Abstract
The convergence of advanced materials science and additive manufacturing technologies has revolutionized medical applications ranging from orthodontic devices to patient-specific bone fixation systems. Despite these advancements, the clinical translation of 3D-printed orthopedic implants remains constrained by suboptimal biocompatibility of conventional manufacturing materials. This study systematically evaluates dental resin-based composites as alternative biomaterials for bone fixation devices, hypothesizing that their rapid photopolymerization kinetics, mechanical robustness, and inherent biocompatibility can address current limitations. Through stereolithography technology, ceftriaxone sodium-loaded bone fixation devices were developed with customized geometries tailored to diverse anatomical requirements. Material optimization revealed that a 7:3 ratio of ethoxylated bisphenol A dimethacrylate (BPA2EODMA) to triethylene glycol dimethacrylate (TEGDMA) achieved optimal mechanical performance. Cytocompatibility assessment using L929 fibroblasts demonstrated > 70% cell viability, confirming minimal cytotoxicity. The drug-eluting implants exhibited potent antimicrobial efficacy in 8-h elution assays, showing inhibition rates of 49.14 ± 4.89% (Staphylococcus aureus), 48.38 ± 5.88% (Escherichia coli), and 26.79 ± 2.69% (Candida albicans). These results validate the dual functionality of antibiotic-loaded dental resin constructs, positioning them as promising candidates for patient-specific orthopedic solutions that simultaneously address mechanical stability, osseointegration, and infection prevention.
Similar content being viewed by others
Introduction
The management of critical-sized bone defects remains a persistent clinical challenge in contemporary orthopaedic practice1,2. While autologous and allogeneic bone grafts are frequently employed, they are associated with limitations such as donor site morbidity, immunological incompatibility, infection risks, and restricted availability of viable grafts3,4. Consequently, synthetic bone substitutes have emerged as promising alternatives, offering tunable physicochemical properties and batch-to-batch consistency. Commonly used materials include bioglass5,6, calcium phosphate ceramics7,8, tricalcium phosphate9,10, hydroxyapatite11, calcium phosphate cements12,13 often exhibit suboptimal mechanical strength and biodegradation profiles14.
The rapid advancement of additive manufacturing techniques has revolutionized the scaffold fabrication by precise spatial control of architectural parameters, offering an innovative approach to mimic the complex structure and mechanical properties of natural bone2,15,16,17. With continuous advancements in printing materials ranging from metal to nonmetal and even biological cells, 3D printed implants have demonstrated their potential to replace various anatomical structures including the collarbone, sternum and ribcage, upper and lower jawbones, ear ossicles, spine, and skull18. Furthermore, flexible implants such as vascular sten19, tissue engineered blood vessel20, and nerve graft21 have been successfully fabricated.
Stereolithography (SLA), recognized as one of the earliest 3D printing process, is valued for its high precision, cost-effectiveness, and relatively low requirements for resin fluidity22,23,24. This technology relies on light-curing resins, which undergo cross-linking and polymerization upon exposed to ultraviolet (UV) light, with the type and concentration of the photosensitive resin significantly influencing the curing process25,26. Commonly used materials in SLA include propylene fumarate (PPF), polycaprolactone (PCL), poly-(D, L-Lactide) (PDLLA), and polypropylene fumarate-diethyl fumarate (PPF-DEF)27. However, the clinical application of these materials is often restricted due to concerns regarding their inadequate biocompatibility28. Therefore, we turned our attention to dental resin materials, which offer several advantages over commonly used SLA materials, such as lower shrinkage rates, faster curing times, and enhanced biocompatibility. Notable examples include dimethacrylate carbamate (UDMA), ethoxylated bisphenol A dimethacrylate (BPA2EODMA), triethylene glycol dimethacrylate (TEGDMA), and ethylene glycol dimethacrylate (EGDMA)29,30,31,32. UDMA and BPA2EODMA are macromolecular monomers characterized by high viscosity. They form the primary components of the resin crosslinking network and directly affect the performance of the final product. BPA2EODMA exhibits significantly higher viscosity than UDMA, enhancing the adhesion of the liquid resin. Conversely, TEGDMA and EGDMA are small molecule monomers that improve fluidity and can serve as diluents to adjust the viscosity of the matrix resin. However, TEGDMA is considered safer and more stable than EGDMA.
To impart antimicrobial functionality, the incorporation of ceftriaxone sodium (CRO) into the implant matrix was explored33,34. As a third-generation cephalosporin antibiotic, CRO exerts its bactericidal effect by inhibiting bacterial cell wall synthesis, demonstrating potent antibacterial activity against both Gram-positive and Gram-negative bacteria, including Pseudomonas aeruginosa and anaerobic bacteria35. Additionally, CRO possesses high stability against β-lactamase and low nephrotoxicity, making it a suitable antibiotic for preventing perioperative infections. It is widely utilized in clinical setting for treating pneumonia, abdominal, infections and urogenital system infections.
