile line (Fig. S11).
Expression levels of BrEPFL genes in ogura sterile lines based on qRT-PCR. (A) and (B), Transverse sections of anther development in six periods (microspore mother cell, meiosis II, tetrad, uninucleate, binucleate, and mature pollen period) from B. rapa (Wucai) maintainer and ogu-CMS lines, respectively. AP, abortive pollen; AT, abnormal tapetum; DM, degenerated microspore; E, epidermis; En, endothecium; ML, middle layer; Mmc, microspore mother cell; MPG, mature pollen gains; Ms, meiocytes; Msp, microspores; T, tapetum; Tds, tetrads. Scale bar = 100 μm. C, qRT-PCR analysis of BrEPFL genes in meiosis II, tetrad, uninucleate, and binucleate period. Different lowercase letters above the bars indicate statistically significant differences among stages based on Duncan’s new multiple range test (p < 0.05).
Regulatory subnetworks involving BrEPFLs and other genes
To further elucidate the potential roles of BrEPFL genes in anther development, a protein-protein interaction (PPI) network was constructed using the STRING database for the seven abnormally expressed BrEPFL genes and their coexpressed partners (Fig. 8A). Among the five coexpressed genes identified, three genes (TMM, ERECTA, and SDD1) were identified in five male-sterile lines RNA-Seq analysis (Fig. 8B) and qRT-PCR validation (Fig. 8C) demonstrated that these genes were consistently upregulated in male-sterile lines compared to their corresponding maintainer lines, particularly during the tetrad and uninucleate stages of anther development. Gene Ontology (GO) enrichment analysis showed that the coexpressed genes were primarily involved in postembryonic morphogenesis (Fig. 8D), indicating that these BrEPFL genes may contribute to pollen development through regulation of these biological processes.
Coexpression, RNA-seq data, and qRT-PCR analyses of BrEPFL coexpressed genes. (A), PPI network of BrEPFL genes with their coexpressed genes. Nodes are connected by lines indicating interactions between genes. (B), Heatmap of BrEPFL coexpressed genes across five male sterile lines; the color bar on the top represents fold change values. (C), qRT-PCR analysis of BrEPFL coexpressed genes in B. rapa ogu-CMS plants (Wucai). (D), GO enrichment analysis of genes coexpressed with BrEPFLs. Different lowercase letters above the bars indicate statistically significant differences among stages based on Duncan’s new multiple range test (p < 0.05).
Discussion
The epidermal pattern-building factor-like (EPFL) gene family encodes cysteine-rich secretory polypeptides that perform diverse functions in plant growth and development. These include the regulation of stomatal density and formation, control of shoot apical meristem and inflorescence structure, and mediation of pollen tube elongation2,6,30,42,43. Given the functional importance of EPFL genes in plants, we conducted a genome-wide identification of 204 EPFL genes in the 15 Cruciferae plants, with gene numbers ranging from 5 to 33 per species (Table S2). The variation in gene number among different diploid plants might be the result of whole-genome polyploidization events during species evolution and divergence44. Phylogenetic tree analysis suggested that all of these EPFL genes were separated into five subgroups except for three members (Fig. 1A), and Group IV contained the fewest members per species, suggesting it may represent an ancestral lineage (Fig. 1A).
In Brassica rapa, 14 BrEPFL genes were identified and analyzed for their basic characteristics. Most genes exhibited similar sizes and isoelectric points (pIs) (Fig. S3), along with conserved gene structures and motifs (Figs. 2, 3, and S6). Similar results were observed with BnEPFL6 in B. napus7 and TaEPFL1 in Triticum aestivum1. As secretory proteins, CRPs typically contain an N-terminal signal peptide2. However, four BrEPFL members, although possessing CRP domains (Fig. S2), lacked signal peptides (Fig. S4), potentially due to partial sequence deletions and subsequent functional divergence during gene replication45,46.
