Abstract
Long-term remission in Crohn’s disease (CD) remains challenging. While ustekinumab effectively induces remission, strategies to enhance its maintenance efficacy are urgently needed. This study evaluated therapeutic drug monitoring (TDM)-guided ustekinumab optimization for sustained CD management. A retrospective observational study was conducted involving 158 patients [TDM: n = 87, age(16 39), male = 64; Non-TDM: n = 71, age(16–40), male = 57] with moderate-to-severe CD who achieved clinical remission following ustekinumab therapy, between October 2020 and November 2024, sourced from three inflammatory bowel disease centers. All patients received 8 weekly ustekinumab maintenance treatment with or without therapeutic drug monitoring (TDM)-guided optimization. The clinical outcomes and disease relapse were evaluated at year 1 and 2. The non-TDM group had a slightly higher endoscopic response and mucosal healing rate at baseline, there were no statistically significant differences between two cohorts at baseline with respect to demographic and clinical characteristics. In this multicenter retrospective study of 158 CD patients in clinical remission, TDM-guided dosing (n = 87) significantly improved 1 year (83.9% vs. 70.4%, p = 0.042) and 2 year remission rates (71.3% vs. 46.5%, p = 0.002) compared to standard therapy (n = 71). Subgroup analyses confirmed benefits in endoscopic responders and mucosal healing cohorts. TDM patients exhibited higher ustekinumab trough levels (3.00 vs. 1.46 μg/mL at year 1, p < 0.001) and lower relapse rates (p = 0.003). Neither the TDM nor the non-TDM cohorts reported any severe adversative events. TDM-guided optimization of ustekinumab maintenance treatment is an efficacious and safe strategy for CD patients with ustekinumab induced clinical remission.
Similar content being viewed by others
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease may affect the entire gastrointestinal tract, characterized by progressive and destructive segmental transmural lesions, which can lead to a spectrum of clinical presentations, including recurrent abdominal pain, diarrhea, intestinal obstruction, and perianal disease1. The introduction of various biological agents since the turn of the twenty-first century has revolutionized the therapeutic strategies to CD, with early biological intervention now considered the preferred first-line therapeutic choice. Achieving sustained clinical remission, or even sustained mucosal or histological healing was a considerable challenge to clinical practice.
Ustekinumab, a fully human immunoglobulin monoclonal antibody targeting the p40 subunit of interleukin-12 (interleukin, IL) and IL-23, was approved for moderate to severe CD treatment2. Clinical trials and real-world evidence demonstrated its efficacy and safety in inducing disease remission and maintenance treatment3. Although the IM-UNITI Trial five-year efficacy and safety of Ustekinumab treatment in crohn’s disease described that 34.4% of patients in the every-8-weeks group and 28.7% in the every-12-weeks group were in clinical remission at week 252. Corresponding remission rates among patients who entered the LTE were 54.9% and 45.2%4 real-world data revealed that around half of patients did not achieve clinical remission after one year of maintenance therapy5. In a systematic review of 38 studies of ustekinumab in patients with CD, 60% and 34% of patients achieved clinical response and remission with induction therapy, and only 31% sustaining remission at 1 year6. In long-term treatment, 84.0% of patients who received standard 8-weekly ustekinumab therapy experienced dose intensification by shortening dose intervals to 4 weeks in a 3-year cohort study5.
Drawing on the experience of anti-TNF, investigations have established that rigorous therapeutic drug monitoring (TDM) of serum trough drug concentrations was a crucial clinical instrument for maintenance therapy of CD patients. In clinical practice, it was considered imperative to tailor CD treatment based on the TDM7. Similar to anti-TNF, there was inter-individual variation in clearance of ustekinumab and higher serum trough levels were observed in patients with clinical and endoscopic response, compared with non-responders8. A substantial amount of clinical data delineated a tenable association between ustekinumab serum trough levels and therapeutic efficacy, albeit there remained discernible variability concerning the therapeutic trough concentration thresholds deemed efficacious for sustained remission9,10,11. Studies in China elucidated that trough concentrations of ustekinumab exceeding 1.12 μg/ml were associated with endoscopic remission from week 16 to 20, and levels surpassing 2.11 μg/ml of were correlated with improved clinical remission for CD patients with peripheral fistulas12,13, Rosa Gómez Espín, et al. further explored a significant correlation between trough concentrations of ustekinumab and both clinical and biochemical remission14. Recent meta-analysis advocated for target trough levels of ustekinumab to be no less than 1–3 µg/mL during the maintenance treatment phase15. Although dose escalation, including intravenous reinduction and/or intensification to every 4 to 6 weeks subcutaneous dosing, utilized in patients with suboptimal response with variable success16, to date, there were seldom studies investigating TDM-guided optimized treatment in ustekinumab maintenance therapy. Our previous findings indicated that in maintenance phase, trough levels of ustekinumab were associated with sustained clinical outcomes, and an optimal cutoff level above 3.0 µg/ml would be more advantageous for achieving prolonged clinical remission. (The relevant data have been presented in the abstract of the 2025 AOCC Conference). Despite emerging evidence links ustekinumab trough levels to endoscopic and clinical outcomes, TDM-guided optimization remains underexplored.
