Abstract
The objective of this study is twofold: (i) to evaluate the bony changes of an early medieval skeleton (UF2) from Barcelona, which presents lesions suggestive of Still’s disease and (ii) to contribute to the debate concerning the existence of autoimmune joint diseases in Europe prior to Columbus’s voyages. Macroscopic examination reveals a male between 45 and 50 years of age. Palaeopathological and radiological evaluation reveals the left carpal and carpometacarpal ankylosis, affecting the proximal epiphysis of the second to fifth left metacarpals, and the partial fusion of the C2 and C3 vertebral arches. Differential diagnosis of these lesions, their macroscopic and radiological appearance and the presence of C2-C3 fusion without odontoid erosions, as well as the asymmetry in the joint involvement, indicated Still’s disease. This case provides a new evidence for autoimmune joint diseases in Europe prior to Columbus’s voyages. The apparent increase in these diseases in Europe after the 17th century, could be attributed to the significant changes in lifestyle and diet that occurred in the early modern period. Although individual predisposition is crucial, other factors such as nutrition and lifestyle, which can both play an environmental role as triggers or therapeutic elements for these diseases, are also relevant.
Similar content being viewed by others
Introduction
Still’s disease is a rheumatic condition of unknown aetiology. It is a rare type of arthritis, well known as a multi-systemic autoinflammatory disorder with autoimmune elements1. This disease was described for the first time in 1896 by George Still based on 22 children2. Later, in 1971, Bywaters3 reported on 14 adult patients affected by a disease closely resembling Still’s disease in children. Bywaters named it “Adult Onset Still Disease”3.
Currently, Still’s disease is included in the umbrella term of juvenile chronic arthritis (JCA), or, what is the same, juvenile rheumatoid arthritis or juvenile idiopathic arthritis, which encompasses a spectrum of seven heterogeneous subtypes with distinct clinical features, including: (i) juvenile-onset adult rheumatoid arthritis (RA), which is seropositive; (ii) seronegative chronic arthritis (Still’s disease), which exhibits three variants of presentation (the classic systemic disease, and the polyarticular or oligo-monoarticular forms); and other seronegative arthropathies including (iii) ankylosing spondylitis, (iv) psoriatic arthritis and (v) arthritis associated with inflammatory intestinal disease4.
Clinically, Still’s disease is characterized by a high spiking fever, an evanescent skin rash, polyarthralgia, sore throat, leucocytosis, and hyperferritinemia5,6,7. The onset of symptoms is acute. Patients typically present two daily peaks of fever (> 39 °C), predominantly in the evening. All patients present arthralgias, which tend to be exacerbated during the febrile peak. Arthritis affects 94% of cases8 and the number of joints involved is usually small. Two-thirds of the cases have the cervical spine affected9,10. Intervertebral diarthrodial joints and vertebral bodies can become fused. Occasionally, the atlantoaxial joint can become subluxed and/or fused. While wrists, ankles, carpal and tarsal joints are often affected, the other joints of the hands and feet are frequently unaffected. In the cases that involve the interphalangeal joints, in 15% of them the distal interphalangeal joints are implicated9,10. Ankylosis happens more frequently in carpal and tarsal joints than in large joints10. Radiological characteristics include periarticular osteopenia, periostitis of hand and foot tubular bones, early fusion of the growth cartilage, epiphyseal overgrowth and ankylosis11.
The two morphological features that help to distinguish between Still’s disease (juvenile chronic arthritis and adult-onset Still’s disease) from rheumatoid arthritis are asymmetry and spine fusion (particularly in the cervical area) in the former, while erosion is the norm in rheumatoid arthritis4,11. These differences are very useful in both palaeopathological and clinical cases. This is because in erosive arthropathies overlap exist in diagnostic criteria10. This overlap and the lack of carefully established clinical and palaeopathological diagnostic criteria were the cause of the interpretation problem in earlier reports on the possible evidence for Still’s disease and rheumatoid arthritis in archaeological human skeletal remains10,11. Later, Rogers and collaborators12,13 provided some useful criteria, remarking on the need for caution in the diagnosis of any of the erosive arthropathies.
The first two palaeopathological cases of possible Still’s disease were described in the late of 1970 and early 1980 and were originally from North America. The first corresponds to a possible case of polyarticular erosive arthropathy occurring in the skeletal remains of a female aged between 30 and 35 years, coming from the pre-Columbian period on Kodiak Island, Alaska, displaying destruction of joint surfaces, periarticular cystic erosion, porosity and hypertrophic bone formation in multiple joints, particularly the knee, ankle, elbow, and hands and feet14. It was supposed to be late changes of JCA with onset during childhood10.
The second case came from the Pueblo Indian site at Puye, New Mexico, USA, corresponding to an adult female dated between AD 1559 and 167215. This individual displayed severe erosive joint changes in almost all joints. All the bones of the upper and lower limbs were ankylosed, including both hips. The spine was also partially ankylosed. Considerably remodelling was displayed for those joints that had not undergone ankyloses. The first diagnosis was ankylosing spondylitis15. However, according to Ortner10 either juvenile-onset adult-type rheumatoid arthritis or juvenile seronegative polyarticular chronic arthritis can be diagnosed.
At present, understanding of the patho-physiological basis of Still’s disease remains limited, as it is a disorder of unknown aetiology, scarcely studied compared with other rheumatic diseases6. However, there is evidence that different mechanisms can contribute to its pathogenesis, such as (i) environmental triggers, (ii) genetic susceptibility and (iii) activation of inflammation and its deficient resolution. The environmental triggers and the autoimmune genes act together at the start of the autoimmune response16,17. A significant trigger for the disease is exposure of the immune system to certain pathogens, which can be viral (rubella, echovirus 7, mumps, Epstein-Barr, cytomegalovirus, parainfluenza, parvovirus B19, coxsackie, adeno, influenza, herpes, and hepatitis B and C viruses) or bacterial (Yersinia enterocolitica and Mycoplasma pneumoniae)19– 20. In addition, it seems that stressful life events that occurred during the year preceding the onset of the disease can also be related to its development21. Genetic susceptibility is due to the human leucocyte antigens genetic linkage factors (HLA DRB1*1201 and 1501, B35, DR2 DR5), which are located in the major histocompatibility complex region on chromosome 68.