In this study, BPA2EODMA was selected as the resin matrix, while TEGDMA served as the active diluent aiming to overcome the limitations in biocompatibility of current additive manufacturing materials. Diphenyl (2,4,6-trimethyl benzoyl) phosphine oxide (TPO) was incorporated as a photoinitiator. While TPO has raised concerns regarding its potential cytotoxicity and genotoxicity, it remains widely employed as a photoinitiator in light-curable resin systems. Studies have indicated that its toxicity can be substantially minimized under optimized curing conditions36. Given its high quantum yield and rapid photopolymerization efficiency—critical for achieving effective and uniform curing—TPO was selected based on a balanced consideration of safety and functional performance. The formulation was optimized by adjusting the ratio of the two resins and the concentration of the photoinitiator, thereby enhancing both the printing process and the performance of the final product. Subsequently, a CRO solution was combined with PEG300 and mixed with PEG400DA solution containing TPO to create a drug-loaded resin. After injecting this drug-loaded resin into the implant cavity, it was photocured to produce the final drug-loaded implant. Overall, by integrating structural performance with therapeutic functionality, this study presents a novel approach for fabricating multifunctional 3D printed implants that are not only mechanically reliable and biocompatible, but also capable of mitigating infection risks in clinical settings.
Material and methods
Materials
Ethoxylated bisphenol A dimethacrylate (BPA2EODMA, average MW 453) (Fig. 1A) was provided by DSM Chemical Co., Ltd. (Shanghai, China). Polyethylene glycol diacrylate (PEGDA, average MW 600), Polyethylene glycol (PEG, average MW 300), and Triethylene glycol dimethacrylate (TEGDMA, average MW 286) (Fig. 1B) were purchased from Ryoji Chemical Co., Ltd. (Guangdong, China). Diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide (TPO) (Fig. 1C), and CRO were obtained from Aladdin Chemical Co., Ltd. (Shanghai, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Sigma-Aldrich.
3D Print scaffold design
The scaffold architecture was optimized through computational modeling to satisfy dual mechanical and biological requirements. Specifically, the structures were required to exhibit sufficient mechanical strength and structural integrity for load-bearing applications, while maintaining an internal porosity that facilitates nutrient diffusion and cell infiltration. High dimensional accuracy and printability were also essential to guarantee consistency and reproducibility of the printed products37. SolidWorks 2020 (Dassault Systèmes, Waltham, MA, USA) was utilized to design multiple sizes implant models for subsequent validation shown in Fig. 1D.
Photocurable resin preparation
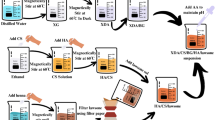
The 3D printer used in this study was the commercially available Anycubic Photon 3D printer (Anycubic Technology Co., Ltd., Shenzhen, China). The photopolymer resin was formulated by combining ethoxylated bisphenol A dimethacrylate (BPA2EODMA, 70 wt%) and triethylene glycol dimethacrylate (TEGDMA, 30 wt%), achieving a 7:3 monomer-to-diluent ratio. Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (TPO, 0.1 wt%) was incorporated as the photoinitiator. The mixture was homogenized via magnetic stirring (500 rpm, 25 °C, 12 h) under light-protected conditions to ensure complete dissolution of TPO and prevent premature photopolymerization.
For antibiotic incorporation, poly(ethylene glycol) diacrylate (PEGDA, Mn = 400 g/mol) and TPO (0.1 wt%) were first dissolved at 90 C to enhance miscibility. After cooling the solution to 25 °C, the candidate model drug, water and PEG were incorporated. The final mixture was subjected to ultrasonication (40 kHz, 30 min) followed by mechanical stirring (300 rpm, 2 h) to ensure uniform drug distribution and resin homogeneity prior to printing.
Preparation of tensile test splines and mechanical properties
The dumbbell-shaped sample was designed using SolidWorks2020 (Fig. 2A). Specimens were fabricated via stereolithography (SLA) using standardized printing parameters: 50 μm layer thickness and 10 s/layer exposure time. To meet diverse functional demands for the implants, material ratios were evaluated to optimize tensile strength. The base resin consisted of BPA2EODMA as the primary monomer and TEGDMA as the reactive diluent, blended at seven discrete mass ratios (9:1, 8:2, 7:3, 6:4, 5:5, 4:6, and 3:7). Post-printing, all specimens underwent uniform photopolymerization under a 405 nm UV mercury lamp (50% intensity) for 30 min to ensure complete curing. The tensile strength of the splines was assessed using a high and low temperature double column testing machine (Instron 5966, Instron, Norwood, MA, USA) at a strain rate of 0.6 mm/min. The experiments were performed in accordance with ASTM D638 standards, with each formulation tested in triplicate. All tests were conducted under controlled laboratory conditions (25 °C, 35% relative humidity).