In terms of the safety profile of UST in the treatment of CD, a review of the literature published in the PubMed database over the last 5 years was conducted. The review discusses the available data on the efficacy and safety of UST, as well as its comparison with other biologic therapies, such as infliximab and adalimumab. UST, although not significantly greater to adalimumab, has lower immunogenicity and higher treatment retention17. Another study summarized the final cumulative safety data of ustekinumab for the treatment of CD over 5 years and UC over 4 years, showing that its safety profile is superior to that of placebo and continues to support the well-established safety profile across all approved indications18.
Here, we reported on real-world cohort to explore the efficacy and safety of TDM-based optimization of maintenance therapy in patients with moderate-to-severe active CD who had achieved clinical remission by treatment with ustekinumab.
Methods
Patients
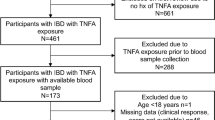
This was a multicenter retrospective and observational cohort study conducted between October 2020 and November 2024 at three IBD centers in Hubei Province, China, including Renmin Hospital of Wuhan University, First Hospital of Jinzhou City, and Central Hospital of Yichang City. IBD database were retrospectively reviewed for those patients with moderate-to-severe CD [defined as Crohn’s disease activity index (CDAI) > 220 or Harvey-Bradshaw Index (HBI) > 4]19,20who achieved clinical remission (defined as CDAI ≤ 150 or HBI ≤ 4)21post-induction treatment by ustekinumab22. A total of 360 patients were screened, with 158 ultimately selected for inclusion who were stratified into TDM-guided optimization therapy group (TDM group) (n = 87) and standard therapy group (non-TDM group) (n = 71) (Fig. 1A). All patients were devoid of corticosteroid therapy and duration of follow-up was 2 years.
The non-TDM group includes patients who did not undergo drug concentration monitoring or those who did but did not have dose adjustment based on trough levels. At baseline of study, 60 patients achieved endoscopic response including 53 patients with mucosal healing. Non-TDM patients received fixed subcutaneous 90 mg ustekinumab every 8 weeks.
In TDM cohort, 57 patients acheived endoscopic response [defined as ΔSimple Endoscopic Score for Crohn’s Disease (SES-CD) ≥ 6] including 50 patients with mucosal healing (MH:defined as a lack of any visible ulcerations) at baseline. During the follow-up, serum ustekinumab trough levels were systematically monitored and dosing was adjusted based on trough levels: subcutaneous 90 mg q8w if ≥ 3.0 μg/mL; weight-based intravenous reinduction(≤ 55 kg: 260 mg; 55–85 kg: 390 mg; > 85 kg: 520 mg) if < 3.0 μg/mL. (Fig. 1B).
Exclusion criteria encompassed patients who did not exhibit moderate-to-severe disease activity according to CDAI or HBI prior to ustekinumab induction therapy, those not in clinical remission at the baseline of the study during ustekinumab maintenance treatment, as well as individuals diagnosed with ulcerative colitis, presence of pouch or ostomy, or a history of extensive bowel resection.
Data collection
Data were meticulously collected through retrospective review of medical records and database of IBD centers, including baseline demographics such as age, gender, disease duration, lesion location and behavior, history of prior treatments, and initial disease severity. The disease phenotype was considered according to the Montreal classification23. The investigation documented details regarding the regimen and dosing of ustekinumab during the maintenance phase, alongside the occurrence of adverse events and the timing of TDM results. Additionally, levels of biochemical markers including fecal calprotectin (FC), clinical assessments, the concurrent use of medications including steroids, immunosuppressants, other biological agents, and endoscopic evaluations, were recorded at baseline and subsequent follow-up visits.
