Abstract
Given the high mortality rate of chronic obstructive pulmonary disease (COPD) complicated by heart failure (HF), early identification of high-risk patients and timely intervention are crucial. There is currently no in-hospital mortality risk prediction model for COPD complicated by HF patients with different Body Mass Index (BMI). This study aims to explore the risk factors of COPD complicated by HF and construct an in-hospital mortality risk prediction model. Method: Select a population that meets the diagnostic criteria for COPD complicated by HF from the Medical Information Mart for Intensive Care IV (MIMIC-IV) and analyze the baseline characteristics of the patients. Univariate Cox regression analysis and multivariate Cox regression analysis were used to determine the risk factors for mortality in patients with different BMIs and to construct a prediction model. Evaluate the model’s consistency, discriminability, and clinical application value using the calibration curve, area under the curve (AUC), and decision curve analysis (DCA), respectively. Result: A total of 907 patients with COPD complicated by HF were included, and risk factors such as age, heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), white blood cell count (WBC), heart rate(HR), respiratory rate (RR), blood urea nitrogen (BUN), prothrombin time (PT), activated partial thromboplastin time (aPTT), diabetes, peripheral vascular disease, sequential organ failure assessment (SOFA), and Glasgow Coma Scale(GCS) were included in the prediction model. AUC, calibration, and decision curves indicate that most models have good discrimination, calibration, and clinical application value. Conclusion: The in-hospital mortality risk prediction model for COPD complicated by HF based on MIMIC-IV has good recognition ability and significant clinical reference value for patient prognosis risk assessment and intervention treatment.
Similar content being viewed by others
Introduction
With the increasing aging of the global population, the issue of COPD complicated by HF is receiving growing attention. COPD and HF have a high degree of comorbidity, and patients with COPD complicated by HF often have poor prognosis1. In the intensive care unit (ICU), patients with COPD complicated by HF often have a higher risk and consume more medical resources. Therefore, quickly assessing the severity of the patient’s disease and predicting their risk of death have good guiding significance for clinical doctors’treatment2.
BMI is a commonly used indicator internationally to measure the degree of body fat and thinness, as well as to determine whether the weight is healthy. Previous studies have shown that obesity is one of the independent risk factors for developing COPD and HF. However, an increasing number of clinical studies and evidence-based medicine have shown that overweight and obese COPD and HF patients have lower mortality rates compared to normal-weight and low-weight patients3,4,5. The counterintuitive epidemiological relationship between this survival outcome and traditional risk factors is known as“reverse epidemiology”or the“obesity paradox”6.
At present, there is still controversy over the relationship between BMI and survival in patients with COPD complicated by HF and further research is urgently needed. Moreover, there is currently no prognostic prediction model for COPD complicated by HF patients with different BMI.
Therefore, this study explored the impact of BMI on the prognosis of patients with COPD complicated by HF and constructed a prognosis prediction model for patients with different BMI to help clinicians accurately assess the patient’s condition and take personalized measures to improve the patient’s prognosis on time.
Data and methods
Data sources
MIMIC-IV-3.1 is a free and open big clinical database that collects 94,458 unique ICU stay records from 2008 to 2019 at Beth Israel Deaconess Medical Center in Boston, Massachusetts Institute of Technology. All patient information is stored in the MIMC-IV database after privacy processing7. Any researcher who accepts the data usage agreement and completes the"Protecting Human Subjects"training can apply to access the data without the need for patient-informed consent. The author of this article, Shaoqiang Wang, has obtained authorization to use the database (authentication number: 13993436).
Selection of research subjects
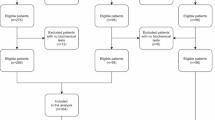
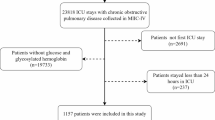
Inclusion criteria: (1) Adult patients (aged ≥ 18 years); (2) The same patient has multiple ICU admission records, only the first record will be included; (3) The diagnosis of patients with COPD complicated by HF is based on the diagnostic codes of the International Classification of Diseases (ICD-10), where the ICD-10 code for HF is I50 and the ICD-10 code for COPD is J44. Exclusion criteria: (1) Patients without BMI information or unable to calculate BMI; (2) Pregnant or lactating patients. The screening process diagram is shown in Fig. 1.