The history and antiquity of autoimmune joint diseases remain controversial. The discussion mainly focuses on when and where autoimmune joint diseases, particularly rheumatoid arthritis and juvenile idiopathic arthritis (Still’s disease) first appeared1. In their clinical textbook, Boyle and Buchanan22 suggested that they could be recent human diseases. In fact, rheumatoid arthritis was first referred to in the 17th century and was first clearly described by Landre-Beauvais (1772–1840) in his thesis1. In addition, as was pointed out before, Still’s disease in children was described at the end of the 19th century and Adult Onset Still’s disease in the second half of the 20th century. There is no unequivocal description of this type of diseases in ancient Greek and Roman medical writings23. Rothschild and collaborators24,25 based on the apparent non-existence of rheumatoid arthritis in ancient Egypt and Europe before the Age of Explorations and high prevalence of polyarticular erosive arthritis, consistent with rheumatoid arthritis among Archaic Amerindians, postulated that rheumatoid arthritis originated in the pre-Columbian New World and then spread to the Old World and subsequently throughout the rest of the world1. It is true that in the Old World in the 17th century there was an apparent increase in the incidence of autoimmune joint diseases. However, if they originated in the Americas and spread to the world at large after the return of Christopher Columbus, these diseases would have been acting as infectious diseases, which is not the case. According to Buchanan et al.1insufficient data have been obtained through research on documentary sources, visual art, historical figures, or ethnographic and palaeopathological evidence to clarify the origin of these diseases. Most of the archaeological cases described during 19th and 20th centuries in skeletal remains from Europe and ancient Egypt remain controversial since differential diagnosis of rheumatoid arthritis and Still’s disease can be difficult on dry bone26. However, despite of this difficulty, recently several convincing palaeopathological studies have been published17,18,26,27,28 highlighting the importance of palaeopathological research in conjunction with demographic, documentary and pictorial studies to furnish information on the antiquity and origin of bony inflammatory and progressive autoimmune diseases26,27. Therefore, the objective of this study is twofold: (i) to assess the bony changes observed in the UF (Funerary Unit) 2 medieval individual from the early medieval cemetery at the archaeological site of the Antics Jutjats Municipals de Barcelona (Spain), which presents lesions suggestive of Still’s disease and (ii) to contribute to the debate concerning the existence of autoimmune joint disease in Europe prior Columbus’s voyages.
Results
Skeleton UF2 was well preserved (Skeletal preservation index IP3 = 100%. For its description, see the Methods section). The complete set of remains of this individual can be seen in Fig. 1. The bone surfaces showed very little post-depositional erosion. All the epiphyses were fused, indicating that this was an adult individual. The morphology of the cranium and innominate bones, the robustness of the skeleton and discriminant function analysis applied to measurements of the long bones indicated that the individual was male. The morphology of the auricular surface, pubic symphysis, acetabulum and dental wear suggested that the individual was between 45 and 50 years of age (For references on these methods, see the Methods section).
Pathological lesions
The macroscopic and radiological examination of the individual UF2 showed oral, traumatic and arthritic pathologies. The oral pathologies observed were (i) a large accumulation of tartar on preserved teeth, which were the following: 13, 22, 23, 27, 28, 32, 33 34, 35, 37, 38, 42, 43, 44, 45 and 46. Extensive tooth wear in the occlusal plane of the preserved teeth. Dental pulp caries largely affecting the following teeth: 27, 28, 36, 37, 38 and 46. Periapical fistulas, affecting the alveoli of the following teeth 18, 17, 16, 11, 21, 22, 26, 27, 28 and 47 (Figs. 2 and 3).
In relation to the traumatic lesions (ii), two lesions were observed on the skull (Fig. 2), corresponding to a sharp force trauma on the left parietal bone, oriented longitudinally, already remodelled, and a penetrating stab wound lesion on the left temple, which broke the zygomatic arc. This last lesion does not reveal bone remodelling (Fig. 2). According to the radiological study, the sharp force trauma exhibits reactive, consolidating sclerosis, suggesting an in vivo fracture (Fig. 2). The penetrating stab wound lesion to the left temple shows non-reactive tissue, indicating perimortem trauma (Fig. 2).
Left lateral view of the UF 2 Skull (a). Lateral (b) and anterior-posterior (c) X-ray of the UF 2 skull. A remodelled sharp force trauma, with oblique linear disposition in the left parietal bone (black arrow) and a penetrating stab wound lesion in the left temple (red arrow) with radiating fracture lines (green arrows) are clearly depicted.
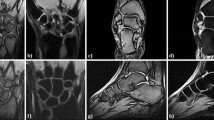
In relation to the arthritic pathologies (iii), the macroscopic and radiologic analysis showed lesions in C2-C3 zygapophyseal joints and left carpal and carpometacarpal bones. In particular, both zygapophyseal joints of the C2 and C3 vertebrae were fused completely (joint spaces not visible) and bone contours remodelled (Fig. 4). The left hand presented carpal and carpometacarpal ankylosis with the proximal epiphysis of the second to fifth metacarpals (MTC) (Figs. 5 and 6). On this left hand, there is a possible subchondral bone erosion with subsequent remodelling at the head of the third MTC and more doubtful at the fourth and fifth MTCs. There were no erosions in other areas of the hand or the skeleton. There were no reactive bone formation and hypervascularity. It is noteworthy the absence of erosions in the contralateral carpus-hand.
During the macroscopic and radiological examination of the UF2 individual, phalangeal bifidity was also observed in the distal phalanges of the right 3rd and 4th fingers (Fig. 6). However, because it is medically considered an anatomical variation29as it does not diminish the functionality of the fingers, we discarded it as pathology.
Discussion
The individual analysed in this study is a male aged between 40 and 50 years. The oral pathologies observed are probably related to deficient oral hygiene and a possible diet rich in low-quality carbohydrates. The sharp force trauma in the parietal bone and the penetrating stab wound lesion in the temple are related to some type of violent interpersonal interaction. The individual analysed survived the lesion in the parietal bone visible by its remodelling and healing; however, the penetrating stab wound lesion in the temple is a perimortem lesion, and it was probably the cause of the individual’s death.