Declaration
We confirm that all methods were performed in accordance with the relevant guidelines and regulations. This study was approved by the ethics committee of Renmin Hospital of Wuhan University (Wuhan, China; WDRY2021-Y076).Informed consent was obtained from all subjects and/or their legal guardian(s) prior to their participation in the study.
Clinical outcomes
Clinical remission (CDAI ≤ 150 or HBI ≤ 4) at year 1 and 2 from the start of ustekinumab maintenance treatment were analyzed. Ustekinumab failure was defined as: (1) withdrawal of biologics due to loss of response (defined as an initial response to ustekinumab with a loss of response during the maintenance phase according to physician assessment) or (2) intolerance, and/or (3) the need for surgery21. Ustekinumab withdrawal for personal reasons, pregnancy or remission was not considered to be a failure.
Measurement of serum ustekinumab trough levels and anti-ustekinumab antibody
Serum ustekinumab trough levels were quantified using a specific biotinylated antibody directed against the idiotype of ustekinumab in an enzyme-linked immunosorbent assay (ELISA) by Guangzhou Huayin Medical Examination Center (Guangzhou, Guangdong Province, China), which assay could measure ustekinumab trough levels from 1 to 60 μg/mL. Two samples of serum were taken for each patient per time, and results were the mean concentrations. All serum samples and results were blindly collected and analyzed. Biologists performing the analysis of ustekinumab trough levels were blinded to the clinical, biological, and endoscopic outcomes of patients. Anti-ustekinumab antibody (AUAs) levels were determined by using an antigen-bridging enzyme immunoassay.
Safety assessments
Safety data were recorded retrospectively from the medical records for each patient. We also collected safety outcomes including patient-reported adverse events, serious adverse events (SAE) requiring hospitalization or treatment discontinuation and CD-related surgery.
Statistical analysis
Descriptive statistics for demographic and clinical characteristics include mean or median (interquartile range [IQR]) for continuous variables and frequency distributions for categorical data. Categorical variables were analyzed by χ2 or Fisher’s exact test, and continuous variables were compared by paired t tests or Wilcoxon matched pairs signed rank tests for parametric and nonparametric data, respectively; The Kaplan–Meier method was used to assess the relapse rate of the disease during the 2 year maintenance treatments. p < 0.05 was considered to be significant.
Results
Patient characteristics
All patients achieved clinical remission after receiving ustekinumab treatment, among whom 87 underwent therapeutic drug monitoring during the maintenance period, while 71 did not (non-TDM group). There were no statistically significant differences between two cohorts at baseline with respect to demographic and clinical characteristics including age and sex as detailed in Table 1. The mean duration of disease, surgical history, extraintestinal manifestations, disease behavior, site of involvement and perianal disease also did not differ between two groups. A significantly higher proportion of patients in non-TDM group accomplished improved endoscopic outcomes, as indicated by endoscopic response [65.5% (57/87) in TDM group versus 84.5% (60/71) in non-TDM group, p = 0.001] and mucosal healing [57.5% (50/87) in TDM group versus 74.6% (53/71) in non-TDM group, p = 0.024]. Approximately half of the patients in both groups exhibited a complicated phenotype, with around 50% presenting with either stricturing or fistulizing disease. Roughly a quarter of the patients had been treated with anti-TNF therapy, and approximately 30% had history of CD-specific surgery. There were no significant disparities in the history of immunosuppressive treatment and proportion of patients with normalized FC and CRP at baseline between TDM and non-TDM groups.
Efficacy of optimized ustekinumab maintenance treatment for patients with clinical remission
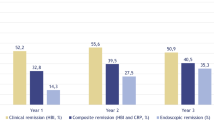
All participants in current cohorts had achieved clinical remission prior to the study onset. At year 1 since maintenance treatment with ustekinumab, patients who received treatment dosing guided by TDM maintained a significant higher rate of clinical remission, with an 83.9% (73/87) success rate, as compared to 70.4% (50/71) in non-TDM group (p = 0.042) (Fig. 2A). This superior clinical outcomes in TDM group was sustained over an extended period of maintenance therapy, with 71.3% (62/87) clinical remission at year 2, significantly exceeding the rate of 46.5% (33/71) observed in non-TDM group (p = 0.002). TDM-guided, optimized maintenance treatment for ustekinumab significantly decreased disease relapse when contrasted with standard maintenance therapy (p = 0.003) (Fig. 3A).