Data extraction
Use Structured Query Language (SQL) and Navicat Premium 16 for data extraction. The extracted variables include demographic characteristics (age, gender, race), vital signs (RR, HR, SBP, DBP, pH value, arterial oxygen saturation (SaO2(%)), laboratory indicators (hemoglobin, platelet count, WBC, bicarbonate, BUN, total calcium, glucose, serum sodium, serum potassium, neutrophil count, monocyte count, lymphocyte count, PT, aPTT), comorbidities (rheumatic disease, kidney disease, peripheral vascular disease, myocardial infarction, mild liver disease, severe liver disease, diabetes, dementia, cerebrovascular disease), GCS and SOFA. In this study, the primary endpoint was in-hospital mortality (including deaths occurring in the ICU and deaths after transfer to general wards). We utilized the hospital expire flag column in the MIMIC-IV database’s admissions table to identify whether patients died during hospitalization (1 = death, 0 = survival). Patient survival time was calculated by determining the time difference between the admission time (admittime column in the admissions table) and the date of death (dod column in the admissions table)."
Statistical processing
Due to the small number of patients with low weight, normal weight, and overweight included, only the training group was set up for all three groups. As more obese patients were included, the data of obese patients were set as the training group and validation group in a 7:3 ratio. Perform statistical analysis using R4.4.2 and SPSS 26.0. The metric data of a normal distribution is represented by \(\overline{x }\)(s). Comparison between two groups using two independent sample t-tests; The measurement data of skewed distribution is represented by M [Q1; Q3]. Comparison between two groups using Mann–Whitney U tests. Use univariate Cox regression analysis to identify clinical factors associated with in-hospital mortality (P < 0.1)8. Clinically relevant factors associated with in-hospital mortality (P < 0.1) identified by univariate Cox regression analysis were included in the multivariate Cox regression model for further screening (P< 0.1). The factors selected through multivariate Cox regression were used to develop a prognostic model, and a nomogram was constructed to represent the results of the multivariate analysis visually9.. In addition, time-dependent receiver operating characteristic (ROC) curves of the nomogram and all independent prognostic variables at 7, 14, and 21 days were generated, and the corresponding time-dependent AUCs were applied to show the discrimination. Calibration curves and DCA of 7, 14, and 21 days were plotted to evaluate the nomogram. According to the median risk score, all patients with COPD complicated by HF were divided into high-, and low-risk groups. Kaplan–Meier (K-M) survival curves with the log-rank test were performed to show the difference in overall survival (OS) status between the two groups (low-risk group and high-risk group)10.
Results
Baseline characteristics of patients
A total of 907 patients with COPD complicated by HF were included, including 27 patients with low weight (< 18.5), 244 patients with normal weight (18.5 ≤ BMI < 25), 216 patients with overweight (25 ≤ BMI < 30), and 420 patients with Obesity (30 ≥ BMI). The Kaplan–Meier survival curve showed a significant difference in survival time among the four groups (P = 0.0028 < 0.1), as shown in Fig. 2. Meanwhile, univariate Cox regression showed that for all included patients, BMI was an independent risk factor for mortality in COPD patients complicated by HF (P = 0.04 < 0.1), and high BMI was a protective factor for mortality in COPD patients complicated by HF, as shown in Table 1 and Fig. 3. The specific baseline characteristics of the included patients are listed in Table 2.
Univariate and multivariate analysis of in-hospital mortality risk in patients with different BMI
Univariate Cox regression analysis showed that WBC, aPTTs, dementia, diabetes, and GCS were independent risk factors for death in the univariate model of patients with low weight (P < 0.1). The statistically significant factors in the univariate Cox regression analysis were included in the multivariate Cox regression analysis. Risk factors with statistical significance (P < 0.1) identified by univariate Cox regression analysis were included in the multivariate Cox regression analysis. Ultimately, WBC and diabetes were incorporated into the prediction model, and a nomogram was constructed, as listed in Table 3.
Univariate Cox regression analysis showed that Age, HR, PH, BUN, Lymphocytes, Monocytes, aPTT, Renal disease, SOFA, and GCS were independent risk factors in the univariate model of patients with normal weight (P < 0.1). Risk factors with statistical significance (P < 0.1) identified by univariate Cox regression analysis were included in the multivariate Cox regression analysis. Ultimately, age, HR, and aPTTs were incorporated into the prediction model, and a nomogram was constructed, as listed in Table 4.
Univariate Cox regression analysis showed that Age, HR, RR, Temperature, SaO2(%), Bicarbonate, BUN, Glucose, Serum sodium, Lymphocytes, aPTT, Dementia, Myocardial infarct, Peripheral vascular disease, Severe liver disease, SOFA, and GCS were independent risk factors for death in the univariate model of patients with overweight (P < 0.1). Risk factors with statistical significance (P < 0.1) identified by univariate Cox regression analysis were included in the multivariate Cox regression analysis. Ultimately, HR, aPTT, SOFA, and GCS were incorporated into the prediction model, and a nomogram was constructed, as listed in Table 5.