These described lesions are independent of the carpal and metacarpal ankylosis. Therefore, the differential diagnosis should focus on the complete ankylosis of the left carpal, affecting the proximal epiphyses of the second to fifth metacarpals, and the complete fusion of both zygapophyseal joints of the C2 and C3 vertebrae. Certainly, infectious diseases and trauma may result in ankylosis. However, (i) the absence of inflammatory processes, (ii) the no evidences of carpal or metacarpal fractures, (iii) the lack of any bone destruction and (iv) the non-appearance of large bony proliferations (sclerosis and periostitis) allow us to discard traumatic or infectious origin for these lesions.
In this individual the latter three pathological entities (ankylosing spondylitis, psoriatic arthritis and arthritis associated with inflammatory intestinal disease) are very unlikely, as there is no evidence to suggest sacroiliac involvement, prominent syndesmophytes or dactylitis.
Accordingly, the differential diagnosis should basically focus on rheumatoid arthritis (RA) versus Still’s disease in some of its variants. Therefore, adult-onset Still’s disease, juvenile chronic arthritis and rheumatoid arthritis were considered for the differential diagnosis.
In Adult-onset Still’s disease, as previously mentioned, the number of joints involved is usually small, most frequently affecting knees, fingers and wrists. Shoulders and ankles are less affected. The carpometacarpal joints (CMC) may be selectively affected as in the juvenile disease. The third (31.3%) and second (27.5%) carpometacarpal articulations are much more frequently affected than the others (fourth CMC: 5.4%; fifth CMC: 4.6%; first CMC: 0.4%). In addition, erosions are not common. Involvement of the carpometacarpal and intercarpal joints, with the relative exception of the radiocarpal joint, is characteristic and usually monoarticular8. Ankylosis of the zygapophyseal joints of C2-C3 in 60% of cases is described4,30. Erosions and sclerosis of the sacroiliac joints8,31 or periarticular calcifications30 have been described in some series. The prevalence of this disease in the Caucasian population is 1:100,000 adults. It most frequently affects young adults between 16 and 35 years old, although cases may occur in people over 70 years of age. In the population of Japan the prevalence is higher: 1.47:100,000 adults. In Europeans, it is more prevalent in males, in contrast to the Japanese, among whom it affects more females32.
Although not pathognomonic, in adult Still’s disease there is a predilection for the carpometacarpal and intercarpal joints and, in a patient from whom laboratory data cannot be obtained, the finding of carpal ankylosis can be an invaluable clue for diagnosis and ultimately for pathogenesis and aetiology33. The case under analysis is an adult male individual with a left carpometacarpal ankylosis and the complete fusion of both zygapophyseal joints of C2-C3 vertebrae, so adult-onset Still’s disease cannot be ruled out.
Juvenile chronic arthritis (JCA) begins in individuals under the age of 16 years. Like Still’s disease, juvenile chronic arthritis primarily affects the synovial joints of the appendicular skeleton4,34and it is predominantly monoarticular. As far as frequency is concerned, from the most to the least often recorded, these lesions appear in the joints of the hands and feet (particularly the carpus), and then in the knees, elbows, hips, and temporomandibular joint. Ankylosis is common in the carpal and tarsal areas, rather than in the long bones. Involvement of C2-C3 zygapophyseal joints is described4,30. In 50% of cases, it affects the temporomandibular joint and can often lead to micrognathia35. A significant coexistence between carpometacarpal and cervical zygapophyseal ankylosis can be observed36. Therefore, the left carpometacarpal ankylosis and the complete fusion of both zygapophyseal joints of C2 and C3 vertebrae in this case are consistent with juvenile chronic arthritis.
Rheumatoid arthritis (RA) usually begins between 25 and 40 years of age, although it can develop prematurely in female individuals (4 women for every man affected). It is polyarticular and usually bilaterally symmetrical. There is bone erosion at the ends of the bone that generates cysts in the subchondral bone, bone destruction and ankylosis, producing irregularities and deformations on the bone surface10.
The first lesions usually affect small joints such as the interphalangeal or metacarpophalangeal joints of the hands and feet. Subsequently, in order of incidence, the temporomandibular joints, knees, elbows, hips, and vertebrae are affected, particularly the cervical vertebrae, where the odontoid process is frequently affected by erosions (1/3 of cases)11,35. In the hands, tendon dislocation and deformities occur due to tendon ruptures, with the appearance of what is known swan neck finger (or buttonhole or hammer finger) and in cases where the ulnar nerve is injured, due to its entrapment in the groove constituted by the olecranon and the posterior area of the medial epicondyle, ulnar claw appears35. In the case analysed, the absence of bilateral lesions, in particular ankylosis only being present in the left hand, as well as the lack of involvement of the temporomandibular joint, the absence of erosive lesions and the fact that it was a male, allow us to rule out rheumatoid arthritis.
Therefore, the arthritic bone lesions outlined in this skeleton seem to represent juvenile chronic arthritis and adult-onset Still’s disease. In fact, both diseases are very similar, and currently both are considered to be the same disease, Still’s disease4. In addition, differentiating between them is difficult in palaeopathology10. Therefore, it can be concluded that the most probable diagnosis for the arthritic pathology manifested by UF 2 was adult-onset Still’s disease or juvenile chronic arthritis, rather than rheumatoid arthritis.
The autoimmune joint diseases, prior to the 17th century, Still’s disease and rheumatoid arthritis, were unknown. If they existed in Europe before Columbus’s voyages, even though they were not manifested in a mild form, they would probably have been misdiagnosed as gout, an error that still occasionally occurs even today1,37. In the Middle Ages and previously, due to the lack of medical knowledge and good clinical definitions of certain diseases, they were confused with each other, as was the case of syphilis and leprosy38. This could be one of the reasons why Still’s disease and rheumatoid arthritis are not described in any ancient medical text.