Long-term clinical remission for patients in TDM and non-TDM cohorts. (A) clinical remission for patients achieved clinical remission at baseline. (B) Clinical remission for patients reached endoscopic response at baseline. (C) Clinical remission for patients with mucosal healing at baseline. TDM group, patients received optimized ustekinumab maintenance treatment guided by TDM; non-TDM group, patients received routinely subcutaneous ustekinumab maintenance treatment.
Long-term relapse for patients in TDM and non-TDM cohorts. (A) Non-relapse rate for patients achieved clinical remission at baseline. (B) Non-relapse rate for patients reached endoscopic response at baseline. (C) Non-relapse rate for patients with mucosal healing at baseline. TDM group, patients received optimized ustekinumab maintenance treatment guided by TDM; non-TDM group, patients received routinely subcutaneous ustekinumab maintenance treatment.
Efficacy of optimized ustekinumab maintenance therapy for patients with endoscopic response at baseline
In current study, 57 patients in TDM cohort and 60 in non-TDM cohort had demonstrated endoscopic response at baseline. Subsequently, we conducted a comparative analysis to evaluate the efficacy of TDM-guided treatment optimization on this specific patient subset. At the first year of follow-up, TDM group exhibited a trend towards higher rates of clinical remission, albeit not statistically significant, when compared to non-TDM group [86.0% (49/57) versus 76.7% (46/60), p = 0.198] (Fig. 2B). However, by the second year, TDM-guided ustekinumab optimization cohort achieved a significantly greater proportion of patients in clinical remission compared to non-TDM cohort [77.2% (44/57) versus 55.0% (33/60), p = 0.011]. Moreover, TDM group demonstrated a significantly lower relapse rate than non-TDM group (p = 0.007) (Fig. 3B).
Efficacy of optimized ustekinumab maintenance treatment for patients with mucosal healing at baseline
Additional analysis conducted on patients who exhibited mucosal healing at the outset demonstrated the therapeutic advantage of TDM-guided optimization for ustekinumab maintenance therapy in patients experiencing profound disease remission. Within these subgroups, 50 patients were managed under the TDM protocol, while 53 received non-TDM treatment. At the first year, patients in TDM cohort achieved clinical remission rates comparable to those in non-TDM cohort [90.0% (45/50) vs. 84.9% (45/53), p = 0.437]. However, by the second year, TDM group exhibited a significantly higher remission rate [84.0% (42/50) vs. 64.2% (34/53), p = 0.022] (Fig. 2C). Moreover, TDM-guided maintenance therapy significantly reduced the relapse rate compared to the standard maintenance regimen (p = 0.024) (Fig. 3C).
Trough concentration for subcutaneous ustekinumab during maintenance treatment
At baseline, there was no significance in trough levels of ustekinumab concentrations between patients with clinical remission in TDM and non-TDM cohorts [The baseline drug concentration in the non-TDM group was a one-time test conducted free of charge for patients (with informed consent obtained), and no further therapeutic drug monitoring was performed during the subsequent maintenance period] (Fig. 4). However, subsequent maintenance optimization treatment guided by TDM obviously increased trough concentrations of subcutaneous ustekinumab during both short-term [3.00 (IQR, 1.62–4.64) vs. 1.46 (IQR, 0.12–3.25) μg/ml at year 1, p < 0.001] and long-term [2.72 (IQR, 1.19–4.61) vs. 0.89 (IQR, 0.36–1.16) μg/ml, p < 0.001] maintenance period . We next assessed the times of intravenous ustekinumab per year for patients in TDM group. In the first year, patients received 2.1 ± 0.5 intravenous doses of ustekinumab while in the second year, intravenous doses significantly decreased to 1.8 ± 0.8 (p = 0.018). In both groups, no serum anti-ustekinumab antibody was detected during the follow-up.
Treatment discontinuation and adverse events
Of the patients who received TDM-guided maintenance treatment, a retention rate of 86.2% (75 out of 87 patients) on ustekinumab treatment was observed by the second year, contrasting with a significantly higher rate of discontinuation among patients undergoing conventional maintenance therapy [49.3% (35 out of 71 patients), p < 0.001]. Importantly, there were no reports of severe adverse events in either TDM or non-TDM treatment groups.