We also identified EPFL gene family members in B. nigra (BB) and B. oleracea (CC) (Table S2). Interestingly, B. nigra had fewer EPFL genes than B. rapa and B. oleracea, in contrast to the pattern observed in the PERK gene family among these species34. Phylogenetic analysis of the EPFL genes in Brassica crops and A. thaliana revealed five clusters (Fig. 1B), and Group 3 might be an ancient clade, which consistent with results from the broader Cruciferae dataset (Fig. 1A), supporting the hypothesis that Brassica species share a common ancestor47,48. Further synteny and evolutionary rate analysis showed that the Ka/Ks ratios for four paralogous and 43 orthologous gene pairs (with B. nigra, B. oleracea, and A. thaliana) were generally below 0.35, except for three pairs. This indicates strong purifying selection and functional conservation of these genes34,49,50.
Previous studies on Arabidopsis have indicated that some EPFL gene family members act as regulators of inflorescence growth13,26,27. For example, misexpression of EPFL4-6 impairs filament cell proliferation, resulting in failed pollination and male sterility12. In B. rapa, transcriptomic analysis revealed that most BrEPFL genes were highly expressed in stem and flower tissues, particularly the genes BraA01g001190.3 C (homolog of AtEPFL2) and BraA01g021960.3 C (homolog of AtEPFL4), which were relatively highly expressed in floral organs (Fig. 6A). These results were consistent with previous reports of A. thaliana EPFL2 and EPFL4 genes26,27, suggesting that BraA01g001190.3 C and BraA01g021960.3 C are involved in flower organ development in B. rapa.
In wheat, the pistillody mutant HTS-1 exhibits an alteration in the high expression level of TaEPFL1 in pistillody stamens, and its overexpression in Arabidopsis leads to abnormal stamen development1. Similarly, BnEPFL6 had high expression in six male-sterile lines of B. napus7. In rye (Secale cereale L.), EPFL1, EPFL7, EPFL9, and EPFL10 displayed significantly higher expression levels in spikelets51. Based on these studies, we hypothesized that EPFLs might be involved in stamen development. To test this hypothesis, we explored BrEPFL gene expression in several male-sterile lines based on RNA-Seq31,33,37,38,39,40,41, revealing upregulation of several BrEPFL genes in five CMS lines and one mutant (ftms) (Fig. 6B and C). However, three BrEPFL genes were consistently differentially expressed in both fsm and msm mutant (Fig. S10). These results suggested that these BrEPFL genes might be related to fertility, although DEGs specific to individual male-sterile lines may also play critical roles in anther or pollen development.
To further examine this hypothesis, we analyzed the expression of BrEPFL genes during anther development in the B. rapa ogu-CMS line31,34. The cellular contents from tapetum degeneration support pollen wall formation and subsequent pollen release52. In this ogu-CMS line, tapetum development was abnormal after the tetrad period, which led to pollen abortion (Fig. 7A and B). Most BrEPFL genes were highly expressed during the tetrad to mature pollen stages (Fig. 7C), suggesting their involvement in tapetum degradation and anther development. This conclusion aligns with previous phenotypic analyses in Arabidopsis, where EPFL gene knockouts showed additive effects on fertility53.
Coexpression network analysis provides valuable insights into genes with shared cellular functions and regulatory pathways54,55. A PPI network identified three coexpressed genes TMM, ER, and SDD1 (Table S5), which were upregulated in the tetrad and uninucleate stages of sterile buds (Fig. 8C). These Arabidopsis homologs are known regulators of stomatal development56,57,58 and are also implicated in anther formation and male fertility59,60,61,62. In particular, the GO annotations of these three coexpressed genes suggest that postembryonic morphogenesis is influenced by these BrEPFL genes, indicating that these BrEPFL genes and their coexpressed genes are necessary for proper pollen development and that upregulation of BrEPFL genes may cause male sterility.