The scarcity of palaeopathological cases of Still’s disease and rheumatoid arthritis in juvenile or adult skeletons from archaeological sites could be due to the low clinical prevalence of these diseases. The current clinical incidence for Still’s disease [0.16–0.4 per 100,000]39 and for rheumatoid arthritis [0.5–1%]40 is relatively low. If these diseases had a similar incidence in pre-antibiotic periods to today, these low values would make the probability of finding any palaeopathological case in any archaeological sample decrease41. In addition, in pre-antibiotic populations the incidence of these pathologies was probably lower than today, because of the high child mortality (50% of newborns died at birth or during the first month of life) and the low life expectancy (30–35 years of age) of the population as a whole, particularly in females due to the high death rate arising from childbirth42. This would decrease the probability of suffering an autoimmune joint disease in past populations. This low frequency of cases would be reflected in the scarcity of palaeopathological cases identified.
The few existing European and Egyptian palaeopathological cases of Still’s disease or/and rheumatoid arthritis published before the 1980s were pointed out by several authors as misdiagnoses24,25. This, together with the scarcity of Old World archaeological cases in comparison with those found in the New World increased the idea that Still’s disease and rheumatoid arthritis originated in the Americas41. However, in recent years, more convincing cases from the Old World have been published, thereby challenging the New World origin hypothesis. Most of these convincing new archaeological cases of Still’s disease and rheumatoid arthritis from the Old World date back to the medieval and post-medieval periods, prior to the first clinical description of rheumatoid arthritis in the 17th century43. The results of the present study add new evidence to the corpus of autoimmune joint diseases from Europe before the colonization of the Americas, particularly Still’s disease.
In relation to Still’s disease, a part from the present case, these convincing new cases from Europe include (i) a prehistoric adult individual from Menorca, Spain35; (ii) a late Roman female (3rd to 5th centuries CE) in young adulthood from Tarragona, Spain44; (iii) the late medieval (1490–1660 CE) male aged between 45 and 50 years from Croatia17; (iv) a young Renaissance-period individual from Italy28; and (v) a post-medieval female juvenile individual (17th to 18th centuries) from Spain45.
As regards rheumatoid arthritis, the oldest of these convincing Old World cases is from (i) ancient Egypt, dated between 1750 and 1550 BCE26followed by (ii) a late Roman (3rd to 4th century CE) mature female from Amiens, France27; (iii) a young adult female (4th century CE) and a mature male (10th to 11th centuries), both also from France46; (iv) a mature male (1278 to 1538) from England47; and (v) an adult female datable to the latter half of the 15th century also from England48. To this, we should also add some cases (vi) from prehistoric to early modern Asia49,50. All of these examples indicate that autoimmune joint diseases, particularly Still’s disease and rheumatoid arthritis, had existed since prehistory in Europe prior to Columbus’s voyages and the Old World in general prior to its first clinical description in the 17th century43.
The apparent increase in the incidence of Still’s disease and rheumatoid arthritis in Europe after the 17th century could be attributed to the existence of a mild version of these inflammatory diseases, which might have been exacerbated at the beginning of the early modern era, marked by the discovery of the Americas in 1492. This new historical period brought about significant social, economic, political, cultural and scientific changes in the Old World. In Europe, this period started in 1500, following the Middle Ages (post-classical era) and has lasted to the present day. The initial stages of this new period were marked by many significant events, such as the fall of Constantinople (1453), Gutenberg’s moveable type printing press (1450s) and Christopher Columbus’s voyage to the Americas (1492), among others. This historical period implied a substantial change in lifestyle and diet in Europe. Paracelsus (1493–1541) started to use chemical and mineral remedies to treat some illnesses. Fracastoro (1478–1553) suggested that epidemics may have been caused by pathogens outside the body; however, the identification of bacteria and the development of vaccines did not occur until the 18th century1. The discovery of the Americas led to an interchange of a wide variety of crops and livestock, which brought about increases in both food production and the population in Europe. The new products and food that were introduced included tobacco, cocoa beans, potatoes, tomatoes, haricot, peanuts, maize, beans, pumpkin, pineapple and the turkey, among others1,51. These new products and foods could have exposed the European population to certain health risk factors and new food intolerances and allergens. In fact, nutrition plays a key role in determining the severity of autoimmune joint diseases1. Nutritional factors seem to explain the low incidence of these diseases among the Inuit in Alaska52 and certain Chinese populations53. Total fasting results in the reduction of joint inflammation54and the low incidence of rheumatoid arthritis in rural Africans, compared with urban Africans, may arise from a low protein intake among the former55. Consumption of dairy foods and a high intake of carbohydrates56 have pro-inflammatory effects and can be associated with an increase in rheumatoid arthritis. In contrast, the ingestion of red meat, poultry and fish seems not to be related to the risk of rheumatoid arthritis57.
Recent literature demonstrates that diet is of great importance in the therapy of rheumatoid arthritis, through the management of inflammation, immunity and oxidative stress58. The substantial change in diet, that the modern era supposed may have triggered the severity of autoimmune joint diseases in Europe. Populational predisposition is relevant; however, in genetically susceptible individuals nutritional factors can have the function of environmental triggers, generating and directing the systemic immune-inflammatory response that leads to the development of the autoimmune joint disease59. Actually, food, acting as an environmental factor, can be both a disease trigger or a ‘moderator’. There are foods that either exacerbate (e.g. dairy foods, salt, excessive food intake) or reduce inflammation (e.g. fatty fish, fruit and vegetables)60. Indeed, in the Western world in contraposition to the Eastern world and developing countries, there is a trend towards a higher prevalence of autoimmune diseases, including rheumatoid arthritis. This entails different environmental triggers including the hygiene hypothesis, which indicates that high standards of hygiene can limit the exposure of the immune system to beneficial pathogens, thus increasing susceptibility to autoimmune conditions, psychosocial stress, smoking, increased alcohol consumption, dietary habits and obesity61. In particular, a ‘Western’ diet, characterized by a high intake of saturated and trans fats, a low ratio of omega-3/omega-6 fatty acids and an excessive intake of refined carbohydrates and sugar-sweetened drinks, increases the risk of autoimmune joint diseases by both directly increasing inflammation61 and indirectly through increasing insulin resistance, obesity and associated co-morbidities62.