Discussion
Despite established efficacy in trials and real-world settings, ustekinumab maintenance therapy faces persistent challenges, including secondary loss of response and suboptimal long-term remission rates. Results From the SUCCESS Consortium showed that in ustekinumab maintenance treatment with subcutaneous dosing every 8 weeks, only 34.4% of patients achieved clinical remission, and lower proportion (28.7%) in the every-12-weeks cohort reached the same endpoint. After 12 months of ustekinumab maintenance therapy, a majority of patients exhibited active disease, with the cumulative incidence rates of 40% clinical remission, 32% steroid-free remission, 39% endoscopic remission, and 30% radiographic remission24. Furthermore, the annual risk for secondary loss of response (SLOR) to ustekinumab was observed to reach 21%25. The phenomenon of drug discontinuation due to loss of response is very common in the treatment of CD with biologics. A multicenter retrospective study shows that the discontinuation rate after one year of ustekinumab treatment is about 38.8%26.These results collectively implied that the long-term therapeutic efficacy of ustekinumab in CD management was suboptimal.
The secondary loss of efficacy may be attributed to multifaceted factors, including serum drug trough levels, the presence of antidrug antibodies, autoantibody generation, and heightened inflammatory responses27. Patients with active disease frequently presented with elevated serum concentrations of pro-inflammatory cytokines, IL-12 and IL-23, which could potentiate the binding affinity of ustekinumab to IL-12/23, thereby expanding its volume of distribution and leading to reduced drug trough levels9,28. These findings underscored a direct association between drug concentration and decreased therapeutic response during ustekinumab maintenance therapy. Therefore, expeditious therapeutic modifications were imperative to mitigate secondary loss of efficacy, optimizing drug trough levels and potentially rejuvenating therapeutic response in patients experiencing diminished efficacy.
Several studies explored the possibility and efficacy of optimized maintenance treatment with ustekinumab by shortening intervals or re-induction with intravenous injection29,30,31. Shortening intervals between subcutaneous doses to 4 or 6 weeks elicited steroid-free clinical remission to approximately 50% and 60% of patients at 1- and 2-year follow-up, respectively. For patients still experienced partial response or eventual loss of response after optimization treatment by subcutaneous doses every 4 weeks, re-induction with intravenous ustekinumab administration facilitated to achieve 31% steroid-free remission in short-term (around 14 weeks), and 62.5% endoscopic remission in medium-term (around 30.9 weeks)32,33. Our previous studies indicated that compared to standard therapy with ustekinumab, optimization by two initial intravenous inductions was more effective for patients with severe CD34. These all suggested the effectiveness of ustekinumab optimization strategy, albeit the specific plan remained to be further explored. Enhancing the sustained therapeutic efficacy of ustekinumab and mitigating the likelihood of secondary non-response constituted the central objective of current investigation.
Our findings aligned with prior evidence linking ustekinumab trough levels to sustained remission. By maintaining concentrations ≥ 3.0 μg/mL, TDM mitigated pharmacokinetic variability and secondary loss of response. Notably, even patients with baseline endoscopic improvement benefited from TDM, suggesting its role in preventing late relapse.
In the present investigation, minimal differences in demographic and clinical attributes were observed between the two cohorts, with the exception that a higher prevalence of patients in the non-TDM cohort achieved endoscopic response and mucosal healing compared to the TDM cohort. Despite initial reduced endoscopic outcomes, TDM cohort exhibited a significant benefit in maintaining remission and preventing disease relapse over a 2-year follow-up. For patients who achieved endoscopic response or mucosal healing at research baseline, TDM-guided maintenance optimization demonstrated higher, albeit non-significant rates of clinical remission at year 1 compared to non-TDM group. However, by year 2, TDM cohort achieved significant benefits in sustained remission, with higher proportions in subgroups of endoscopic response .These findings suggested that TDM-guided optimized maintenance therapy with ustekinumab conferred substantial advantages in sustained clinical remission, even for patients with improved endoscopic outcomes. The study further explored poSZtential mechanisms underlying TDM efficacy, highlighting that TDM-based therapeutic optimization significantly elevated trough concentrations of ustekinumab, maintaining serum levels consistently above 3.0 µg/mL, whereas trough concentrations in the non-TDM cohort were predominantly below 3.0 µg/ml. This maintenance of drug concentrations at or above 3.0 µg/mL was associated with sustained clinical remission and a reduction in disease relapse.