Manufacturing of scaffolds
The Anycubic Mono X stereolithography (SLA) printer (Anycubic Technology Co., Ltd., Shenzhen, China) was employed for scaffold fabrication. Specimen geometries (Fig. 2B) were designed in SolidWorks 2020 (Dassault Systèmes, Waltham, MA, USA) and subsequently sliced using Photon Workshop V2.1.24 (Anycubic) to achieve a uniform layer resolution of 50 µm. These designs were then imported into the printer to fabricate 3D printed structures and evaluate printing capabilities. The scaffolds were printed using pre-selected resin with the following parameters: 10-s exposure time per layer, 12 s exposure time for the bottom layer, 3 s extinguishing time, a 50 µm layer height and 50 bottom layer. Printing was conducted layer by layer under 50% light intensity of 405 nm ultraviolet light.
A 1% TPO content was found to maintain implant integrity and prevent overcuring caused by excess photo absorber. Insufficient TPO content resulted in scaffold detachment, while excessive content led to diminished or absent internal cavity features. The finalized resin formulation comprised a 7:3 mass ratio of BPA2EODMA (base monomer) to TEGDMA (reactive diluent), with 1 wt% TPO, successfully fabricated load-bearing orthopedic components (screws, bone fixation plates) and porous scaffolds (70–85% porosity) tailored to defect-specific anatomical requirements (Fig. 2C).
Mechanical properties test
Compression tests on scaffolds were conducted using a high and low temperature double column testing machine (Instron 5966, Instron, Norwood, MA, USA) at a strain rate of 0.6 mm/min under controlled laboratory conditions (25 °C, 35% relative humidity) to evaluate their compressive strength.
Fabrication of drug-loaded scaffolds
To prepare the CRO solutions, 1000 mg, 500 mg, and 100 mg of CRO were each dissolved in 1 mL of purified water. Each CRO solution (1 mL) was homogenized with 4000 mg polyethylene glycol 300 (PEG300) via probe ultrasonication (20 kHz, 150 W, 10 min, 4 °C). Concurrently, a photocurable resin base was formulated by combining 4000 mg poly(ethylene glycol) diacrylate (PEG400DA) with 100 mg TPO in light-shielded microcentrifuge tubes. The drug-resin composite was synthesized by mixing CRO/PEG300 and PEG400DA/TPO solutions at a 1:4 volumetric ratio under vortex agitation (2500 rpm, 5 min). Final formulations were stored in amber vials at −20 °C until use. Prior to biological testing, scaffolds were post-cured under 405 nm UV light for 90 s to ensure sterility while preserving drug activity.
Characterization of drug-loaded resins
X-ray diffraction (XRD)
X-rays were the essential analytical technique for structural analysis. Drug-containing resins with concentration of 1%, 5% and 10% were cured and then ground into a powder. The samples were analyzed under Cu Kα radiation (λ = 0.1541 nm) using XRD spectra (Malvern Panalytical, Empyrean, Netherlands) obtained from a powder diffractometer. The operating voltage and current were set to 40 kV/40 mA, respectively, with a measurement range of 2θ from 10° to 80°.
Scanning electron microscopy (SEM)
Under vacuum conditions, the dried test samples were fixed onto a gold-sputtered copper plate holder using conductive adhesive. Subsequently, the surface morphology of the drug-loaded implants was observed, photographed, and documented using field emission scanning electron microscopy (FE-SEM, S-4700, Hitachi Ltd., Japan), the accelerating voltage was set to 15 kV. This procedure was conducted to further investigate the surface characteristics of the implants.
Cytocompatibility assessment of non-loaded scaffolds
The cytotoxicity of the printed scaffolds was evaluated in vitro according to the ISO 10993-5 (elution test method)38. Scaffold extracts were prepared by incubating sterilized samples (n = 3) in phenol red-free DMEM (1 cm2/mL surface area-to-volume ratio) at 37 °C under orbital shaking (100 rpm, 24 h). High-density polyethylene served as the negative control, while a 0.64% phenol solution was used as the positive control. L929 murine fibroblast cells (American Type Culture Collection, ATCC® CCL-1™, USA) between passages 10–15 were used for all experiments. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C under 5% CO2. L929 cells were seeded in a 96-well plate at a density of 1.5 × 104 cells per well and allowed to adhere for 24 h. After removing the original culture medium, the negative control group, the positive control group, and the scaffold soaking solution in culture medium were each co-cultured for an additional 24 h at intervals for 24, 72, and 120 h. Subsequently, 20 μL of MTT solution (5 mg/mL) was added to each well and incubated for 4 h. The plates were then read by a micro-plate reader at an absorbance wavelength of 490 nm. The relative cell viability (%) was calculated by the following formula: cell viability = (As-Ab)/(An-Ab), where As, An and Ab represent the absorbance of samples, negative control (24 h) and blank sample (cell-free), respectively. All measurements were performed in triplicate.