Why Still’s disease and rheumatoid arthritis were only first convincingly described after the 17th century remains a mystery. It may have been a consequence of the low level of medical development, the new lifestyle in the early modern era and the introduction of new types of products and foods from the Americas. However, what seems to have been true is that autoimmune joint diseases existed in Europe and Egypt prior to the discovery of the Americas.
Conclusions
The macroscopic and radiological evaluation of the early medieval (9th to 10th centuries) adult male (UF 2) individual from Barcelona reveals left carpal and carpometacarpal ankylosis, affecting the proximal epiphyses of the second to fifth left metacarpal, and the complete fusion of both zygapophyseal joints of C2 and C3 vertebrae, without odontoid erosions. Their differential diagnosis and macroscopic and radiological appearance, with asymmetry in the joint involvement, indicated Still’s disease.
The results of the present study contribute new evidence to the corpus of autoimmune joint diseases from Europe before the time of Columbus, particularly Still’s disease. According to recent evidence provided by different researchers, it is clear that, since prehistory, autoimmune joint diseases, particularly Still’s disease and rheumatoid arthritis, had existed in Europe before the time of Columbus prior to its first clinical description in the 17th century. The apparent increase in their incidence in Europe after the 17th century could be attributed to the existence of a possible mild version of these inflammatory diseases, which might have been exacerbated at the start of the modern era because of the significant lifestyle and dietary changes that it implicated. Although population predisposition is relevant, nutrition plays an important role in determining the severity of autoimmune joint diseases.
This study also furnishes information on the state of health of this early medieval male individual, who suffered from carpometacarpal ankyloses and complete fusion of both C2-C3 zygapophyseal joints. In addition, his deficient hygiene and dental care; his nutrition, which seems to have been largely based on low quality carbohydrates; and the presence of blade injuries –one in the temple without bone remodelling, indicating a probable cause of death–; suggest the possibility that this individual had experienced harsh living conditions and limited access to medical care. Particularly noteworthy are the injuries caused by a bladed weapon, which are probably related to violent interpersonal interactions, reinforcing the idea of abuse, maltreatment or punishment in the case of the individuals buried in the Antics Jutjats Municipals de Barcelona cemetery.
Materials and methods
The material of this study corresponds to an early medieval individual (UF2) buried in the medieval cemetery at the Antics Jutjats Municipals archaeological site in Barcelona63. In this burial area, the individual (Fig. 1) was found in sector 3 and in stratigraphic unit 93 (UE93) of the site. It was buried in a supine (face up) position, without grave goods, in an anthropomorphic tomb covered by six stone slabs, arranged transversely across the tomb.
The former municipal courts of Barcelona site
The Antics Jutjats Municipals de Barcelona is an archaeological site excavated by ABANS Serveis Culturals in 2018 and 2019 as a rescue excavation63. It is located in the city of Barcelona (Spain) in the Ciutat Vella district (Fig. 7), occupying a surface area of approximately 5,300m2between the streets known as Carrer del Comerç, Passeig Pujades and Passeig Lluís Companys. It consists of an early medieval burial area (radiocarbon dated between the 9th and 10th centuries), which lay outside the city of Barcino (the Roman name of modern-day Barcelona), but between the Roman and medieval walls of the city, closer to the medieval defences63. However, these medieval fortifications were built in the 13th century, which means that, at its time of use, this cemetery was located outside the city, specifically in the suburbium (peripheral area) close to the territorium (countryside around the city). The burials (44 adults and 71 immatures) found at the Antics Jutjats Municipals site followed the Christian ritual, characterized by their simplicity and absence of grave goods63. The range of burial types was diverse: earth-cut anthropomorphic tombs covered with flat stone slabs; graves lined with head, side and foot stones or tegulae (re-used Roman flat roof tiles); anthropomorphic tombs with stone and/or tegula covers; cist burials and, finally, simple earth-cut graves63. The cemetery displayed spatial organization, following axes that may have structured the burial area, which, however, could not be traced. The tombs of adult individuals defined the basic layout of the burial space while the tombs of immature individuals filled the free spaces that must have been left between the tombs of adult individuals63. There was no case of a grave cut by another, nor any case of reuse. No specific burial areas related to age, sex, diseases and tomb typology were observed63.
Map showing the location of Spain in Europe (a); Barcelona in Spain (b); and the map of Barcelona (c), showing the Roman (green), medieval and early modern walls (black), with the bastions; in red excavations with burials corresponding to the 9th and 10th centuries, the southernmost corresponds to the Antics Jutjats site.
To date, the Antics Jutjats site is unique in Barcelona. Apart from its organization and the number of burials and type of funeral ritual, it represents an ample funerary space of great importance in the area known as the eastern suburbium64which appears not to have been linked to any religious centre. This last characteristic differentiates this cemetery from other funerary areas in this period65. From the 6th century onwards, the Christian funerary world was predominantly organized around urban religious buildings, basilicas or monasteries64. On the contrary, this seems to have been a planned burial area, at some considerable distance from the late Roman city wall.