Conclusions
In this study, we identified 204 EPFL gene family members in 15 Brassicaceae plants. Among these genes, 14 BrEPFL genes were consistently identified across different versions of the B. rapa genome. Comprehensive analyses of gene structure, conserved motif, cis-acting element, chromosome distribution, phylogenetic tree, gene duplication, and collinearity were performed on the BrEPFLs genes. Our results suggest that genome duplication events have contributed to the expansion of the BrEPFL gene family. Additionally, expression pattern analysis based on RNA-Seq data showed that some BrEPFL members exhibit tissue-specific expression and may be involved in reproductive development. Further analyses of RNA-Seq datasets from seven male-sterile lines of B. rapa revealed that six BrEPFL genes were upregulated, while one was downregulated, potentially indicating their crucial roles in anther development. Expression profiling of these seven BrEPFL genes validated the transcriptome results, suggesting their involvement in pollen development and that their abnormal expression might result in male sterility. These findings provide a foundation for future functional studies targeting key male sterility genes in Brassica rapa.
Materials and methods
Identification and structural analysis of EPFL genes
The protein sequences of A. thaliana EPFL gene family members were obtained from the TAIR website (https://www.arabidopsis.org/) and used to identify EPFL homologs in other 14 Brassicaceae species, which were downloaded from the BRAD website (http://brassicadb.cn). According to Chen et al.34,63, the candidate protein sequences were obtained by all-BLAST-all searching and verified using several tools.
The physicochemical properties of BrEPFLs were analyzed through ProtComp (v6), ExPASy64, SignalP65, PSORT Prediction, and TMHMM Servers (v2.0). The domains, conserved motifs and gene structure of BrEPFLs were identified using the CDD66. InterProScan67, and MEME68 tools. The cis-acting elements in the promoter regions of BrEPFL were predicted by the PlantCARE database69. The tertiary structures of BrEPFL proteins were predicted by the SWISS-MODEL tool70.
Chromosomal distribution and collinearity analyses
All BrEPFL genes distribution was mapped to B. rapa chromosomes using TBtools71. The gene duplication events of the paralogous BrEPFL gene pairs were analyzed by the Multiple Collinearity Scan toolkit (MCScanX)72, and the syntenic blocks of the orthologous pairs among the Brassica crops and Arabidopsis were conducted through syntenic analysis.
Phylogenetic and Ka/Ks analyses
All identified EPFL protein sequences from 15 Brassicaceae were aligned by ClustalW and used to generate a phylogenetic tree by the neighbor-joining (NJ) method of MEGA 7.0 73, with the following parameters: Poisson model, pairwise deletion, and 1000 bootstrap replications. The software Ka_Ks Calculator74 was used to perform nonsynonymous substitution (Ka) and synonymous substitution (Ks) installment analyses, and Ks > 2.0 was excluded to avoid substitution saturation risk and calculate separation time.
Expression and interaction network analyses of the BrEPFL gene family
Transcriptomic data75 were used to estimate the expression levels of BrEPFLs in different tissues. Several reported RNA-Seq datasets (GMS-AB01, ftms, CMS-ogu1, CMS-ogu2, CMS-ogu3, CMS-poly)31,33,37,38,39,40,41 and our own dataset (CMS-ogu4) of male-sterile materials were taken to examine the function of BrEPFLs in anther development. The raw data were filtered and mapped to the B. rapa genome (V3.0), and the gene expression levels were calculated using FPKM (fragments per kilobase of transcript per million mapped reads) values. Finally, the differentially expressed genes (DEGs) were analyzed using DEGseq software. The heatmaps and venn diagrams were generated with TBtools.