In vitro drug releasev kinetics
The drug release profiles for CRO implant were assessed through a release test. Phosphate-buffered saline (PBS, pH 7.2) was pre-warmed at 37 °C for 15 min to serve as the release medium, and then the CRO implant was added. At specific time intervals (3 h, 6 h, 12 h, 24 h, 48 h, 96 h, 120 h, 144 h, 168 h, 192 h, 216 h, 240 h), 3 mL of the dissolved medium was withdrawn and replaced with an equal amount of fresh medium. The absorbance of the resulting solution was measured at a wavelength of 241 nm using UV–Vis spectrophotometry. The concentration of the sample drug at each time point was determined by referencing the absorbance value to a standard curve. The cumulative release amount and cumulative release percentage at each time point were calculated and the drug release curve was plotted. This method was used to study the effects of drug concentration (1%, 5%, 10%) (n = 3), screw shape (tension screws, locking nail, coarse grain screw) (n = 9), and light curing time (2 min, 3 min, 4 min) (n = 3) on drug release. The calculation formula is as follows:
(Ci indicates the concentration of drug in the release medium measured at each time point, Vmedium indicates the volume of media (in 10 mL), Vsampling indicates the sampling volume (3 mL)).
In-vitro antibacterial evaluation
The bacteriostatic ring test of the implants was conducted using the plate spreading method with preloaded bacterial solution. Bacterial solutions of Staphylococcus aureus, Escherichia coli and Candida albicans, each at a concentration of 1 × 106 CFU/mL, were evenly spread on a solid medium. In the experimental group, a CRO implant containing 10% drug was placed at the center of the petri dish, while an unloaded implant was used in the control group. The experimental group was incubated in a biochemical incubator at 37 °C for 2, 4, and 8 h, whereas the control group was incubated for 8 h. After incubation, the petri dishes were removed and photographed. The images were then processed using Image J software to quantify the antibacterial effect of the implants.
Intracellular bactericidal activity
L929 cells were seeded in six-well plates at a density of 106 cells per well and cultured until they adhered. Subsequently, the cells exposed to activated Staphylococcus aureus at concentrations of 108, 5 × 107, 107, 5 × 106, and 2.5 × 106 CFU/mL in complete culture media for 4 h to induce bacterial infection. After removing the bacterial culture, fresh culture media and CRO implants were added. The control group received an equivalent dosage of pure drug solution. Co-cultivation continued for 24 h, after which cell viability was assessed using the MTT assay.
To evaluate the broad-spectrum antibacterial properties of the drug-loaded implants, the experiment was repeated using Escherichia coli, Candida albicans, and Staphylococcus aureus at a concentration of 107 CFU/mL.
Statistical analysis
Data analysis utilized OriginPro 2021 (v9.8.5.204). Continuous variables are expressed as mean ± SD (n ≥ 3). Between-group comparisons employed one-way ANOVA with Tukey’s post-hoc test (α = 0.05). Drug release modeling used nonlinear least-squares regression (R2 > 0.95).
Results and discussion
Rheological and mechanical characterization
Systematic evaluation of the viscoelastic properties of BPA2EODMA/TEGDMA formulations revealed critical structure-process-property relationships that significantly impact scaffold printability. Rheological characterization at 25 °C demonstrated markedly different viscosity profiles for the pure monomers: 500–700 cps for BPA2EODMA versus 10.2 cps for TEGDMA. This substantial viscosity difference directly influenced formulation flowability, prompting the exclusion of 8:2 and 9:1 (BPA2EODMA:TEGDMA) ratios due to insufficient viscosity for optimal processing.
The fractional strength of the 3D printed scaffolds is shown in Fig. 3A. The mechanical performance demonstrated a non-monotonic relationship with monomer composition, exhibiting a statistically significant variation (p < 0.05). Ultimate tensile strength reached a maximum value of 36.61 ± 2.03 MPa at the 7:3 BPA2EODMA:TEGDMA mass ratio. While the proportion of BPA2EODMA increases beyond the 7:3 ratio, the viscosity of the mixture becomes significantly higher, reducing the resin’s flowability. This decrease in flowability may lead to poor interlayer adhesion and incomplete curing during the 3D printing process, resulting in a less homogeneous polymer network. Additionally, higher viscosity can limit the material’s ability to adequately fill molds or form a uniform structure, which can result in weak spots or inadequate bonding between polymer chains. These factors contribute to the reduction in the tensile strength of the scaffolds at higher BPA2EODMA concentrations. Choi et al.39 reached a similar conclusion in their study on 3D printing materials, where they observed that high viscosity can degrade the performance and printing resolution of the resin. When the viscosity was too high, missing layers were observed. This finding aligns with our results, suggesting that an optimal balance between viscosity and flowability is crucial for ensuring the mechanical integrity and resolution of 3D printed scaffolds. Therefore, a BPA2EODMA to TEGDMA ratio of 7:3 was selected as the optimal formulation for subsequent experiments.
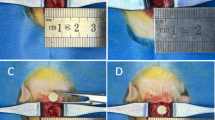
Mechanical properties and 3D printed semi-hollow scaffolds. (A) Fraction strength of the 3D printed scaffolds at different concentration (mean ± SD, n = 5), *, p < 0.05; ****, p < 0.0001. (B) Compression test result of the implant fabricated with a BPA2EODMA to TEGDMA ratio of 7:3. (C) 3D printed semi-hollow scaffolds (a) and drug-loaded implants (b).