The individuals analysed from the Antics Jutjats site (n ₌ 115), adults (n ₌ 44) exhibited a low prevalence of dental hypoplasia (29.4%, n = 13/44) and a mean stature (153.6 cm for females and 166.6 cm for males) similar to their contemporaries63. They had long bone diaphyses with a relatively circular shape and a low prevalence of physical activity markers, indicating they did not undertake a large amount of physical exertion63. In fact, at the time when the cemetery existed, an increase in the population of the city was recorded66. This phenomenon might denote good access to certain foods, which could be reflected in the sample by a low representation of dental hypoplasia, which is below the values found in other contemporary populations, as well as individual statures64. In 44 adult individuals, 22 of them have preserved orbits, only two cases of cribra orbitalia were found (2/22). In contrast, the immature individuals (n ₌ 71) buried in this cemetery clearly faced some difficulties as they grew, similar to those recorded among pre-industrial individuals from Coimbra and Lisbon63. In addition, these 71 immature individuals (33 of them have preserved orbits, 68 have preserved humeri and 69 have preserved femurs) manifested certain types of cribra (20/71), such as cribra orbitalia (8 individuals, 8/33), cribra femoralis (8 individuals, 8/68) and cribra humeralis (1 individual, 1/69) or a combination of cribra orbitalia and femoralis (2 individuals, 2/71) and cribra femoralis and humeralis (1 individual, 1/71)63. Nevertheless, in both adults and immature individuals from this site, subperiosteal new bone formation was also observed (in 10 adults and 16 immatures)63. This new bone formation can be related to many different types of diseases, indicating biological stress, which, if the previous observations on the adults are taken into account, indicate that this biological stress could have occurred among this population relatively recently63. In the Antics Jutjats population, what stands out is the large number of healed and non-healed traumatic lesions observed, particularly in the adult population. More precisely in 28 adult individuals, although there were also three immature individuals with traumatic injuries. It should be noted that, of the 31 individuals (28 adults plus 3 immatures) with trauma, 18 of them have multiple traumas (all of them adults). As a result, 27% (n = 31/115) of the total population have some type of trauma and 16% (n = 18/115) have multiple traumas. It is relevant that of the 31 individuals with trauma, 19 had cranial perimortem trauma of penetrating stab wounds, including one of the three non-adult individuals63.
The high frequency and wide variety of types of fracture, together with the subperiosteal new bone formation, lead us to consider the likelihood, that the period in which this population lived was in some way difficult63. The fact that the Antics Jutjats cemetery was located at some considerable distance from the city clearly differentiates this population from the rest of the inhabitants of Barcelona. Most of the adults from Antics Jutjats appear to have had a good quality of life when young; however, for some reason, at the moment of their death, both they and their children faced far more difficult conditions63. The regular organization of the cemetery and the range of radiocarbon dates indicate that this was not a mass burial of the victims of a single Islamic or Frankish attack, of which there were a considerable number from between 801 and 98564. In these two centuries, Barcelona experienced a period of particular political instability, the city being captured several times.
Methods
The anthropological and palaeopathological study of this material was carried out at the Human Bone Laboratory of the Barcelona City History Museum (Museu d’Història de la Ciutat de Barcelona) in the Zona Franca of Barcelona (Spain) in where the remains are curated, according to DECRET 78/2002 Law of Heritage. The skeletal material arrived at the laboratory in a box. First, the bones were dry-cleaned with soft brushes and wooden chopsticks. Secondly, skeletal preservation (IP3) was estimated by applying the methodology of Alesan et al.67. The IP₃ shows the percentage of preserved elements [IP3= (Number bones present /22) x 100]. The number of bones considered is 22 in total: the long bones plus both girdles together with the cranium and mandible. The presence of a part of a bone is considered as a whole bone. Therefore, it does not inform us about the physical state of the bone, but it serves to calculate the number of bones preserved. The sex of the skeleton was estimated using the morphology of the cranium and innominate bones, the robustness of the skeleton and discriminant function analysis applied to measurements of the long and innominate bones68,69. Age at death was estimated from the morphology of the auricular surface, pubic symphysis, acetabulum and dental wear69,70.
The distribution of any abnormal bone formation and destruction was recorded macroscopically and by plain film radiography. This last analysis was carried out in the Radiology Department of the Hospital Clínic in Barcelona. The X-rays were taken in two orthogonal projections, anterior-posterior and mediolateral, using digital X-ray system YSIO X.pree, Siemens (Germany) equipment, at 50 kilovolts (kV) and 15 milliampere/second (mAs) settings, with a focus-plate distance of 120 cm.
Data availability
No datasets were generated or analysed during the current study.
References
Buchanan, W. W., Kean, C. A., Kean, W. F. & Raindsford, K. D. Rheumatoid Arthritis Inflammopharmacology, 32, 3–11 (2024).
Still, G. F. On a form of chronic joint disease in children. Med. Chir. Trans. 80, 47–60 (1897).
Bywaters, E. G. L. Still’s disease in the adult. Ann. Rheum. Dis. 30, 121–132 (1971).
Resnick, D. & Niwayama, G. Diagnosis of bone and joint disorders. 2nd editionW B Saunders Company, Toronto,. (1988).
Mahroum, N., Mahagna, H. & Amital, H. Diagnosis and classification of adult still’s disease. J. Autoimmun. 48–49, 34–37 (2014).
Giacomelli, R., Ruscitti, P. & Shoenfeld, Y. A comprehensive review on adult onset still’s disease. J. Autoimmun. 93, 24–36 (2018).
Wang, M. Y., Jia, J. C., Yang, C. D. & Hu, Q. Y. Pathogenesis, disease course, and prognosis of adult-onset still’s disease: an update and review. Chin. Med. J. 132, 2856–2864 (2019).
Pouchot, J. et al. Adult still’s disease: manifestations, disease course and outcome in 62 patients. Medicine 70, 118–136 (1991).
Ansell, B. & Bywaters, E. Rheumatoid arthritis (Still’s disease). Pediatr. Clin. North. Am. 10, 921–940 (1963).
Ortner, D. J. Identification of Pathological Conditions in Human Skeletal Remains. Second editionAcademic Press, London,. (2003).
Aufderheide, A. C. & Rodríguez-Martín, C. The Cambridge Encyclopaedia of Human Paleopathology (Cambridge University Press, 1997).
Rogers, J., Waldron, T., Dieppe, P. & Wat, I. Arthropaties in paleopathology: the bases of classification according to the most probable cause. J. Archaeol. Sci. 14, 113–120 (1987).
Rogers, J. & Waldron, T. A Field Guide To Joint Disease in Archaeology (Wiley, 1995).
Ortner, D. J. & Utermohle, C. J. Polyarticular inflammatory arthritis in a pre-Columbian skeleton from Kodiak island, Alaska. USA Am. J. Phys. Anthrop. 56, 23–31 (1981).
Steinbock, R. & Thomas Paleopathological diagnosis and interpretation. (Springfield. IL, (1976).
Davidson, A. & Diamond, B. General features of autoimmune disease. In The Autoimmune Diseases (eds (eds Rose, N. R. & Mackay, I. R.) 25–36 (Elsevier, St. Louis, (2006).