The CMS-ogu4 line (4-2B) and its maintainer line (4–2 A) of wucai (Brassica campestris syn. B. rapa L. ssp. chinensis var. rosularis Tsen) were bred by Vegetable Breeding Laboratory of the College of Horticulture, Anhui Agricultural University, and used as the plant materials in this study. The male sterile line 4-2B was generated from an ogu-CMS line of nonheading Chinese cabbage through continuous backcrossing for more than ten generations with 4–2 A of wucai31,32. The microscopy observations were carried out as previously described31,35. Their bud materials from six different stages of anther development were applied to analyze the expression pattern in detail, including microspore mother cell, meiosis II, tetrad, uninucleate, binucleate, and mature pollen periods34,63,76. Total RNA was isolated and reverse-transcribed into cDNA with the related TaKaRa kits36,63. qRT-PCR was carried out with a SYBR® Premix Ex Taq™ II Kit using specific primers (Table S1). Three biological replicates for each sample and three technical replicates for each gene were analyzed. Relative fold gene expression levels were calculated using the 2−ΔΔCt method. The Actin gene was used as internal controls36. The interaction network among BrEPFL genes was constructed using the STRING database77 and Cytoscape software78. Gene Ontology (GO) annotations for BrEPFL coexpressed genes were obtained via Blast2GO79.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Sun, Q. et al. TaEPFL1, an EPIDERMAL PATTERNING FACTOR-LIKE (EPFL) secreted peptide gene, is required for stamen development in wheat. Genetica 147, 121–130 (2019).
Marshall, E., Costa, L. M. & Gutierrez-Marcos, J. Cysteine-rich peptides (CRPs) mediate diverse aspects of cell-cell communication in plant reproduction and development. J. Exp. Bot. 62, 1677–1686 (2011).
Lin, H. et al. Molecular traits and functional analysis of rapid alkalinization factors (RALFs) in four Gossypium species. Int. J. Biol. Macromol. 194, 84–99 (2022).
Silverstein, K. et al. Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant J. 51, 262–280 (2010).
Lin, Z. et al. Ectopic expression of a self-incompatibility module triggers growth arrest and cell death in vegetative cells. Plant Physiol. 183, 1765–1779 (2020).
Bessho-Uehara, K. et al. Loss of function at RAE2, a previously unidentified EPFL, is required for awnlessness in cultivated Asian rice. PNAS 113, 8969 (2016).
Huang, Y. et al. BnEPFL6, an epidermal patterning factor-like (EPFL) secreted peptide gene, is required for filament elongation in Brassica napus. Plant Mol. Biol. 85, 505–517 (2014).
Li, P. et al. Genome-wide analyses of member identification, expression pattern, and protein–protein interaction of EPF/EPFL gene family in Gossypium. BMC Plant Biol. 24, 554 (2024).
Sugano, S. et al. Stomagen positively regulates stomatal density in Arabidopsis. Nature 463, 241–246 (2010).
Lin, Z., Du, W. & Zhao, F. Genome-wide identification and phylogenetic and expression pattern analyses of EPF/EPFL family genes in the Rye (Secale cereale L). BMC Genom. 25, 532 (2024).
Hara, K. et al. Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves. Plant Cell Physiol. 50, 1019–1031 (2009).
He, Y. et al. An EPFL peptide signaling pathway promotes stamen elongation via enhancing filament cell proliferation to ensure successful self-pollination in Arabidopsis thaliana. New Phytol. 238, 1045–1058 (2023).
Hunt, L., Bailey, K. & Gray, J. The signalling peptide EPFL9 is a positive regulator of stomatal development. New Phytol. 186, 609–614 (2009).
Richardson, L. & Torii, K. Take a deep breath: peptide signalling in stomatal patterning and differentiation. J. Exp. Bot. 64, 5243–5251 (2013).
Jangra, R. et al. Duplicated antagonistic EPF peptides optimize grass stomatal initiation. Development 148, 199780 (2021).
Lee, J. S. et al. Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature 522, 439–443 (2015).
Zeng, S. M., Lo, E. K., Hazelton, B. J., Morales, M. F. & Torii, K. U. Effective range of non-cell autonomous activator and inhibitor peptides specifying plant stomatal patterning. Development 147, dev192237 (2020).
Yang, X. et al. The transcription factor ZmNAC49 reduces stomatal density and improves drought tolerance in maize. J. Exp. Bot. 72, 1399–1410 (2021).