Univariate Cox regression analysis showed that Race, SBP, DBP, HR, RR, Temperature, WBC, Bicarbonate, BUN, Calcium, Glucose, Serum sodium, Serum potassium, Monocytes, Neutrophils, PT, Peripheral vascular disease, and SOFA were independent risk factors for death in the univariate model of patients with overweight (P < 0.1). Risk factors with statistical significance (P < 0.1) identified by univariate Cox regression analysis were included in the multivariate Cox regression analysis. Ultimately, Race, SBP, DBP, HR, RR, BUN, PT, Peripheral vascular disease, and SOFA were incorporated into the prediction model, and a nomogram was constructed, as listed in Table 6.
Construction of nomogram
Based on the results of multivariate Cox regression analysis, construct a nomogram for in-hospital mortality in patients with COPD complicated by HF. When using the nomogram, a vertical line should be drawn up from each variable to the“Points”line to obtain the point, and these values should be added up to obtain the total points. A vertical line should be drawn down from the“Total Points”to obtain the in-hospital mortality probability of patients with COPD complicated by HF, as shown in Fig. 4. The K-M OS curves showed great discrimination among the three risk groups, as shown in Fig. 5.
Evaluation of the predictive value of the prediction model
The calibration curve showed that, except for the group of patients with low weight, the predicted in-hospital mortality rate and actual in-hospital mortality rate of the other groups had good consistency, as shown in Fig. 6. Moreover, this study further calculated the AUC by plotting ROC curves, and the results showed that the AUCs of the clinical prediction models constructed in this study were all greater than 0.7. The C-index predicted by the prediction models for in-hospital mortality in low-weight patients, normal-weight patients, overweight patients, obesity patients (Training set) and Obesity patients (Validation set) were 0.773 [95% CI (0.644–0.902)], 0.768 [95% CI (0.692–0.844)], 0.781 [95% CI (0.659–0.903)], and 0.864 [95% CI (0.819–0.909)], respectively. This indicated that the prediction model had good discriminability, as shown in Fig. 7. The DCA results showed that, except for the 7-day mortality risk prediction model in the low-weight group and the 14-day mortality risk prediction model in the normal-weight group, all other models had good performance in clinical practice, as shown in Fig. 8.
Discussion
HF is the terminal stage of various cardiovascular diseases, with high incidence, high risk of death, and huge patient costs. It is an important part of global cardiovascular disease prevention and treatment. The latest epidemiological survey shows that there are approximately 68 million HF patients worldwide, with a prevalence rate between 1 and 2%11. According to the 2023 Global Initiative for Chronic Obstructive Lung Disease guidelines, COPD causes 3 million deaths annually and currently ranks as the third leading cause of death worldwide12. As COPD patients age and the course of the disease prolongs, their condition worsens, leading to a gradual increase in the incidence of HF, which seriously affects the quality of life and physical health of elderly patients13. Meanwhile, studies showed that COPD patients who died due to cardiovascular disease account for over half of all COPD patient deaths14. Moreover, data from the Clinical Practice Research Datalink in the UK showed that the 1-year mortality rate of COPD patients with HF was three times higher than that of COPD patients without HF15.
Clinical risk prediction models analyze and model clinical sample data to establish mathematical models that predict the probability of specific individuals developing a certain disease or clinical outcome. Among these, the nomogram—an intuitive and convenient predictive tool in clinical prediction modeling—translates complex multivariable regression equations into graphical scoring systems. This allows clinicians to rapidly assess individual risk without complex calculations, thus enabling swift evaluation of patient risk levels and supporting the development of more personalized treatment plans. Furthermore, from the perspective of doctor-patient communication, its visual interface facilitates a clear explanation of risk factor contributions to patients, thereby improving treatment adherence16,17. Consequently, developing an accurate model to predict mortality risk in patients with COPD complicated by HF holds significant clinical importance. Moreover, significant differences in mechanical load18, inflammatory responses19, and pharmacokinetics between obese and non-obese patients result in distinct pathological progression and treatment responses, necessitating differentiated monitoring and intervention strategies for COPD patients complicated by HF in these two populations20.