Šikanjić, P. R. & Vlak, D. Autoimmune joint diseases in late medieval sample from Croatia. Rheumatol. Int. 30, 349–356 (2010).
Huang, S. H. & DeCoteau, W. E. Adult-onset still’s disease: an unusual presentation of Rubella infection. Can. Med. Assoc. J. 122, 1275 (1980).
Colebunders, R., Stevens, W. J., Vanagt, E. & Snoeck, J. Adult still’s disease caused by Yersinia enterocolitica infection. Arch. Intern. Med. 144, 1880e2 (1984).
Wouters, J. M., van der Veen, J., van de Putte, L. B. & de Rooij, D. J. Adult onset still’s disease and viral infections. Ann. Rheum. Dis. 47, 764e7 (1988).
Sampalis, J. S. et al. Risk factors for adult still’s disease. J. Rheumatol. 23, 2049e54 (1996).
Boyle, J. A. & Buchanan, W. W. Clinical Rheumatology (Blackwell Scientific, 1971).
Short, C. L. An antiquity of rheumatoid arthritis. Arthr Rheum. 17, 193–205 (1974).
Rothschild, B. M., Turner, K. R. & Deluca, M. A. Symmetrical erosive polyarthritis in the late archaic period of Alabama. Science 24, 1498–1501 (1988).
Woods, R. J. & Rothschild, B. M. Population analysis of symmetrical erosive arthritis in Ohio woodland Indians (1200 years ago). J. Rheumatol. 15, 1258–1263 (1988).
Mant, M., Pitre, M. C., Dancer, S. & Gatto, M. C. A case of rheumatoid arthritis in a Nubian woman from the site of Sheikh mohamed, near aswan, Egypt. Int. J. Paleopathol. 44, 78–84 (2024).
Kacki, S. Erosive polyarthopathy in a late Roman skeleton from Northern france: a new case of rheumatoid arthritis from the pre-Columbian old world?? Int. J. Paleopathol. 3, 59–63 (2013).
Riccomi, J., Minozzi, S., Aringhieri, G. & Giuffra, V. A possible case of juvenile idiopathic arthritis from renaissance Lucca (Tuscany, central Italy). Int. J. Paleopathol. 33, 72–83 (2021).
Keats, T. Atlas of Normal Roentgen Variants that May Simulate Disease (Elsevier, 2012).
Reginato, A. J., Schumacher, H. R., Baker, D. G., JR., O’Connor, C. R. & Ferreiros, J. Adult onset still’s disease: experience in 23 patients and literature review with emphasis on organ failure. Semin Arthritis Rheum. 17, 39–57 (1987).
Olivéa, A., Holgadoa, S. & Valls, M. Enfermedad de still Del Adulto. Rev. Esp. Reumatol. 28, 32–37 (2001).
Wakai, K. et al. Estimated prevalence and incidence of adult still’s disease: findings by a nation-wide epidemiological survey in Japan. J. Epidemiol. 7, 221–225 (1997).
Medsger, T. A. & Christy, W. C. Carpal arthritis with ankylosis in late onset still’s disease. Arth Rheumatol. 19, 232–242 (1976).
Cunha, E. Aproximación paleopatológica a Algunas enfermedades reumáticas. In Paleopatología La Enfermedad No Escrita (eds (eds Isidro, A. & Malgosa, A.) 209–220 (Masson, Barcelona, (2003).
Campillo, D. Introducción a La Paleopatologia (Edicions Bellaterra, SL., 2001).
Maldonado-Cocco, J. A., García-Morteo, O., Spindler, A. J., Hübscher, O. & Gagliardi, S. Carpal ankylosis in juvenile rheumatoid arthritis. Arthr Rheumatol. 23, 1251–1255 (1980).
Talbott, J. H., Altman, R. D. & Yu, T. F. Gouty arthritis masquerading as rheumatoid arthritis and vice versa. Arthr Rheumatol. 8, 77–114 (1977).
Hirsch, A. Handbook of Geographical and Historical Pathology. Translation from German Second Edition by Creighton ChVol. IIP. 44 (The New Sydenham Society, 1985).
Qudsiya, Z. & Baker, L. D. The great Still-Usion: unmasking Adult-Onset still’s disease masquerading as upper respiratory tract infection. Cureus 15(3), e35880 (2023). https://doi.org/10.7759/cureus.35880
Smolen, J. S., Aletaha, D. & McInnes, L. C. Rheumatoid arthritis. Lancet 388 (10055), 2023–2038 (2016).
Rogers, J. & Dieppe Rogers, J. & dieppe, P. Skeletal palaeopathology and rheumatic diseases: where are we now? Ann. Rheum. Dis. 49, 885–886 (1990). (1990).
Hall, L. Polyarthritis in Kenya. East. Aft Med. J. 43, 161–170 (1996).
Yeap, S. S. Rheumatoid arthritis in paintings: a Tale of two origins. Int. J. Rheum. Dis. 12 (4), 343–347 (2009).
Campillo, D. Etude des restes squelettiques d’un individu de l’époque tardi-romaine Attein de polyarthrite rhumatoïde. Anthropol. Et Préhis. 101, 71–83 (1990).
Garralda, M. D. & Herrerin, J. Juvenile rheumatoid arthritis in a woman from the necropolis (XVII-XVIII centuries, AD) of El burgo de Osma Cathedral (Soria, Spain). Int. J. Paleopathol. 15, 133–152 (2003).
Blondiaux, J. et al. Two Roman and medieval cases of symmetrical erosive polyarthropathy from normandy: anatomicopathological and radiological evidence for rheumatoid arthritis. Int. J. Osteoarchaeol. 7, 451–466 (1997).
Mays, S., Watt, I. & Loe, L. An unusual erosive arthropathy from medieval England. Int. J. Osteoarchaeol. 27, 693–699 (2017).
Hacking, P., Allen, T. & Rogers, J. Rheumatoid arthritis in a medieval skeleton. Int. J. Osteoarchaeol. 4, 251–255 (1994).
Inoue, K., Hukuda, S., Nakai, M., Katayama, K. & Huang, J. Erosive peripheral polyarthritis in ancient Japanese skeletons: a possible case of rheumatoid arthritis. Int. J. Osteoarchaeol. 9, 1–7 (1999).