Liu, R. et al. Investigation on the potential functions of ZmEPF/EPFL family members in response to abiotic stress in maize. Int. J. Mol. Sci. 25, 16 (2024).
Li, S. et al. SlTLFP8 reduces water loss to improve water-use efficiency by modulating cell size and stomatal density via endoreduplication. Plant Cell Environ. 43, 2666–2679 (2020).
Li, H., Yang, Y., Wang, H., Liu, S. & Xia, X. The receptor-like kinase ERECTA confers improved water use efficiency and drought tolerance to Poplar via modulating stomatal density. Int. J. Mol. Sci. 22, 7245 (2021).
Franks, P. J., Doheny-Adams, T. W., Britton-Harper, Z. J. & Gray, J. E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New. Phytol. 207, 188–195 (2015).
Hughes, J., Hepworth, C., Dutton, C., Dunn, J. A. & Gray, J. E. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant. Physiol. 174, 776–787 (2017).
Hao, J. H. et al. CRISPR/Cas9-mediated SiEPF2 mutagenesis attenuates drought tolerance and yeld in Foxtail millet (Setaria italica). Plant. Cell. Environ. https://doi.org/10.1111/pce.15597 (2025).
Guo, T. et al. Competitive binding of small antagonistic peptides to the OsER1 receptor optimizes rice panicle architecture. Plant Commun. 6, 101204 (2025).
Uchida, N. et al. Regulation of inflorescence architecture by intertissue layer ligand-receptor communication between endodermis and phloem. PNAS 109, 6337 (2012).
Abrash, E. B., Davies, K. A. & Bergmann, D. C. Generation of signaling specificity in Arabidopsis by spatially restricted buffering of ligand-receptor interactions. Plant Cell 23, 2864–2879 (2011).
Abrash, E. & Bergmann, D. C. Regional specification of stomatal production by the putative ligand CHALLAH. Development 137, 447–455 (2010).
Uchida, N. & Tasaka, M. Regulation of plant vascular stem cells by endodermis-derived EPFL-family peptide hormones and phloem-expressed ERECTA-family receptor kinases. J. Exp. Bot. 64, 5335–5343 (2013).
Guo, T. et al. Optimization of rice panicle architecture by specifically suppressing ligand–receptor pairs. Nat. Commun. 14, 1640 (2023).
Chen, G. et al. Comparative transcriptome analysis between fertile and CMS flower buds in Wucai (Brassica Campestris L). BMC Genom. 19, 908 (2018).
Chen, G. et al. Characterization and utilization of a cytoplasmic male sterility line of Wucai (Brassica Campestris L). Hortic. Environ. Biotech. 60, 373–382 (2019).
Tan, C. et al. Pectin methylesterase inhibitor (PMEI) family can be related to male sterility in Chinese cabbage (Brassica rapa ssp. pekinensis). Mol. Genet. Genom. 293, 343–357 (2018).
Chen, G. et al. Genome-wide analysis of proline-rich extension-like receptor protein kinase (PERK) in Brassica rapa and its association with the pollen development. BMC Genom. 21, 401 (2020).
Chen, G. et al. Transgenic Wucai (Brassica Campestris L.) produced via Agrobacterium-mediated anther transformation in planta. Plant Cell Rep. 38, 577–586 (2019).
Liu, X. et al. TMT-label comparative proteomics reveals the vernalization mechanism in Wucai (Brassica Campestris L). J. Proteom. 314, 105398 (2025).
Zhou, X., Liu, Z., Ji, R. & Feng, H. Comparative transcript profiling of fertile and sterile flower buds from multiple-allele-inherited male sterility in Chinese cabbage (Brassica campestris L. ssp. pekinensis). Mol. Genet. Genom. 292, 967–990 (2017).