This study screened 907 patients with COPD complicated by HF from 94,458 unique ICU stay records in the MIMC-IV database and constructed a clinical prediction model through univariate and multivariate COX analysis. For the group of patients with low weight, 2 variables, WBC and diabetes, were included; For the group of patients with normal weight, 5 variables, age, HR, and aPTT, were included. For the group of patients with overweight, 4 variables, age, HR, aPTT, SOFA, and GCS, were included; For the group of patients with obesity, 9 variables, race, SBP, DBP, HR, RR, BUN, PT, peripheral vascular disease, and SOFA, were included. The low-weight group may be affected by the small sample size, and the calibration curve of their mortality risk prediction model showed poor consistency between predicted mortality rates and actual in-hospital mortality rates. The DCA results showed that, except for the 7-day mortality risk prediction model in the low-weight group and the 14-day mortality risk prediction model in the normal-weight group, all other groups had good predictive ability, and performance in clinical practice, and the predicted situation was consistent with the actual situation.
As individuals age, the function of vital organs such as the heart and lungs gradually declines. The heart’s pumping capacity weakens, myocardial contractility decreases, and the elasticity of blood vessel walls diminishes. These changes collectively increase cardiac workload and the risk of HF21. Similarly, pulmonary functions decline with age, including reduced elastic recoil and decreased secretion of alveolar surfactant. This leads to impaired ventilation and gas exchange, exacerbating symptoms in patients with COPD. Furthermore, immune system function weakens with advancing age, rendering elderly individuals more susceptible to infections. Such infections can trigger and worsen acute exacerbations of both HF and COPD22. These cumulative factors result in more rapid disease progression, a narrower therapeutic window, higher complication risks, and poorer prognoses in elderly patients. COPD and HF patients often experience an increase in HR due to hypoxemia and overactivation of the sympathetic nervous system, so HR can often reflect the patient’s sympathetic nervous activity and cardiac burden23. When body weight increases, the blood volume in the body also increases accordingly. This leads to an increase in venous return to the heart, consequently raising the cardiac preload. Obesity is a significant risk factor for atherosclerosis. Atherosclerosis, in turn, increases the patient’s peripheral vascular resistance (afterload). The increase in both cardiac preload and afterload then results in a compensatory increase in HR24. For SBP and DBP, HF patients usually experience a decrease due to changes in cardiac output25. When HF occurs, the heart’s pumping function is already compromised. A decrease in blood pressure further reduces coronary artery blood flow and perfusion. This worsens myocardial oxygen supply, leading to disorders in myocardial energy metabolism and aggravating the deterioration of cardiac function, creating a vicious cycle26. Simultaneously, COPD patients often have pulmonary hypertension. Low blood pressure results in insufficient pulmonary circulation dynamics, worsening pulmonary congestion, and pulmonary edema. This impairs alveolar gas exchange function, leading to exacerbated hypoxia and carbon dioxide retention, which further increases the burden on the heart and worsens HF27. Compared to non-obese patients, obese patients generally have higher blood volume and cardiac output. Maintaining perfusion for their larger body mass requires relatively higher blood pressure28. Therefore, for obese patients, hypotension is often considered an independent risk factor for poor prognosis. RR can reflect the patient’s respiratory burden and oxygenation status and is an important vital sign for COPD patients with HF. A high respiratory rate may indicate that the patient is in an acute exacerbation or compensatory state29.If a patient experiences an increased respiratory rate and reduced tidal volume, pulmonary ventilation function becomes further limited. This leads to exacerbated carbon dioxide retention (hypercapnia) and worsening hypoxia. COPD itself causes increased airway resistance and diminished pulmonary elastic recoil, impairing pulmonary ventilation. Furthermore, obesity—particularly central obesity—restricts diaphragmatic movement, thereby further aggravating ventilation impairment in COPD patients and worsening their clinical condition30. An increase in BUN may indicate insufficient renal perfusion due to low cardiac output, and in the treatment of COPD, chronic hypoxia, and diuretic use can also lead to an increase in BUN. For patients with HF, impaired cardiac pumping function leads to reduced cardiac output and diminished renal blood flow. Inadequate renal perfusion decreases the glomerular filtration rate, hindering urea excretion and resulting in the accumulation of BUN. Concurrently, during the progression of COPD, chronic hypoxia and diuretic use also contribute to elevated BUN levels31,32. Obese patients exhibit a chronic low-grade inflammatory state in which adipose