For compressive strength test, the results were illustrated in Fig. 3B. The stress–strain curves of the scaffolds (n = 3) exhibited linear elasticity up to 4.50 ± 0.53% strain, followed by brittle fracture at ultimate compressive strength (UCS) = 9.54 ± 0.75 MPa. This performance exceeds human trabecular bone (UCS = 2–12 MPa) while remaining below cortical bone thresholds (UCS > 100 MPa), confirming its suitability for non-load-bearing applications such as cranial or maxillofacial reconstruction. Overall, the resin formulation optimized with a BPA2EODMA to TEGDMA ratio of 7:3 not only demonstrated excellent printability but also provided sufficient mechanical strength to support surrounding tissues during the early postoperative stage. Nevertheless, the compressive strain at fracture reflects the brittle nature of the material, suggesting that further structural optimization is needed to improve its mechanical stability.
Fabrication of drug-loaded scaffolds
As illustrated in Fig. 3C, distinct morphological differences were observed between hollow control scaffolds (Fig. 3C(a)) and ceftriaxone sodium (CRO)-loaded constructs (Fig. 3C(b)). Before light curing, the resins containing three different concentrations of CRO appeared yellow. In contrast, the resin solution without CRO was clear and transparent. Upon the addition of CRO, a dark yellow color emerged, intensifying with higher CRO concentrations.
XRD analysis
XRD was employed to analyze the crystallinity of various concentrations of CRO in the resin, with the results presented in Fig. 4A. CRO exhibited multiple large crystal peaks with narrow widths at 2θ = 18–25°, indicative of a typical crystalline morphology. In the drug-containing resins with CRO concentrations of 1%, 5% and 10%, a single crystal peak was observed at 2θ = 18–25°. However, the peak intensity and width increased, deviating from the typical crystal form. The X-ray diffraction patterns of CRO powder at 1% and 5% concentrations in the resin were nearly identical, while the diffraction pattern for the 10% concentration showed reduced intensity and peak width compared to the other two. This suggests that the 1% and 5% CRO concentrations in the resin maintained similar crystalline and amorphous states. In contrast, the 10% CRO concentrations exhibited a lower amorphous form, resulting in a smaller peak width. In summary, the 1% and 5% CRO concentrations in the resin were more amorphous and well dispersed.
Characterization of implants. (A) XRD patterns of drug-loaded resin with CRO content of 1%, 5%, 10% and CRO (100%). (B) SEM image of 3D printed implants: (a) and (b) were the vertical section view, (c) and (d) were the cross section view. (C) In vitro cytotoxicity of the leachate from the implanted scaffold. n = 3, **, p < 0.01; ****, p < 0.0001.
Microstructural characterization of drug-loaded constructs
SEM analysis (Fig. 4B) revealed distinct morphological features between the scaffold surface and drug-loaded regions. The polymer scaffold exhibited a dense, non-porous surface structure, which correlates with enhanced compressive strength40. Such a dense morphology also offers several advantages, including improved mechanical stability, reduced degradation rate, and increased resistance to microbial infiltration—factors that are critical for maintaining implant integrity during the early stages of healing. In contrast, the central region of the implant displayed a granular or porous morphology, which may be attributed to polymorphic transitions of the polymer matrix or the model drug during the curing process. The observed structural differentiation suggests a potential mechanism for controlled drug release, where the dense surface layer may initially retard drug elution while the porous core serves as a drug reservoir.
Cytocompatibility assessment
Considering TPO was recently reclassified by the European Union’s REACH regulation (Registration Evaluation Authorization of Chemicals) with subsequent labeling as a CMR (Carcinogenic, Mutagenic, Reprotoxic) substance of “very high concern”36. In our study, the concentration of TPO added was 1% (w/w), exceeding the 0.1% threshold. However, studies have shown that the toxicity of TPO can be reduced when mixed with resin materials and optimized curing conditions are applied41. Furthermore, many manufacturers use TPO concentrations ranging from 1 to 1.5%36, which provides a useful reference for our selection of TPO dosage. Therefore, it is essential to evaluate its toxicity. To assess the potential toxicity at the selected concentration of 1%, cell-based experiments were conducted. According to ISO 10993-5:200927, a material is considered non-cytotoxic if the relative activity of cells exposed to the highest concentration of the sample extract (100% extract) is more than 70% of that of the control group.
Analysis of Fig. 4C reveals that the cell growth rate decreases as the extract concentration increases. Notably, when the extract was diluted to 25%, the cell growth status was nearly identical to that of the negative control group. Importantly, the average cell relative activity at the highest concentration of the sample extract (100% extract) remained above 70% of that of the negative control group. Therefore, the implant can be considered non-toxic. Furthermore, reports have indicated that the leaching of free monomers such as TEGDA can lead to cell death42. However, no such phenomenon was observed in the cytotoxicity experiments, suggesting that the materials were fully cross-linked and cured. Additionally, the dense structure of the scaffold effectively prevents the leaching of internal monomers, which is consistent with the SEM results. Overall, the fabricated scaffold exhibits acceptable biocompatibility, making it suitable for further research.