Wei, X., Lv, Y., Zhao, Y., Nath, U. K. & Zhang, X. Comparative transcriptome analysis in Chinese cabbage (Brassica rapa ssp. pekinesis) for DEGs of Ogura-, Polima-CMS and their shared maintainer. Physiol. Mol. Biol. Plants 26, 719–731 (2020).
Lin, S. et al. Comprehensive analysis of Ogura cytoplasmic male sterility-related genes in turnip (Brassica rapa ssp. rapifera) using RNA sequencing analysis and bioinformatics. Plos One 14, e0218029 (2019).
Huang, S. et al. Investigation of the genes associated with a male sterility mutant (msm) in Chinese cabbage (Brassica campestris ssp. pekinensis) using RNA-Seq. Mol. Genet. Genom. 295, 233–249 (2020).
Huang, S. et al. Transcriptome analysis of a female-sterile mutant (fsm) in Chinese cabbage (Brassica campestris ssp. pekinensis). Front. Plant Sci. 8, 546 (2017).
Liu, S., Chen, T., Li, X., Cui, J. & Tian, Y. Genome-wide identification and expression analysis of EPF/EPFL gene family in Populus trichocarpa. Fronti. Genet. 15, 1432376 (2024).
Li, P. et al. Genome-wide analyses of member identification, expression pattern, and protein–protein interaction of EPF/EPFL gene family in Gossypium. BMC Plant Biol. 24, 20 (2024).
Dong, X. et al. Identification and expression analysis of the NAC gene family in Coffea canephora. Agronomy 9, 670 (2019).
Khan, N. et al. Genome-wide identification, classification, and expression pattern of homeobox gene family in Brassica rapa under various stresses. Sci. Rep. 8, 16265 (2018).
Cheng, F. et al. Deciphering the diploid ancestral genome of the mesohexaploid Brassica rapa. Plant Cell 25, 1541–1554 (2013).
Wang, X. et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 43, 1035–1039 (2011).
Yang, Y. W., Lai, K. N., Tai, P. Y. & Li, W. H. Rates of nucleotide substitution in angiosperm mitochondrial DNA sequences and dates of divergence between Brassica and other Angiosperm lineages. J. Mol. Evol. 48, 597–604 (1999).
Liu, T. et al. Genome-wide identification, molecular evolution, and expression profiling analysis of pectin methylesterase inhibitor genes in Brassica campestris ssp. chinensis. Int. J. Mol. Sci. 19, 1338 (2018).
Azim, J. B., Khan, M., Hassan, L. & Robin, A. Genome-wide characterization and expression profiling of plant-specific PLATZ transcription factor family genes in Brassica rapa L. Plant Breed. Biotech. 8, 28–45 (2020).
Lin, Z., Du, W. & Zhao, F. Genome-wide identification and phylogenetic and expression pattern analyses of EPF/EPFL family genes in the Rye (Secale cereale L). BMC Genom. 25, 532 (2024).
Dong, X., Jiang, Y. & Hur, Y. Genome-wide analysis of glycoside hydrolase family 1 β-glucosidase genes in Brassica rapa and their potential role in pollen development. Int. J. Mol. Sci. 20, 1663 (2019).
Cai, H. et al. Signaling by the EPFL-ERECTA family coordinates female germline specification through the BZR1 family in Arabidopsis. Plant Cell 35, 1455–1473 (2023).
Usadel, B., Obayashi, T., Mutwil, M., Giorgi, F. M. & Provart, N. J. Co-expression tools for plant biology: opportunities for hypothesis generation and caveats. Plant Cell Environ. 32, 1633–1651 (2010).
Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. PNAS 95, 14863–14868 (1998).
Xia, Y., Han, Q., Shu, J., Jiang, S. & Kang, X. Stomatal density suppressor PagSDD1 is a generalist gene that promotes plant growth and improves water use efficiency. Int. J. Biol. Macromol. 262, 129721 (2024).
Herrmann, A. et al. Chemical genetics reveals cross-regulation of plant developmental signaling by the immune peptide-receptor pathway. Sci. Adv. 11, eads3718 (2025).