tissue secretes various inflammatory factors33. These inflammatory mediators can cause renal damage, impairing the excretion of waste products such as urea nitrogen and consequently raising BUN34. Furthermore, the relatively increased blood volume in obese patients places additional strain on the heart. Compounded by HF, this leads to restricted cardiac diastolic function, elevated atrial pressure, and stimulated secretion of atrial natriuretic peptide (ANP). ANP induces vasoconstriction, increasing peripheral resistance. This further reduces renal blood flow perfusion, exacerbating the renal excretory burden and ultimately contributing to elevated BUN levels35. Both hypoxemia in COPD and hypoperfusion in HF can trigger the HIF signaling pathway, leading to further release of inflammatory factors. At the same time, the hypoxic environment may also amplify the inflammatory response by increasing oxidative stress damage. Therefore, both COPD and HF have the characteristics of chronic inflammation, and WBC can reflect the patient’s inflammatory status36,37. The development of obesity is accompanied by alterations in the adipose tissue microenvironment, wherein macrophage recruitment and infiltration constitute a critical component of the inflammatory response38. Under obese conditions, adipocytes secrete chemokines that attract macrophage infiltration. These infiltrating macrophages subsequently release pro-inflammatory cytokines such as TNF-α and IL-6, triggering chronic low-grade inflammation39. This inflammatory cascade not only disrupts insulin signaling transduction in adipocytes but also exacerbates insulin resistance and metabolic dysregulation, ultimately exacerbating the patient’s clinical condition40.PT and aPTT can reflect the coagulation function of patients. COPD and HF patients usually have an imbalance in the coagulation fibrinolysis system, and abnormal coagulation function may further cause hemodynamic instability in COPD and HF patients, thereby worsening the condition41. Concurrently, critically ill patients frequently require anticoagulant therapy to prevent thrombosis, which inherently prolongs both PT and PTT42. Furthermore, HF, COPD, and obesity are all intrinsically associated with chronic inflammation. Under the stimulation of inflammatory cytokines, activated immune cells release substantial quantities of inflammatory mediators. These mediators inflict damage upon vascular endothelial cells, compromise the physiological function of coagulation factors, and disrupt the coagulation cascade, ultimately resulting in prolonged PT and PTT43. For patients with COPD complicated by HF, atherosclerosis will further intensify due to the occurrence of hypoxemia and systemic inflammation, thus increasing the risk of peripheral vascular disease. At the same time, venous congestion and hypoperfusion caused by HF may also lead to the occurrence of peripheral vascular diseases44,45. Obesity is an independent risk factor for peripheral vascular disease. Obese patients often exhibit components of metabolic syndrome, such as hyperlipidemia, hypertension, and hyperglycemia. These factors can lead to vascular endothelial damage, enhanced inflammatory responses, alterations in hemodynamics, and a hypercoagulable state of the blood. Consequently, they accelerate the formation of atherosclerosis and increase the risk of developing peripheral vascular disease46. SOFA is a scoring tool used to evaluate the organ function status and the degree of multiple organ failure in critically ill patients. The higher the SOFA score, the more severe the organ dysfunction and the higher the risk of death. The score reflects the degree of organ function damage of patients by evaluating the functional status of six major organ systems and is applicable to all critically ill patients47. GCS is a clinical tool used to assess a patient’s level of consciousness. It rapidly quantifies neurological function by evaluating three aspects of responsiveness: Eye Opening, Verbal Response, and Motor Response. This assessment determines the depth of coma and provides insight into the patient’s prognostic outlook48. HF and COPD mutually exacerbate each other, complicating therapeutic management. Obesity further intensifies the burden on both cardiac and pulmonary systems. Elevations in SOFA and GCS reflect this intricate pathophysiological complexity, indicating heightened risk of multi-organ functional deterioration. The“obesity paradox”was similarly observed in this study, which demonstrated that a higher BMI served as a protective factor against mortality in HF complicated by COPD. Obesity is one of the major risk factors for HF. Studies have shown that about 50%—80% of patients with HF are overweight or obese. However, in patients with HF, it was found that compared with normal weight, overweight and obese patients had a lower risk of death49. Moreover, studies on respiratory diseases have shown that overweight or obese patients with COPD have a better prognosis than normal-weight patients and studies have shown that patients with BMI < 25 have the highest incidence of COPD exacerbation within 1 year, and the survival rate of COPD patients with BMI > 25 is much higher than that of patients with BMI < 2050.