In vitro drug release
Influence of drug concentration on release kinetics
The cumulative release of CRO implants was diminished at a 5% drug concentration compared to those at concentrations of 10% and 1% (Fig. 5A). This reduction may result from increased interactions between CRO and the resin matrix at intermediate concentrations. Moreover, CRO implants with a 10% drug concentration exhibited enhanced release at 72 h, likely owing to the higher presence of crystals. As hydrolysis degrades the polymer network, these crystals dissolve, generating microporous channels that accelerate drug diffusion.
All formulations exhibited biphasic release kinetics: an initial burst release (within 24 h), followed by sustained release until plateauing at 96 h. This profile is clinically advantageous, as the rapid initial release delivers a therapeutically effective drug concentration to prevent acute postoperative infections or inflammation, while the prolonged release supports wound healing during the critical early recovery phase.
Effect of light curing duration on drug release
As shown in Fig. 5B, drug-loaded implants cured for 4 min demonstrated an average cumulative release of 60% after 72 h. Conversely, those cured for shorter durations (2 min and 3 min) showed significantly higher drug release, reaching approximately 73% and 78%, respectively. This trend suggests that extended curing times may lead to a more densely crosslinked polymer network at the implant surface, thereby restricting drug diffusion from the bulk matrix. The observed increase in cumulative release with longer curing times could be attributed to enhanced structural integrity of the resin, which may facilitate more controlled drug elution by preventing premature matrix disintegration.
Effect of implant geometry on release behavior
As illustrated in Fig. 5C, the cumulative release profiles showed negligible differences across the three screw designs. This similarity can be attributed to their nearly identical surface areas exposed to the release medium. The consistency was especially pronounced at 5% and 10% drug loadings, implying that release kinetics are governed primarily by drug dissolution and matrix erosion rather than macroscopic implant geometry.
In-vitro antibacterial effect
The results of antibacterial experiment data were presented in Fig. 6A. The antibacterial ring formed in the control group was significantly smaller than those in the experimental group at all time points, indicating that CRO was effectively released from the implants and exerted its antibacterial effect. The drug release was positively correlated with incubation time. The implants exhibited antibacterial effects against all three tested microorganisms. Notably, for Staphylococcus aureus and Escherichia coli, the antibacterial rings formed by the samples in the 8-h group accounted for 49.14 ± 4.89% and 48.38 ± 5.88% of the total area of the culture plate respectively, which was significantly larger than those in the 2-h and 4-h groups. Additionally, the samples also demonstrated inhibitory effects on fungi, with the rings formed accounting for 26.79 ± 2.69% of the total area in the 8-h group (Fig. 6B). These results confirm that the implant can exert an inhibitory effect on microorganisms within a short period. It exhibits strong inhibitory effects against both Gram-positive and Gram-negative bacteria, and also demonstrates antifungal activity.
In-vitro and intracellular antibacterial effect of CRO implant. (A) Antibacterial ring test for CRO implant. (B) Corresponding statistical analysis of antibacterial ring. (C) Therapeutic efficacy to Staphylococcus aureus. (D) Therapeutic efficacy to different types of bacteria at a concentration of 107 CFU/mL. n = 3; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Antimicrobial efficacy and cellular protection
At a bacterial concentration of 5 × 107 CFU/mL, implants containing 5% CRO exhibited rapid drug release, effectively preventing extensive cell mortality resulting from bacterial infections (Fig. 6C). The cell survival rate in the CRO implant group reached 89.35 ± 5.29%, showing no significant difference compared to the pure CRO group. However, as the bacterial concentration exceeded the range treatable by antibiotics, both the implant group and the pure drug group experienced increased cell mortality, with cell survival rates of 40.68 ± 4.68% and 48.56 ± 5.52%, respectively. The time-dependent drug release in the implant group led to a slightly diminished therapeutic effect compared to the pure drug group.
According to therapeutic effects of CRO and CRO implants on L929 cells infected with different microbial strains (Fig. 6D), the CRO implant group showed no significant differences compared to the CRO group across various microorganism strains, demonstrating the applicability of the implants in various microbial treatment scenarios.
Conclusion
This study presents the successful development of a novel light-cured, 3D-printed ceftriaxone (CRO)-eluting implant fabricated from BPA2EODMA/TEGDMA-based dental resin composites. The optimized implant formulation demonstrated exceptional print fidelity, favorable mechanical properties. Importantly, the incorporated CRO exhibited broad-spectrum antimicrobial efficacy against S. aureus, E. coli, and C. albicans. The observed biphasic release kinetics characterized by an initial burst release followed by sustained release, which offers both immediate infection prophylaxis and prolonged therapeutic coverage during the critical postoperative period.