This study provided informative findings regarding the optimization strategy of ustekinumab based on TDM in CD patients with disease remission. However, some limitations of current study need to be considered. Predominant among these were its retrospective design and the limited sample size. It is, however, noteworthy that these data were meticulously gathered from three IBD medical centers in Hubei province, including a regional center for IBD in China with admitted abilities in IBD clinical treatment and investigative research. All these patients received consistent follow-up management. Additionally, the follow-up duration, designed to evaluate long-term maintenance efficacy, were only 2 years. Future studies were imperative to collect and scrutinize data on the efficacy and safety of current optimized strategy for protracted maintenance therapy. An additional limitation was limited endoscopic follow-up. Prospective trials with protocolized TDM thresholds and extended follow-up are warranted to validate this strategy.
In conclusion, our study revealed that the formulation selection of ustekinumab maintenance therapy in CD patients, contingent upon trough levels exceeding of 3.0 μg/ml, constituted an efficacious and practical therapeutic strategy for those with disease remission.
References
Veauthier, B. & Hornecker, J. R. Crohn’s disease: Diagnosis and management. Am. Fam. Phys. 98(11), 661–669 (2018).
Lamb, Y. N. & Duggan, S. T. Ustekinumab: A review in moderate to severe crohn’s disease. Drugs 77(10), 1105–1114 (2017).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375(20), 1946–1960 (2016).
Sandborn, W. J. et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment. Pharmacol. Ther. 48(1), 65–77 (2018).
Barkai, L. J. et al. Efficacy, drug sustainability, and safety of ustekinumab treatment in Crohn’s disease patients over three years. Sci. Rep. 14(1), 14909 (2024).
Honap, S. et al. Effectiveness and safety of ustekinumab in inflammatory bowel disease: A systematic review and meta-analysis. Dig. Dis. Sci. 67(3), 1018–1035 (2022).
Papamichael, K. et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 17(9), 1655–1668 (2019).
Hanauer, S. B. et al. IM-UNITI: Three-year efficacy, safety, and immunogenicity of ustekinumab treatment of crohn’s disease. J. Crohns Colitis 14(1), 23–32 (2020).
Adedokun, O. J. et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology 154(6), 1660–1671 (2018).
Hirayama, H. et al. Ustekinumab trough levels predicting laboratory and endoscopic remission in patients with Crohn’s disease. BMC Gastroenterol. 22(1), 195 (2022).
Shehab, M. et al. Relationship between Ustekinumab trough concentrations and clinical, biochemical and endoscopic outcomes in Crohn’s disease: A multi-center nationwide retrospective study (TARGET STUDY). Medicine (Baltimore) 103(27), e38804 (2024).
Yao, J. et al. Ustekinumab promotes radiological fistula healing in perianal fistulizing Crohn’s disease: A retrospective real-world analysis. J. Clin. Med. 12(3), 9394 (2023).
Yao, J. Y. et al. Ustekinumab trough concentration affects clinical and endoscopic outcomes in patients with refractory Crohn’s disease: A Chinese real-world study. BMC Gastroenterol. 21(1), 380 (2021).
Gomez Espin, R. et al. Association between ustekinumab trough concentrations and biochemical outcomes in patients with Crohn’s disease. A real life study. Rev. Esp. Enferm. Dig. 113(2), 110–115 (2021).
Restellini, S. & Afif, W. Update on TDM (therapeutic drug monitoring) with ustekinumab, vedolizumab and tofacitinib in inflammatory bowel disease. J. Clin. Med. 10(6), 1242 (2021).
Meserve, J. et al. Effectiveness of reinduction and/or dose escalation of ustekinumab in Crohn’s disease: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20(12), 2728–2740 (2022).
Piecuch, D. et al. Ustekinumab in the treatment of Crohn’s disease-a narrative review on clinical efficacy and safety profile. Pharmacy (Basel) 13(3), 73 (2025).
Ghosh, S. et al. Safety of ustekinumab in inflammatory bowel disease: pooled safety analysis through 5 years in Crohn’s disease and 4 years in ulcerative colitis. J. Crohns Colitis 18(7), 1091–1101 (2024).
Frenz, M. B. et al. Comparison between prospective and retrospective evaluation of Crohn’s disease activity index. Am. J. Gastroenterol. 100(5), 1117–1120 (2005).