However, the model still has some limitations: Firstly, due to the sample size, the external validation of random sampling was not carried out for the low weight group, the normal weight group, and the overweight group, and further external validation of the model is needed in the future. Secondly, it may be limited by the small sample size of the low-weight group, the calibration curve of the low-weight group showed that the in-hospital mortality predicted by the low-weight group was less consistent with the actual in-hospital mortality, and the DCA results showed that the performance in clinical practice of the 7-day mortality risk prediction model in the low-weight group was poor. Thirdly, due to dataset limitations, the median hospital length of stay could not be provided in this study. Fourthly, due to the inherent limitations of the database and the scope constraints of this manuscript, we were unable to delve into the impact of dietary habits, smoking behaviors, and physical activity patterns across different populations, nor examine the effects of regional disparities in healthcare accessibility and insurance systems. This limitation may introduce potential bias into our findings. Finally, a key limitation of this study is the use of distinct predictor sets across BMI subgroups in our multivariate Cox models. This approach restricts direct comparability of prognostic factor effects between BMI categories and compromises model consistency. Consequently, the interpretability and generalizability of our findings may be constrained.
Conclusion
In conclusion, this study used the data in the big clinical database MIMIC-IV to build a prediction model for the mortality risk of in-hospital death of HF complicated with COPD with different BMI. This model enables accurate prediction of in-hospital mortality risk in patients with COPD by HF with different BMI categories, providing clinicians with a practical tool for rapid individualized risk assessment.
Data availability
Data can be obtained from the first author and the corresponding authors.
References
Khan, S. S. & Kalhan, R. Comorbid Chronic Obstructive Pulmonary Disease and Heart Failure: Shared Risk Factors and Opportunities to Improve Outcomes. Ann. Am. Thorac Soc. 19, 897–899. https://doi.org/10.1513/AnnalsATS.202202-152ED (2022).
Shi, Q., Xu, J., Zeng, L., Lu, Z. & Chen, Y. A nomogram for predicting short-term mortality in ICU patients with coexisting chronic obstructive pulmonary disease and congestive heart failure. Respir Med. https://doi.org/10.1016/j.rmed.2024.107803 (2024).
Guo, Y. et al. Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine (Baltimore) https://doi.org/10.1097/MD.0000000000004225 (2016).
Horwich, T. B., Fonarow, G. C. & Clark, A. L. Obesity and the Obesity Paradox in Heart Failure. Prog. Cardiovasc Dis. 61, 151–156. https://doi.org/10.1016/j.pcad.2018.05.005 (2018).
Jieyun, Z. et al. Body mass index and mortality of chronic obstructive pulmonary disease: a meta-analysis. Chin. J. Evid. Based Med. 19, 811–817 (2019).
Tutor, A. W., Lavie, C. J., Kachur, S., Milani, R. V. & Ventura, H. O. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 78, 2–10. https://doi.org/10.1016/j.pcad.2022.11.013 (2023).
Liu, S. et al. Reinforcement Learning to Optimize Ventilator Settings for Patients on Invasive Mechanical Ventilation: Retrospective Study. J. Med. Internet. Res. https://doi.org/10.2196/44494 (2024).
Liao, F. et al. Nomogram for predicting electrocoagulation syndrome after endoscopic submucosal dissection of esophageal tumors. Surg. Endosc. 38, 1877–1883. https://doi.org/10.1007/s00464-024-10685-2 (2024).
Wu, J. et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. (Lond) 40, 301–312. https://doi.org/10.1002/cac2.12067 (2020).
Chen, B. et al. Risk Factors, Prognostic Factors, and Nomograms for Distant Metastasis in Patients With Newly Diagnosed Osteosarcoma: A Population-Based Study. Front Endocrinol. (Lausanne) https://doi.org/10.3389/fendo.2021.672024 (2021).
Svahn, S. et al. Prevalence of heart failure and trends in its pharmacological treatment between 2000 and 2017 among very old people. Bmc. Geriatr. 24, 701. https://doi.org/10.1186/s12877-024-05307-4 (2024).
Wu, K. et al. Associations of anxiety and depression with prognosis in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Pulmonology 31, 2438553. https://doi.org/10.1080/25310429.2024.2438553 (2025).
Chunmei, Y., Jing, Z. & Lanying, S. Application value of hs CRP/PAB ratio in elderly patients with chronic obstructive pulmonary disease complicated with heart failure. Exp. Lab. Med. 41, 325–328 (2023).
Mentz, R. J. et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J. Am. Coll. Cardiol. 64, 2281–2293. https://doi.org/10.1016/j.jacc.2014.08.036 (2014).
Axson, E. L. et al. Temporal Trends in the Incidence of Heart Failure among Patients with Chronic Obstructive Pulmonary Disease and Its Association with Mortality. Ann. Am. Thorac. Soc. 17, 939–948. https://doi.org/10.1513/AnnalsATS.201911-820OC (2020).
Jie, L., Liusujuan, R. L. & Zhangwenjing, Y. W. Establishment of a Nomogram model for individualized prediction of the risk of acute spinal cord injury complicated with respiratory dysfunction. China J. Orthop. Traumatol. 38, 525–531 (2025).