While this preliminary investigation demonstrates promising results, several limitations warrant acknowledgement.First, the exclusive reliance on in vitro models precludes comprehensive assessment of crucial biological parameters, including osseointegration potential, host tissue response, and systemic drug pharmacokinetics. Second, the monotherapeutic approach employing ceftriaxone as a single antibiotic agent, raises concerns regarding potential for resistance development, a factor not addressed within the scope of this study. Third, the absence of long-term degradation data limits our understanding of the implant’s structural integrity over clinically relevant timeframes. To facilitate clinical translation of this technology, future research will prioritize: (1) comprehensive in vivo evaluation in appropriate animal models to assess osseointegration kinetics, local and systemic biocompatibility, and infection prevention efficacy; (2) the development of combinatorial therapeutic approaches incorporating anti-inflammatory agents or osteogenic factors to address multifactorial postoperative complications; and (3) the implementation of antibiotic stewardship strategies, potentially through dual-drug delivery systems or surface modification approaches to mitigate resistane. Furthermore, detailed pharmacokinetic profiling and Good Manufacturing Practice (GMP)-compliant scale-up studies will be essential prerequisites for clinical translation. Despite these limitations, this proof-of-concept study establishes a robust foundation for utilizing modified dental resins in the fabrication of multifunctional, drug-eluting orthopedic implants. The convergence of 3D printing technology with antimicrobial-eluting capabilities represents a significant advancement toward personalized, infection-resistant bone fixation devices for high-risk orthopedic interventions.
Data availability
Data will be made available on request.
References
Peng, B. et al. Additive manufacturing of porous magnesium alloys for biodegradable orthopedic implants: Process, design, and modification. J. Mater. Sci. Technol. 182, 79–110 (2024).
Meng, M. et al. 3D printing metal implants in orthopedic surgery: Methods, applications and future prospects. J. Orthop. Translat. 42, 94–112 (2023).
Tammisetti, V. S. et al. Immunosuppressive therapy in solid organ transplantation: Primer for radiologists and potential complications. Radiol. Clin. North. Am. 61(5), 913–932 (2023).
Pham, T. T. et al. Epidemiology and outcomes of bone and joint infections in solid organ transplant recipients. Am. J. Transpl. 22(12), 3031–3046 (2022).
Daskalakis, E. et al. Novel 3D bioglass scaffolds for bone tissue regeneration. Polymers (Basel) 14(3), 1248 (2022).
Gritsch, L. et al. Combining bioresorbable polyesters and bioactive glasses: Orthopedic applications of composite implants and bone tissue engineering scaffolds. Appl. Mater. Today 22, 100923 (2021).
Othman, Z. et al. The role of ENPP1/PC-1 in osteoinduction by calcium phosphate ceramics. Biomaterials 210, 12–24 (2019).
Tang, Z. et al. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regen. Biomater. 5(1), 43–59 (2018).
Thomas, D. et al. Microwave synthesis of functionally graded tricalcium phosphate for osteoconduction. Mater. Today Commun. 9, 47–53 (2016).
Gupta, V. et al. Microsphere-based scaffolds encapsulating tricalcium phosphate and hydroxyapatite for bone regeneration. J. Mater. Sci. Mater. Med. 27(7), 121 (2016).
Arcos, D. & Vallet-Regi, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 8(9), 1781–1800 (2020).
Luo, J., Engqvist, H. & Persson, C. A ready-to-use acidic, brushite-forming calcium phosphate cement. Acta Biomater. 81, 304–314 (2018).
Tronco, M. C., Cassel, J. B. & dos Santos, L. A. alpha-TCP-based calcium phosphate cements: A critical review. Acta Biomater. 151, 70–87 (2022).
Gillman, C. E. & Jayasuriya, A. C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 130, 112466 (2021).
Ahmadpour, L. et al. Knowledge sharing in temporary teams: Exploring the use of 3D printing in orthopaedic surgery. Technovation 123, 102723 (2023).
Garcia-Avila, J. et al. Dynamic topology optimization of 3D-Printed transtibial orthopedic implant using tunable isotropic porous metamaterials. J. Mech. Behav. Biomed. Mater. 153, 106479 (2024).
Misiaszek, J. P. et al. 3D-ink-extruded titanium scaffolds with porous struts and bioactive supramolecular polymers for orthopedic implants. Acta Biomater. 188, 446–459 (2024).
Alqutaibi, A. Y. et al. Advanced additive manufacturing in implant dentistry: 3D printing technologies, printable materials, current applications and future requirements. Bioprinting 42, e00356 (2024).
Li, W. et al. Light-based 3D bioprinting techniques for illuminating the advances of vascular tissue engineering. Mater. Today Bio 29, 101286 (2024).
Jeong, H. J. et al. Dragging 3D printing technique controls pore sizes of tissue engineered blood vessels to induce spontaneous cellular assembly. Bioact. Mater. 31, 590–602 (2024).