Zekri, M. A. & Lang, I. Lack of trichomes and variation in stomata properties influence the quantum efficiency of photosynthesis in Arabidopsis. Environ. Exp. Bot. 227, 105948 (2024).
Bundy, M. G. et al. A mutation in the catalytic subunit of the glycosylphosphatidylinositol transamidase disrupts growth, fertility, and stomata formation. Plant Physiol. 171, 974–985 (2016).
Shpak, E. D., Berthiaume, C. T., Hill, E. J. & Torii, K. U. Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131, 1491–1501 (2004).
Hossain, M. F. et al. Targeted expression of bgl23-D, a dominant-negative allele of ATCSLD5, affects cytokinesis of guard mother cells and exine formation of pollen in Arabidopsis thaliana. Planta 257, 64 (2023).
Shpak, E. D. Diverse roles of ERECTA family genes in plant development. J. Integr. Plant Biol. 55, 1238–1250 (2013).
Liu, X. et al. Genome-wide identification, transcript profiling and functional analyses of PCP gene family in Wucai (Brassica campestris). Sci. Rep. 14, 28236 (2024).
Gasteiger, E. et al. Protein identification and analysis tools on the expasy server. Proteomics Protocols Handbook, 571–607 (2005).
Armenteros, J. J. A. et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 37, 420–423 (2019).
Marchlerbauer, A. et al. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 45, 200–203 (2017).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240 (2014).
Bailey, T. L., Nadya, W., Chris, M. & Li, W. W. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 34, 369–373 (2006).
Magali, L. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in Silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–327 (2002).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, 296–303 (2018).
Chen, C. et al. TBtools-II: a one for all, all for one bioinformatics platform for biological big-data mining. Mol. Plant 16, 1733–1742 (2023).
Wang, Y. et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, e49 (2012).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Wang, D., Zhang, Y., Zhang, Z., Zhu, J. & Yu, J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinf. 8, 79–82 (2010).
Tong, C. et al. Comprehensive analysis of RNA-seq data reveals the complexity of the transcriptome in Brassica rapa. BMC Genom. 14, 689 (2013).
Zhang, S. et al. Functional analysis of a MYB transcription factor BrTDF1 in the tapetum development of Wucai (Brassica rapa ssp). Sci. Horic. 257, 108728 (2019).
Szklarczyk, D. et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, 607–613 (2018).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Conesa, A. et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676 (2005).
Acknowledgements
This work was supported by the Natural Science Foundations of Anhui Province and Hefei City (2308085QC101; 2308085MC96; 202343); Anhui Provincial Program on Major Basic Research Project (2023z04020005); Excellent Young Talents Fund Program of Higher Education Institutions of Anhui Province (2024AH030017).
Funding
This work was supported by the Natural Science Foundations of Anhui Province and Hefei City (2308085QC101; 2308085MC96; 202343); Anhui Provincial Program on Major Basic Research Project (2023z04020005); Excellent Young Talents Fund Program of Higher Education Institutions of Anhui Province (2024AH030017).
Author information
Authors and Affiliations
Contributions
G. C. and X. T. designed the research. K. W and Y. W. carried out the experiments. K. W., X. T. and G. C. wrote the manuscript. N. L. and D. Y. helped to analyze the data. W. W. and L. Y. helped to perform the experiments. J. W., J. H., and C. W. helped to draft the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The CMS-ogu4 line 4-2B and its maintainer line 4–2 A were screened by the corresponding author, and all seed materials were bred and kept in the Vegetable Breeding Laboratory of the College of Horticulture, Anhui Agricultural University. No specific permits were required for the described field studies. The location is not privately-owned or protected in any way, and the field studies did not involve endangered or protected species. We complied with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, K., Wang, Y., Tang, X. et al. Genome wide identification and characterization of EPFL genes and its potential association with male sterility in Brassica rapa. Sci Rep 15, 24033 (2025). https://doi.org/10.1038/s41598-025-09555-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-09555-1