Li, P., Zhong, C., Huang, X., Cai, Z. & Guo, T. Construction of a predictive model for relapse of primary autoimmune hemolytic anemia: a retrospective cohort study. Ann. Med. 57, 2506482. https://doi.org/10.1080/07853890.2025.2506482 (2025).
Baglyas, S. et al. Using intra-breath oscillometry in obesity hypoventilation syndrome to detect tidal expiratory flow limitation: a potential marker to optimize CPAP therapy. Bmc Pulm Med. 23, 477. https://doi.org/10.1186/s12890-023-02777-x (2023).
Chiu, Y. H., Chou, W. L., Ko, M. C., Liao, J. C. & Huang, T. H. Curcumin mitigates obesity-driven dysbiosis and liver steatosis while promoting browning and thermogenesis in white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. https://doi.org/10.1016/j.jnutbio.2025.109920 (2025).
Zhang, M. et al. Effect of Obesity on Pharmacokinetics of Lidocaine and its Active Metabolites in Chinese Patients Undergoing Laparoscopic Bariatric Surgery: A Prospective Clinical Study. Clin. Pharmacokinet. https://doi.org/10.1007/s40262-025-01510-7 (2025).
Fleg, J. L. & Strait, J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev. 17, 545–554. https://doi.org/10.1007/s10741-011-9270-2 (2012).
Devulder, J. V. Unveiling mechanisms of lung aging in COPD: A promising target for therapeutics development. Chin Med. J. Pulm. Crit. Care Med. 2, 133–141. https://doi.org/10.1016/j.pccm.2024.08.007 (2024).
Schafauser, N. S. et al. Influence of heart failure (HF) comorbidity in chronic obstructive pulmonary disease (COPD) and isolated forms of HF and COPD on cardiovascular function during hospitalization. Respir Med. 231, 107731. https://doi.org/10.1016/j.rmed.2024.107731 (2024).
Mille-Hamard, L., Momken, I., Koralsztein, J. P. & Billat, V. L. Cardiovascular, Hemodynamic, and Anthropometric Adaptations Induced by Walking Training at FATmax in Obese Males and Females over 45 Years Old. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph22050701 (2025).
Matsiukevich, D. et al. Characterization of a robust mouse model of heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 325, H203–H231. https://doi.org/10.1152/ajpheart.00038.2023 (2023).
Xiaonan, D., Jun, W., Jianfang, M. & Jinbo, L. Clinical Observation of Xinshuai No.1 Formula Combined with Sacubitril Valsartan in Treatment of Chronic Heart Failure with Hypotension. Chinese Arch. Trad. Chinese Med. 43, 164–167. https://doi.org/10.13193/j.issn.1673-7717.2025.02.035 (2025).
Casselbrant, A. et al. Orthostatic blood pressure reactions and resting heart rate in relation to lung function - the Swedish CArdioPulmonary bioImage Study (SCAPIS). Bmc Pulm Med. 24, 587. https://doi.org/10.1186/s12890-024-03398-8 (2024).
Brignole, M. et al. Corrigendum to “Syncope in hypertrophic cardiomyopathy (part II): An expert consensus statement on the diagnosis and management” [International Journal of Cardiology, 2023, 41:180–186]. Int. J .Cardiol. https://doi.org/10.1016/j.ijcard.2023.131589 (2024).
Hunold, K. M. et al. Diagnosing Dyspneic Older Adult Emergency Department Patients: A Pilot Study. Acad Emerg Med 28, 675–678. https://doi.org/10.1111/acem.14183 (2021).
Dixon, A. E. & Peters, U. The effect of obesity on lung function. Expert Rev. Respir Med. 12, 755–767. https://doi.org/10.1080/17476348.2018.1506331 (2018).
Crisafulli, E. et al. Clinical features and outcomes of elderly hospitalised patients with chronic obstructive pulmonary disease, heart failure or both. Intern Emerg. Med. 18, 523–534. https://doi.org/10.1007/s11739-023-03207-w (2023).
Lindenfeld, J. & Schrier, R. W. Blood urea nitrogen a marker for adverse effects of loop diuretics?. J. Am. Coll. Cardiol. 58, 383–385. https://doi.org/10.1016/j.jacc.2011.01.054 (2011).
Ouchi, N., Parker, J. L., Lugus, J. J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97. https://doi.org/10.1038/nri2921 (2011).