Buckley, C. et al. Hyaluronic acid hybrid formulations optimised for 3D printing of nerve conduits and the delivery of the novel neurotrophic-like compound tyrosol to enhance peripheral nerve regeneration via Schwann cell proliferation. Int. J. Pharm. 661, 124477 (2024).
Han, X. et al. 3D micro-nano printing technology as a transformative tool apply for microneedle drug delivery. J. Drug. Deliv. Sci. Technol. 96, 105709 (2024).
Husna, A. et al. Recent advancements in stereolithography (SLA) and their optimization of process parameters for sustainable manufacturing. Hybrid Adv. 7, 100307 (2024).
Afridi, A., Al Rashid, A. & Koç, M. Recent advances in the development of stereolithography-based additive manufacturing processes: A review of applications and challenges. Bioprinting 43, 00360 (2024).
Elomaa, L. et al. Development of GelMA/PCL and dECM/PCL resins for 3D printing of acellular in vitro tissue scaffolds by stereolithography. Mater. Sci. Eng. C Mater. Biol. Appl. 112, 110958 (2020).
Curti, C., Kirby, D. J. & Russell, C. A. Systematic screening of photopolymer resins for stereolithography (SLA) 3D printing of solid oral dosage forms: Investigation of formulation factors on printability outcomes. Int. J. Pharm. 653, 123862 (2024).
Sultana, N. et al. 3D Printing in pharmaceutical manufacturing: Current status and future prospects. Mater. Today Commun. 38, 107987 (2024).
Chen, J. T. et al. 3D printing for bone repair: Coupling infection therapy and defect regeneration. Chem. Eng. J. 471, 144537 (2023).
Dwivedi, R. et al. Auricular reconstruction via 3D bioprinting strategies: An update. J. Oral Biol. Craniofac. Res. 12(5), 580–588 (2022).
Kang, Y. J. et al. Effect of airborne particle abrasion treatment of two types of 3D-printing resin materials for permanent restoration materials on flexural strength. Dent. Mater. 39(7), 648–658 (2023).
Graca, A. et al. Vat-based photopolymerization 3D printing: From materials to topical and transdermal applications. Asian J. Pharm. Sci. 19(4), 100940 (2024).
Ree, B. J. Critical review and perspectives on recent progresses in 3D printing processes, materials, and applications. Polymer 308, 127384 (2024).
Rony, M. R. I. et al. Influences of alcohols, urea and polyethylene glycol on the cloudy formation nature and physico-chemical parameters of the mixture of triton X-100 and ceftriaxone sodium salt. Colloid Surf. A 677, 132410 (2023).
Bose, S., Sarkar, N. & Jo, Y. Natural medicine delivery from 3D printed bone substitutes. J. Control Release 365, 848–875 (2024).
Jasprica, I. et al. Utilization of a kinetic isotope effect to decrease decomposition of ceftriaxone in a mixture of D(2)O/H(2)O. Eur. J. Pharm. Sci. 187, 106461 (2023).
Messer-Hannemann, P. et al. Residual TPO content of photopolymerized additively manufactured dental occlusal splint materials. Biomedicines 13(1), 44 (2025).
ISO. Implants for surgery-mechanical testing of porous metal materials: ISO 13314, (2018).
ISO. Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity, (2009).
Choi, Y. et al. Development of Bisphenol-A-Glycidyl-Methacrylate- and Trimethylolpropane-Triacrylate-based stereolithography 3D printing materials. Polymers (Basel) 14(23), 5198 (2022).
Zhao, C. Q. et al. Doping lithium element to enhance compressive strength of β-TCP scaffolds manufactured by 3D printing for bone tissue engineering. J. Alloy. Compd. 814, 152327 (2020).
de Melo Soares, V., dos Reis, A. C. & da Costa Valente, M. L. The influence of 2,4,6-trimethylbenzoyldiphenylphosphine oxide on the toxicity of dental resins: A systematic review of in vitro studies. Int. J. Adhes. Adhes. 138, 103922 (2025).
Lin, C. H. et al. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 123(2), 349–354 (2020).
Acknowledgements
Not applicable.
Funding
This work was financially supported by Science and Technology Plan Project of Taizhou, grant number 23ywa37.
Author information
Authors and Affiliations
Contributions
Liwen Zhang: Methodology, Software, Validation, Formal analysis, Writing-original draft. Jinhong Zhao: Conceptualization, Methodology, Validation. Jie Zhang: Software, Validation, Formal analysis. Bang Lou: Methodology, Software, Validation. Huijie Li: Methodology, Software. Fangyuan Guo: Formal analysis, Data curation. Gensheng Yang: Conceptualization, Resources, Writing-review & editing. Weiyong Hong: Conceptualization, Resources, Writing-review & editing, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The author(s) report no competing interests in this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Zhao, J., Zhang, J. et al. 3D printed bone nails loaded with ceftriaxone sodium for localized drug delivery. Sci Rep 15, 24892 (2025). https://doi.org/10.1038/s41598-025-09748-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09748-8