Kim, D. K. et al. Possible rheumatoid arthritis found in the human skeleton collected from the tomb of Joseon dynasty, korea, dating back to the 1700s AD. Int. J. Osteoarchaeol. 21, 136–149 (2011).
Buchanan, W. W. Disease in ancient America. Bull. Roy Coll. Phys. Surg. Glasg. 28, 7–12 (1999).
Beasley, R. P., Retailliau, H. & Healey, L. A. Prevalence of rheumatoid arthritis in Alaskan Eskimos. Arthr Rheumatol. 16, 737–774 (1973).
Chang, N. C. Rheumatic diseases in China. J. Rheumatol. 10, 41–45 (1983).
Hafstrom, I., Ringertz, P., Gyllenhammer, H., Palmblad, J. & Harms-Ringdahl, M. Effects of fasting on disease activity, neutrophil, function, fatty acid composition and leukotriene biosynthesis in patients with rheumatoid arthritis. Arthr Rheumatol. 31, 585–592 (1988).
Beighton, S. W., de la Harpe, A. L., van Staden, D. J., Batenhorst, J. H. & Myers, O. L. The prevalence of rheumatoid arthritis in a rural African population. J. Rheumatol. 15, 405–408 (1988).
Venter, C., Eyerich, S., Sarin, T. & Klatt, K. C. Nutrition and the immune system: a complicated Tango. Nutrients 12, 818 (2020).
Sundstrom, B., Ljung, L. & Di Giuseppe, D. Consumption of meat and dairy products is not associated with the risk for rheumatoid arthritis among women: a population-based cohort study. Nutrients 11, 2825 (2019).
Rondanelli, M. et al. Ideal food pyramid for patients with rheumatoid arthritis: A narrative review. Clin. Nutr. 40, 661–689 (2021).
Philippou, E. & Nikiphorou, E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun. Rev. 17, 1074–1077 (2018).
Cutolo, M. & Nikiphorou, E. Don’t neglect nutrition in rheumatoid arthritis! RMD Open. 4, e000591 (2018).
Procaccini, C. et al. Obesity and susceptibility to autoimmune diseases. Expert Rev. Clin. Immunol. 7, 287–294 (2011).
Qin, B. et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res. Ther. 17, 86 (2015).
Merino, M. & Rissech, C. The early medieval necropolis of the former municipal courts of barcelona: a unique discovery. In Reconstructing Past Monastic Life. Volume 1. Bioarchaeology, Life and Death (eds (eds Lloveras, L., Rissech, C., Nadal, J. & Banks, P.) 55–68 (Oxbow Books, Oxford, (2025).
Beltran, J. La cristianización del suburbium de Barcino. Monografias de arqueología cordobesa, Córdoba, 18, 363–395 (2010).
Ripoll, G. & Molist, N. L’arqueologia funerària a Catalunya de l’antiguitat tardana al món medieval. In Arqueologia funeraria al nord-est peninsular (segles VI al XII) (eds Molist, N. & Ripoll, G.), Monografies d’Olèrdola 3.1, MAC, Barcelona (2012).
Banks, P. El creixement físic de Barcelona, segles X-XIII. Barcelona, Quaderns d’Història 8, 11–33 (2003).
Alesán, A. Estudi d’una població subadulta de l’edat del ferro: demografia, antropometria i creixement. Master Thesis in Human Biology, University Autonoma of Barcelona (UAB), Bellaterra, Barcelona (1990).
Alemán, I., Botella, M. C. & Ruiz, L. Determinación del sexo en el esqueleto postcraneal. Estudio de una población mediterránea actual. Arch. Esp. Morfol. 2, 7–17. Sevilla (1997).
Brickley, M. & Mckniley, J. I. Guidelines To the Standards for Recording Human Remains. British Association for Biological Anthropology and Osteoarchaeology (Institute of Field Archaeologists and BABAO, 2004).
Rissech, C., Estabrook, E. F., Cunha, E. & Malgosa, A. Estimation of age-at-death for adult males using the acetabulum, applied to four Western European populations. J. Forensic Sci. 51, 774–778 (2006).
Acknowledgements
The authors would like to thank the Museu de Història de la Ciutat de Barcelona (MUHBA) and Emili Revilla for enabling the present study and for giving us access to the museum’s facilities to carry out this study. We also would like to thank the Servei de Arqueología de Catalunya and Direcció General de Gestió d’Infraestructures del Departament de Justícia de la Generalitat de Catalunya for making the study of both this individual and the entire cemetery possible. We would like to thank Philip Banks and Diana Morales for their linguistic corrections and patience.SGR Evolució social, cultural i biològica al Pleistocè (StEP). Direcció General de Recerca de la Generalitat de Catalunya. Ref: 2021 SGR 01239MONBONES. Ministerio de Ciencia e Innovación (MICINN). Ref: PID2020-118194RJ-I00.
Funding
Open access funding provided by Department of Basic Medical Sciences. Faculty of Health Sciences and Medicine, University Rovira i Virgili.
Author information
Authors and Affiliations
Contributions
M.M and CR designed the study, produced the analysis, figures and tables and wrote the main manuscript text.M.M made all the photographs and designs in Figs. 1, 2, 3, 4, 5, 6 and 7 herself.M.P, C.R and X.T undertook the radiological study and contributed to the writing process of the manuscript.All of the authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Permission statement
Marta Merino and Carme Rissech were permitted to study the human skeletal material from the Antics Jutjats Municipals de Barcelona archaeological site in the at the Human Bone Laboratory of the Barcelona City History Museum, where the remains are curated in accordance with the Catalan Heritage Law.
Ethical approval
Not applicable. As we used archaeological individuals from High Medieval period, the research did not require approval from the Ethics Committee.
Competing interest
The authors declare no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pérez, M.M., Rissech, C., Porta-Vilaró, M. et al. Contribution to the debate on the origin of autoimmune joint diseases in Europe through an archaeological case of still’s disease. Sci Rep 15, 23531 (2025). https://doi.org/10.1038/s41598-025-09623-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09623-6