Prasad, R., Jha, R. K. & Keerti, A. Chronic Kidney Disease: Its Relationship With Obesity. Cureus https://doi.org/10.7759/cureus.30535 (2022).
Ruifeng, Y., Wen, W., Peng, Z. & Baicheng, L. Research Progress on Obesity-Associated Kidney Diseases. Acta Acad. Med. Sinicae 47, 77–85 (2025).
Chai, T. et al. A narrative review of research advances in hypoxic pulmonary hypertension. Ann. Transl. Med. 10, 230. https://doi.org/10.21037/atm-22-259 (2022).
Yu, B. et al. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacol. Ther. https://doi.org/10.1016/j.pharmthera.2022.108186 (2022).
Michaud, A., Drolet, R., Noël, S., Paris, G. & Tchernof, A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism 61, 689–698. https://doi.org/10.1016/j.metabol.2011.10.004 (2012).
Permana, P. A., Menge, C. & Reaven, P. D. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem. Biophys. Res. Commun. 341, 507–514. https://doi.org/10.1016/j.bbrc.2006.01.012 (2006).
Gao, D., Madi, M., Ding, C., Fok, M. & Bing, C. Interleukin-1β mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am. J. Physiol. Endocrinol. Metab. 307, E289 (2014).
Ashitani, J., Mukae, H., Arimura, Y. & Matsukura, S. Elevated plasma procoagulant and fibrinolytic markers in patients with chronic obstructive pulmonary disease. Intern Med. 41, 181–185. https://doi.org/10.2169/internalmedicine.41.181 (2002).
Owusu-Bediako, K. et al. Association of Severe Obesity and Chronic Obstructive Pulmonary Disease With Pneumonia Following Non-Cardiac Surgery. J. Clin. Med. Res. 14, 237–243. https://doi.org/10.14740/jocmr4741 (2022).
Salvino, N. et al. Is the obesity paradox in outpatients with heart failure reduced ejection fraction real?. Front Cardiovasc. Med. 10, 1239722. https://doi.org/10.3389/fcvm.2023.1239722 (2023).
Hebert, K. et al. Peripheral vascular disease and erectile dysfunction as predictors of mortality in heart failure patients. J. Sex Med. 6, 1999–2007. https://doi.org/10.1111/j.1743-6109.2009.01278.x (2009).
Kim, V. et al. Risk Factors for Venous Thromboembolism in Chronic Obstructive Pulmonary Disease. Chronic. Obstr. Pulm. Dis. 1, 239–249 (2014).
Lempesis, I. G. et al. Obesity and Peripheral Artery Disease: Current Evidence and Controversies. Curr. Obes. Rep. 12, 264–279. https://doi.org/10.1007/s13679-023-00510-7 (2023).
Qiu, X., Lei, Y. P. & Zhou, R. X. SIRS, SOFA, qSOFA, and NEWS in the diagnosis of sepsis and prediction of adverse outcomes: a systematic review and meta-analysis. Expert Rev. Anti. Infect. Ther. 21, 891–900. https://doi.org/10.1080/14787210.2023.2237192 (2023).
Rostami, E. et al. Prognosis in moderate-severe traumatic brain injury in a Swedish cohort and external validation of the IMPACT models. Acta Neurochir. (Wien) 164, 615–624. https://doi.org/10.1007/s00701-021-05040-6 (2022).
Simati, S., Kokkinos, A., Dalamaga, M. & Argyrakopoulou, G. Obesity Paradox: Fact or Fiction?. Curr. Obes. Rep. 12, 75–85. https://doi.org/10.1007/s13679-023-00497-1 (2023).
Spelta, F., Fratta, P. A., Cazzoletti, L. & Ferrari, M. Body weight and mortality in COPD: focus on the obesity paradox. Eat. Weight. Disord. 23, 15–22. https://doi.org/10.1007/s40519-017-0456-z (2018).
Acknowledgements
This study was generously supported by Jingding Medical Tech, to whom we extend our sincere gratitude. We especially thank them for providing authorization and technical support for the JD_PCPM software. The team at Jingding Medical Tech offered invaluable assistance in data processing.
Author information
Authors and Affiliations
Contributions
Jihong Zhou contributed to the study design; Jingwen Zhu and Yifan Wang contributed to the writing of the manuscript; Shaoqiang Wang and Jingwen Zhu contributed to the data visualization and data collection.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, J., Wang, Y., Wang, S. et al. Development and validation of a BMI stratified mortality prediction model for patients with COPD complicated by HF using the MIMIC-IV database. Sci Rep 15, 23765 (2025). https://doi.org/10.1038/s41598-025-09605-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-09605-8