Foti, P. V. et al. Crohn’s disease of the small bowel: evaluation of ileal inflammation by diffusion-weighted MR imaging and correlation with the Harvey-Bradshaw index. Radiol. Med. 120(7), 585–594 (2015).
Sandborn, W. J. et al. Five-year efficacy and safety of ustekinumab treatment in crohn’s disease: the IM-UNITI trial. Clin. Gastroenterol. Hepatol. 20(3), 578–590 (2022).
Gajendran, M. et al. A comprehensive review and update on Crohn’s disease. Dis. Mon. 64(2), 20–57 (2018).
Xiang, J. et al. Clinical features of Crohn’s disease stratified by age at diagnosis according to montreal classification. J. Inflamm. Res. 16, 737–746 (2023).
Johnson, A. M. et al. The real-world effectiveness and safety of ustekinumab in the treatment of Crohn’s disease: Results from the success consortium. Am. J. Gastroenterol. 118(2), 317–328 (2023).
Yang, H. et al. Systematic review with meta-analysis: Loss of response and requirement of ustekinumab dose escalation in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 55(7), 764–777 (2022).
Liefferinckx, C. et al. Long-term clinical effectiveness of ustekinumab in patients with crohn’s disease who failed biologic therapies: A national cohort study. J. Crohns Colitis 13(11), 1401–1409 (2019).
Wong, U. & Cross, R. K. Primary and secondary nonresponse to infliximab: Mechanisms and countermeasures. Expert Opin. Drug Metab. Toxicol. 13(10), 1039–1046 (2017).
Straatmijer, T. et al. Ustekinumab trough concentrations are associated with biochemical outcomes in patients with Crohn’s disease. Dig. Dis. Sci. 68(6), 2647–2657 (2023).
Biemans, V. B. C. et al. Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn’s disease patients with prior failure to anti-TNF treatment. Aliment. Pharmacol. Ther. 52(1), 123–134 (2020).
Fumery, M. et al. Effectiveness and safety of ustekinumab intensification at 90 mg every four weeks in Crohn’s disease: A multicenter study. J. Crohns Colitis 15, 222 (2020).
Dalal, R. S., Pruce, J. C. & Allegretti, J. R. Long-term outcomes after ustekinumab dose intensification for inflammatory bowel diseases. Inflamm. Bowel Dis. 29(5), 830–833 (2023).
Heron, V. et al. Efficacy of intravenous ustekinumab reinduction in patients with crohn’s disease with a loss of response. J. Can. Assoc. Gastroenterol. 5(5), 208–213 (2022).
Sedano, R. et al. Intravenous ustekinumab reinduction is effective in prior biologic failure Crohn’s disease patients already on every-4-week dosing. Clin. Gastroenterol. Hepatol. 19(7), 1497–1498 (2021).
Ren, H. et al. Efficacy of ustekinumab optimization by 2 initial intravenous doses in adult patients with severe Crohn’s disease. Inflamm. Bowel Dis. 30(8), 1295–1302 (2024).
Funding
This study was funded by the National Natural Science Foundation of China [Grant Number 81302131 and 82170632 (to Ping An)], National Natural Science Foundation of China [Grant Number 82200598(to Haixia Ren)],Emergency Scientific Research Project of Wuhan Municipal Health Commission [Grant Number EX20B04 (to Ping An)] and Teaching and Research Project of Wuhan University School of Medicine [Grant Number 2021033 (to Ping An)].
Author information
Authors and Affiliations
Contributions
Haixia Ren, Juan Su , Xinsheng Gao ,Jian Kang, Jing Wang, Wei Wang: data curation, patient management and investigation, endoscopy, project administration, and writing–review & editing. Haixia Ren: methodology, manuscript preparation. Anning Yin, Jiao Li, Jing an, Qian Zhou, Wei Chen, Pengbo Wu: patient management and investigation, endoscopy, project administration. Zhishun Tang, Huipeng Wan,Xinin Wang: data curation. Ping An: methodology, patient management, manuscript preparation, data curation, formal analysis, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent for publication
Obtained.
Ethics approval
This study was approved by the ethics committee of Renmin Hospital of Wuhan University (Wuhan, China; WDRY2021-Y076).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, H., Su, J., Yin, A. et al. Long-term efficacy of therapeutic drug monitoring-guided optimization of ustekinumab maintenance therapy for Crohn’s disease patients. Sci Rep 15, 25540 (2025). https://doi.org/10.1038/s41598-025-09802-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-09802-5